

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (2): 327.doi: 10.7503/cjcu20170341
• Physical Chemistry • Previous Articles Next Articles
DENG Guixian1,2, LI Kongzhai2,3,*( ), CHENG Xianming2, GU Zhenhua2, LU Chunqiang2, ZHU Xing2
), CHENG Xianming2, GU Zhenhua2, LU Chunqiang2, ZHU Xing2
Received:2017-06-01
Online:2018-02-10
Published:2018-01-11
Contact:
LI Kongzhai
E-mail:kongzhai.li@foxmail.com
Supported by:CLC Number:
TrendMD:
DENG Guixian, LI Kongzhai, CHENG Xianming, GU Zhenhua, LU Chunqiang, ZHU Xing. Red Mud as Oxygen Carrier for Chemical Looping Combustion of Methane: Reactivity and Cyclic Performance†[J]. Chem. J. Chinese Universities, 2018, 39(2): 327.
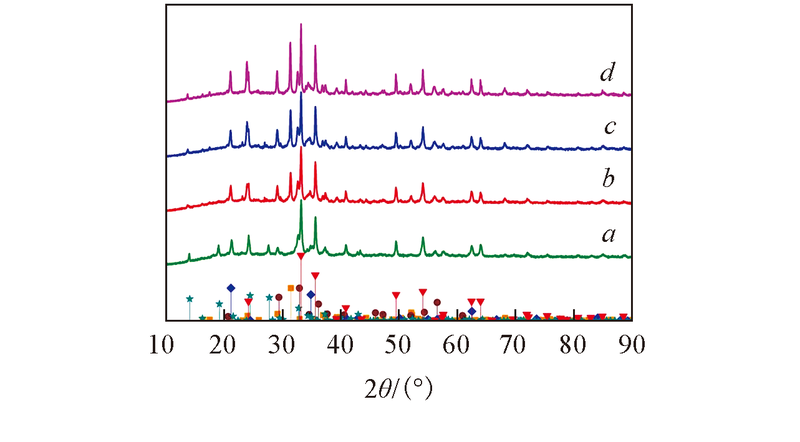
Fig.2 XRD patterns of samples 800-RM(a), 850-RM(b), 900-RM(c) and 950-RM(d)◆ Na6Al4Si4O17, JCPDS No.76-2385;■ Ca2Al2SiO7, JCPDS No.35-0755;▼ Fe2O3, JCPDS No.33-0664;● Ca3TiFeSi3O12, JCPDS No.47-1877;★ Na6CaAl6Si6(CO3)O24·2H2O, JCPDS No.48-1862.
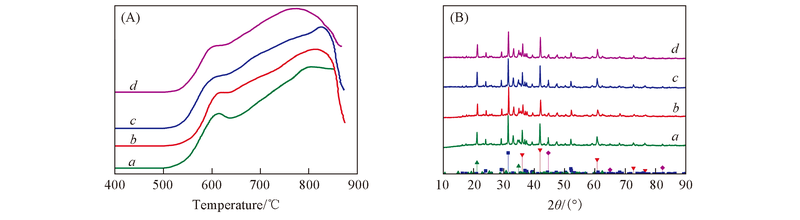
Fig.3 H2-TPR profiles(A) and XRD(B) patterns of samples 800-RM(a), 850-RM(b), 900-RM(c) and 950-RM(d) ▼ FeO, JCPDS No.06-0615; ▲ Na1.45Al1.45Si0.55O4, JCPDS No.49-0002; ◆ Fe, JCPDS No.06-0696; ■ Ca2Al2Si4O17, JCPDS No.35-0755.
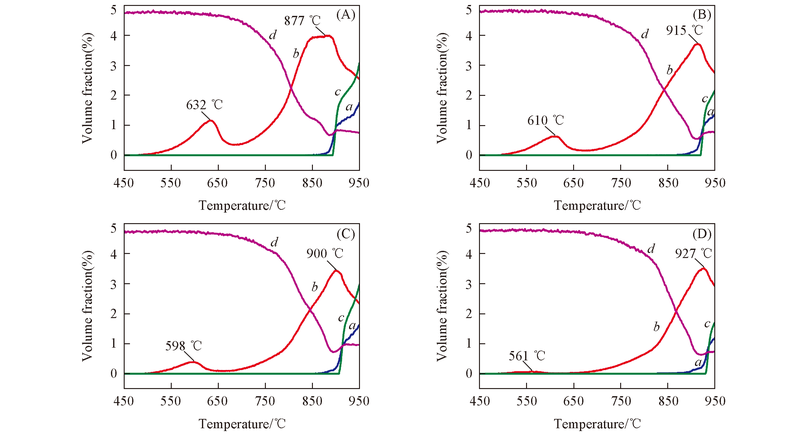
Fig.4 Typical curves of the main products and reactants during the temperature programmed reactions with CH4 over the red mud oxygen carriers (A) 800-RM; (B) 850-RM; (C) 900-RM; (D) 950-RM. a. CO; b. CO2; c. H2; d. CH4.
