

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (5): 940.doi: 10.7503/cjcu20190063
• Physical Chemistry • Previous Articles Next Articles
CHENG Wenmin, XIA Wensheng*( ), WAN Huilin
), WAN Huilin
Received:2019-01-23
Online:2019-05-06
Published:2019-03-27
Contact:
XIA Wensheng
E-mail:wsxia@xmu.edu.cn
Supported by:CLC Number:
TrendMD:
CHENG Wenmin,XIA Wensheng,WAN Huilin. Influence of Surface Reactivity of Lanthanum Oxide on the Activation of Methane and Oxygen†[J]. Chem. J. Chinese Universities, 2019, 40(5): 940.
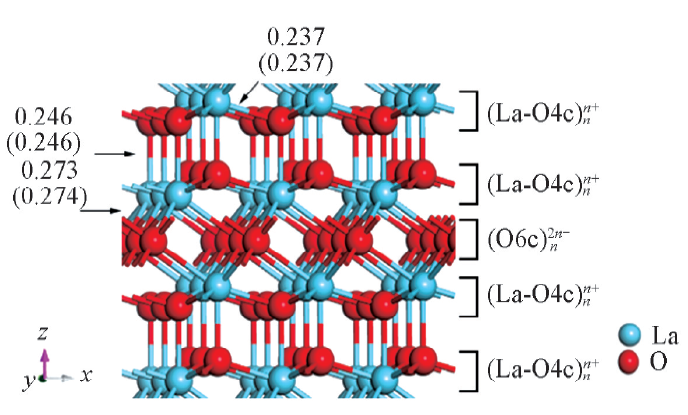
Fig.1 Structures for bulk La2O3 and the experimental and calculated(in parentheses) values for the three unique La-O bond lengths in nmO4c and O6 crepresent 4- and 6-coordinated oxygen atoms, respectively.
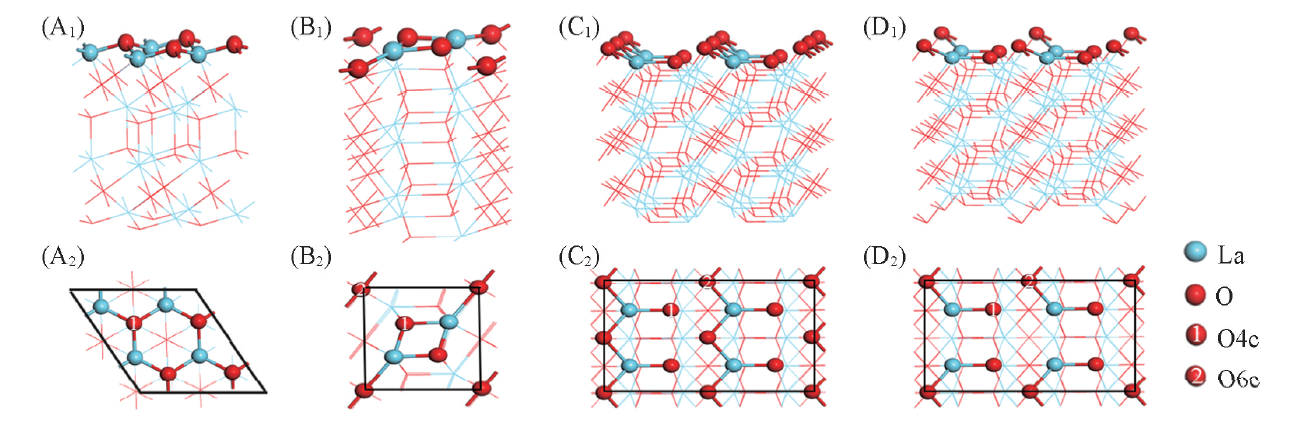
Fig.2 Side(A1—D1) and top(A2—D2) views of La2O3 surfaces(A1, A2)(2×2)(001);(B1, B2)(1×1)(110);(C1, C2)(2×2)(100), before dipole correction;(D1, D2)(2×2)(100), after dipole correction; O4c and O6c represent 4- and 6-coordinated oxygen atoms, respectively.