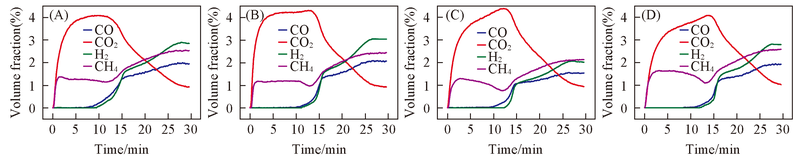
Fig.5 Typical curves of the main products and reactant during the isothermal reactions with CH4 over the red mud oxygen carriers (A) 800-RM; (B) 850-Rm; (C) 900-RM; (D) 950-RM.
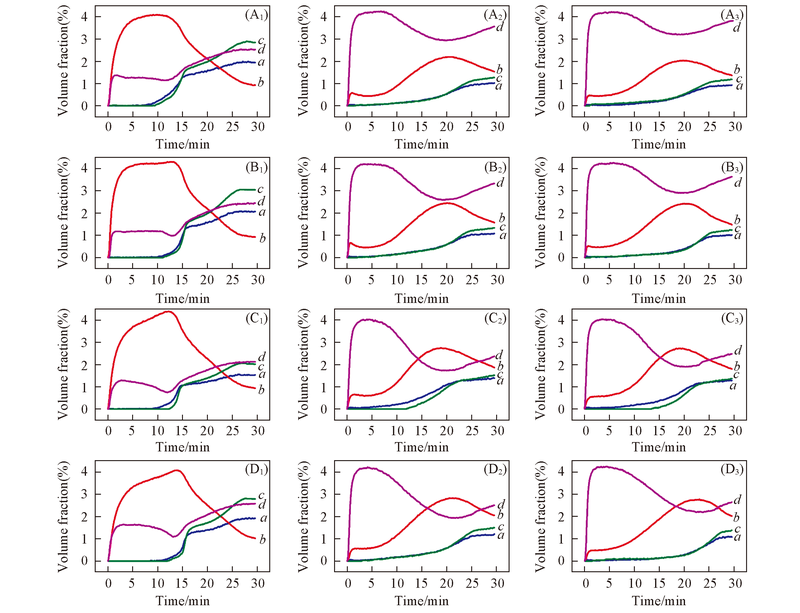
Fig.6 Typical curves of the main products and reactant during 5 cycles of redox reaction(A) 800-RM; (B) 850-RM; (C) 900-RM; (D) 950-RM. (A1)~(D1) The first; (A2)~(D2) the third;(A3)~(D3) the fifth. a. CO; b. CO2; c. H2; d. CH4.
| [1] | Lyngfelt A., Bo L., Mattisson T., Chem. Eng. Sci., 2001, 56(10), 3101—3113 |
| [2] | Hossain M. M., de Lasa H. I., Chem. Eng. Sci., 2008, 63(18), 4433—4451 |
| [3] | Ishida M., Zheng D., Akehata T., Energy,1987, 12(2), 147—154 |
| [4] | Fan L. S., Zeng L., Wang W. L., Luo S. W., Energ. Environ. Sci., 2012, 5(6), 7254—7280 |
| [5] | Bhavsar S., Najera M., Veser G., Chem. Eng. Technol., 2012, 35(7), 1281—1290 |
| [6] | Fan L. S., Li F. X., Ind. Eng. Chem. Res., 2010, 49(21), 10200—10211 |
| [7] | Gu H., Shen L., Xiao J., Zhang S., Song T., Chen D., Ind. Eng. Chem. Res., 2013, 52(5), 1795—1805 |
| [8] | Zeng L. P., Huang F., Zhu X., Zheng M., Li K. Z., Chem. J. Chinese Universities, 2017, 38(1), 115—125 |
| (曾良鹏, 黄樊, 祝星, 郑敏, 李孔斋. 高等学校化学学报, 2017,38(1), 115—125) | |
| [9] | Qin W., Lin C. F., Cheng W. L., Xiao X. B., Chem. J. Chinese Universities, 2015, 36(1), 116—123 |
| (覃吴, 林常枫, 程伟良, 肖显斌. 高等学校化学学报, 2015,36(1), 116—123) | |
| [10] | Gu Z., Li K., Wang H., Qing S., Zhu X., Wei Y., Appl. Energ., 2016, 163, 19—31 |
| [11] | Adánez J., Diego L. F. D., Garcíalabiano F., Gayán P., Abad A., Energ. Fuel., 2004, 18(2), 371—377 |
| [12] | Richter H. J., Knoche K. F., ACS Symp. Ser., 1983, 235, 71—85 |
| [13] | Ishida M., Zheng D., Akehata T., Energy,1987, 12, 147—154 |
| [14] | Bao J., Chen L., Liu F., Zhen F., Heather S. N., Liu K., Ind. Eng. Chem. Res., 2016, 55(29), 8046—8057 |
| [15] | Adanez J., Abad A., Garcia-Labiano F., Gayan P., de Diego L. F., Prog. Energy Combust. Sci., 2012, 38(2), 215—282 |
| [16] | Xu L., Wang J., Li Z., Cai N., Energ. Fuel., 2013, 27(3), 1522—1530 |
| [17] | Xu L., Edland R., Li Z., Leion H., Zhao D., Cai N., Energ. Fuel., 2014, 28(11), 7085—7092 |
| [18] | Leion H., Mattisson T., Lyngfelt A., Energ. Fuel., 2009, 23(4), 2307—2315 |
| [19] | Mendiara T., Abad A., de Diego L. F., García-Labiano F., Gayán P., Adanez J., Energ. Fuel., 2012, 26(2), 1420—1431 |
| [20] | Mei D., Mendiara T., Abad A., de Diego L. F., García-Labiano F., Gayán P., Adánez J., Zhao H., Energ. Fuel., 2015, 29(10), 6605—6615 |
| [21] | Tian H., Siriwardane R., Simonyi T., Poston J., Energ. Fuel., 2013, 27(8), 4108—4118 |
| [22] | Rubel A., Zhang Y., Neathery J., Liu K., Energ. Fuel., 2012, 26(6), 3156—3161 |
| [23] | Wang B., Yan R., Zheng Y., Zhao H., Zheng C., Fuel,2011, 90(7), 2359—2366 |
| [24] | Xiao R., Song Q., Song M., Lu Z., Zhang S., Shen L., Combust. Flame., 2010, 157(6), 1140—1153 |
| [25] | Gu H., Shen L., Xiao J., Zhang S., Song T., Energ. Fuel., 2010, 25(1), 446—455 |
| [26] | Xiao R., Song Q., Zhang S., Zheng W., Yang Y., Energ. Fuel., 2010, 24(2), 1449—1463 |
| [27] | Leion H., Jerndal E., Steenari B. M., Hermansson S., Israelsson M., Jansson E., Johnsson M., Thunberg R., Vadenbo A., Mattisson T., Lyngfelt A., Fuel,2009, 88(10), 1945—1954 |
| [28] | Linderholm C., Lyngfelt A., Cuadrat A., Jerndal E., Fuel,2012, 102(6), 808—822 |
| [29] | Xu L., Sun H., Li Z., Cai N., Appl. Energ., 2016, 162, 940—947 |
| [30] | Wen Y., Li Z., Xu L., Cai N., Energ. Fuel., 2012, 26(6), 3919—3927 |
| [31] | Leion H., Mattisson T., Lyngfelt A., Int. J. Greenh. Gas Con., 2008, 2(2), 180—193 |
| [32] | Berguerand N., Lyngfelt A., Fuel,2008, 87(12), 2713—2726 |
| [33] | Chen L., Zhang Y., Liu F., Liu K., Energ. Fuel., 2015, 29(1), 305—313 |
| [34] | Zhu X. B., Wang L. I., Guan X. M., T. Nonferr. Metal. Soc., 2015, 25(9), 3139—3145 |
| [35] | Brunori C., Cremisini C., Massanisso P., Pinto V., Torricelli L., J. Hazard. Mater., 2005, 117(1), 55—63 |
| [36] | Panda I., Jain S., Das S. K., Jayabalan R., Int. Biodeter. Biodegr., 2017, 119, 368—376 |
| [37] | Yu Z. L., Shi Z. X., Chen Y. M., Niu Y. J., Wang Y. X., Wan P. Y., T. Nonferr. Metal. Soc., 2012, 22(2), 456—460 |
| [38] | Xue S. G., Zhu F., Kong X. F., Wu C., Huang L., Huang N., William H., Environ. Sci. Pollut. R., 2016, 23(2), 1120—1132 |
| [39] | Ayres R. U., Holmberg J., Andersson B., MRS Bull., 2001, 26(6), 477—480 |
| [40] | Brunori C., Cremisini C., Massanisso P., Pinto V., Torricelli L., J. Hazard. Mater., 2005, 117(1), 55—63 |
| [41] | Mattisson T., Johansson M., Lyngfelt A., Energ. Fuel., 2004, 18(3), 628—637 |
| [42] | Cabello A., Dueso C., García-Labiano F., Gayán P., Abad A., de Diego L. F., Adánez J., Fuel., 2014, 121(4), 117—125 |
| [43] | Cabello A., Abad A., García-Labiano F., de Diego L. F., Adánez J., Chem. Eng. J., 2014, 258, 265—280 |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [3] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [4] | HU Chuanchuan, PANG Jingxiang, HE Chuangchuang, LI Wei, SUN Shutao. Sc(OTf)3 Catalyzed 1,6-Conjugate Allylation of δ-CN p-QMs: Synthesis of Allyl Substituted Diarylacetonitrile Compounds [J]. Chem. J. Chinese Universities, 2021, 42(9): 2805. |