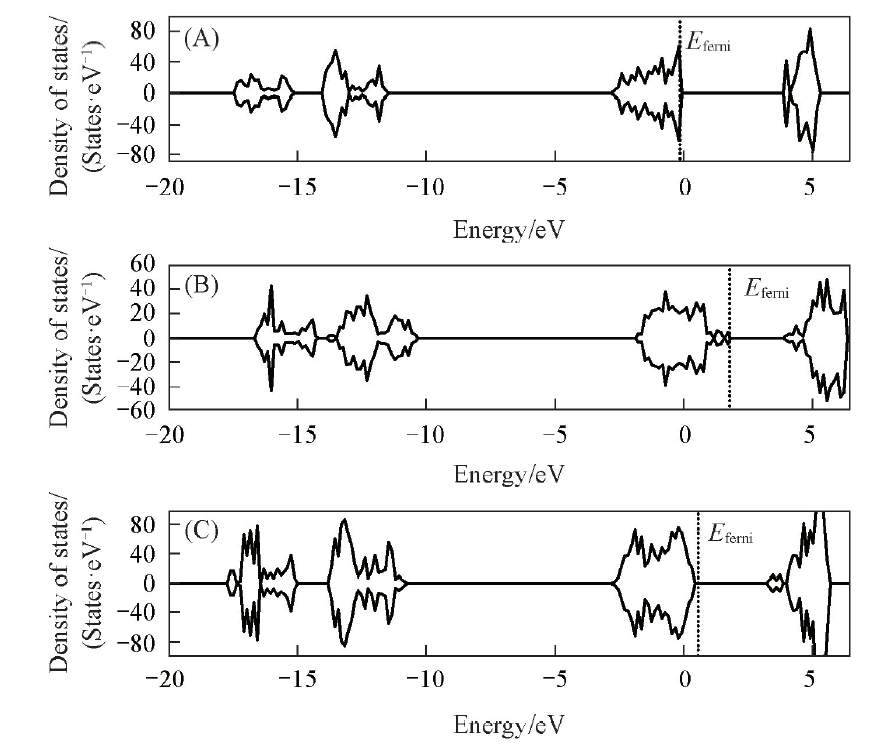
Fig.3 Density of states(DOS) of La2O3 surfaces (001)(A), (110)(B) and (100)(C)The densities of states above 0 are for orbitals with spin up, and those below 0 are for orbitals with spin down.
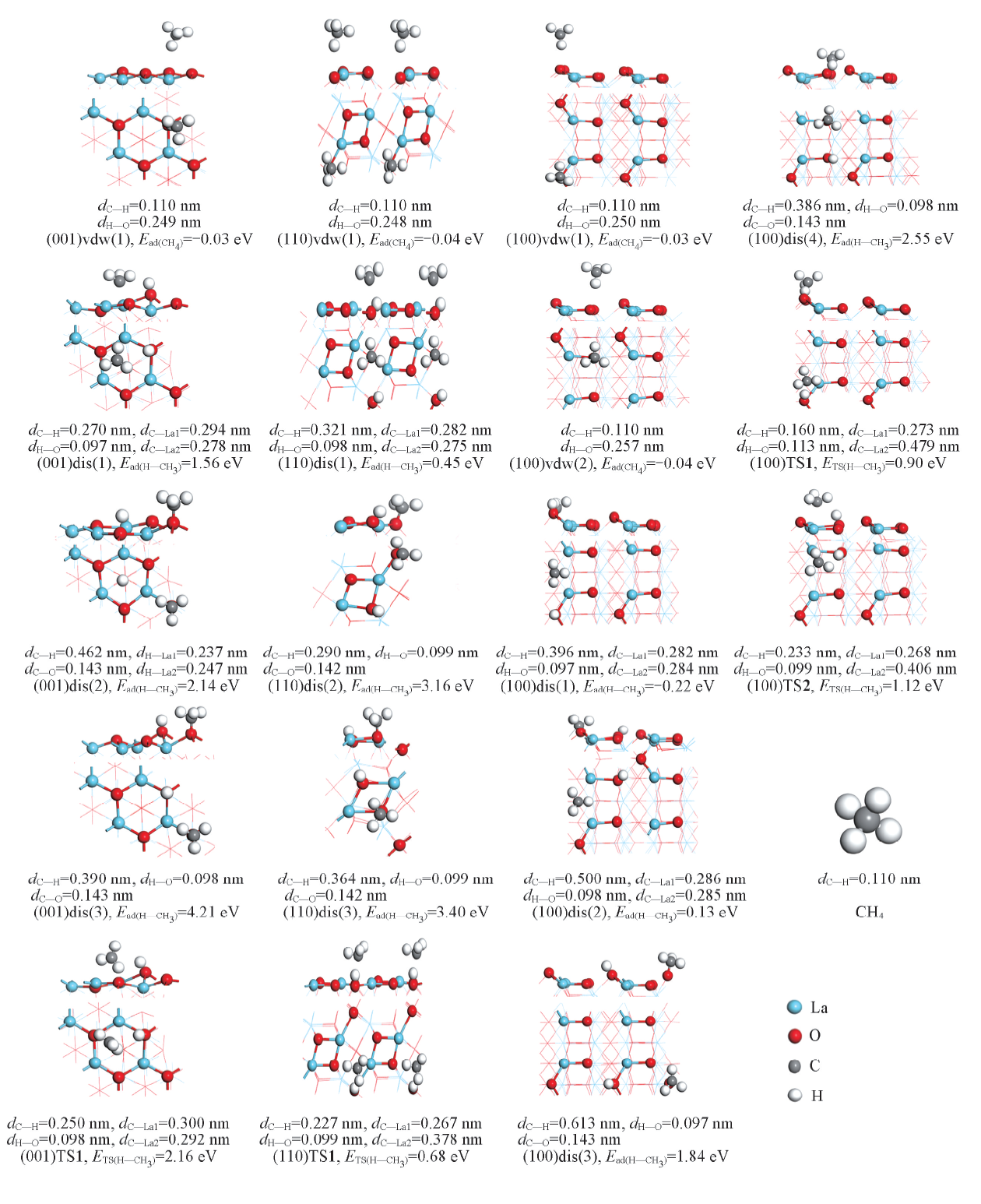
Fig.4 Structures, energies and related bond length of associative(vdw) and dissociative(dis) adsorption