| [5] | LIU Huazheng, PAN Xiaoguang, LI Hua, WAN Renzhong, LIU Xigong. Na2CO3-catalyzed 1,6-Conjugate Addition of Trimethylsilyl Azide to δ-CF3-δ-Aryl-disubstituted Para-Quinone Methides: Efficient Construction of Diarylmethanes Bearing CF3- and N3-Substituted Quaternary Stereocenters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2772. |
| [6] | LI Jian, YU Mingming, SUN Yuan, FENG Wenhua, FENG Zhaochi, WU Jianfeng. Effect of Aqueous Solution pH on the Oxidation of Methane to Methanol at Low Temperature [J]. Chem. J. Chinese Universities, 2021, 42(3): 776. |
| [7] | SHEN Wenjie. Molecular-fence Catalysts for Low-temperature Oxidation of Methane to Methanol [J]. Chem. J. Chinese Universities, 2020, 41(3): 375. |
| [8] | WU Hao, WANG Changzhen, QIU Yuan, TIAN Yani, ZHAO Yongxiang. Effect of Steric Confinement Dimension on Metal Site Anti-carbon Deposition Ability of Ni-SiO2 Catalysts in CH4-CO2 Reforming [J]. Chem. J. Chinese Universities, 2020, 41(11): 2488. |
| [9] | MA Jinyu, LIU Shuanglei, ZHANG Zhenguo, JIN Junyang, JIA Zhenhua. B(C6F5)3-Catalyzed Synthesis of 3,3′-Bisindolylmethane Derivatives [J]. Chem. J. Chinese Universities, 2020, 41(10): 2225. |
| [10] | RAN Shiya,SHEN Haifeng,LI Xiaonan,WANG Zilu,GUO Zhenghong,FANG Zhengping. Effect and Mechanism of Rare Earth Trifluoromethanesulfonate on the Thermal Stability of Polypropylene† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1333. |
| [11] | CHENG Wenmin,XIA Wensheng,WAN Huilin. Influence of Surface Reactivity of Lanthanum Oxide on the Activation of Methane and Oxygen† [J]. Chem. J. Chinese Universities, 2019, 40(5): 940. |
| [12] | CHEN Tao,FANG Lei,LUO Wei,MENG Yue,XUE Jilong,XIA Shengjie,NI Zheming. Theoretical Study of Dry Reforming of Methane Catalyzed by Bimetallic Alloy Cluster M12Ni(M=Pt, Sn, Cu) † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2135. |
| [13] | TONG Bo, ZHANG Zhongxiang, LIU Zhenjie, PENG Zhangquan, ZHOU Zhibin. Novel Electrolyte Containing Li[(CF3SO2)(n-C4F9SO2)N] for High Voltage LiNi0.5Mn1.5O4-based Cell† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1518. |
| [14] | CHENG Weiliang, ZHU Mengqian, QIN Wu, HOU Cuicui. Chemical Looping Combustion Characteristics of Fe2O3(104) and CO Under Synergistic Action of ZrO2/TiO2 Carrier† [J]. Chem. J. Chinese Universities, 2018, 39(3): 506. |
| [15] | BAI Yan, XIA Wensheng, WENG Weizheng, LIAN Mengshui, ZHAO Mingquan, WAN Huilin. Influence of Phosphate on La-based Catalysts for Oxidative Coupling of Methane† [J]. Chem. J. Chinese Universities, 2018, 39(2): 247. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||