states and corresponding transition states(TS) for CH4 on La2O3 surfaces(001), (110) and (100)
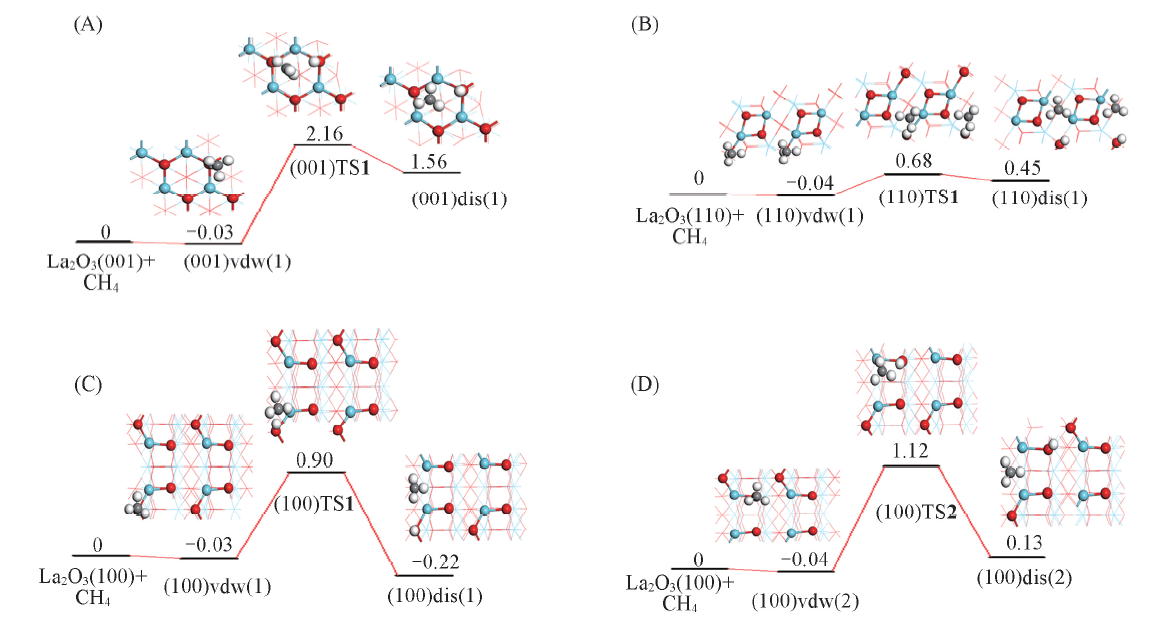
Fig.5 Diagram of potential energy surface for methane C—H activation on La2O3 surfaces(A) (001), H is bound to O4c; (B) (110), H is bound to O6c; (C) (100), H is bound to O6c; (D) (100), H is bound to O4c.The energy is in eV.
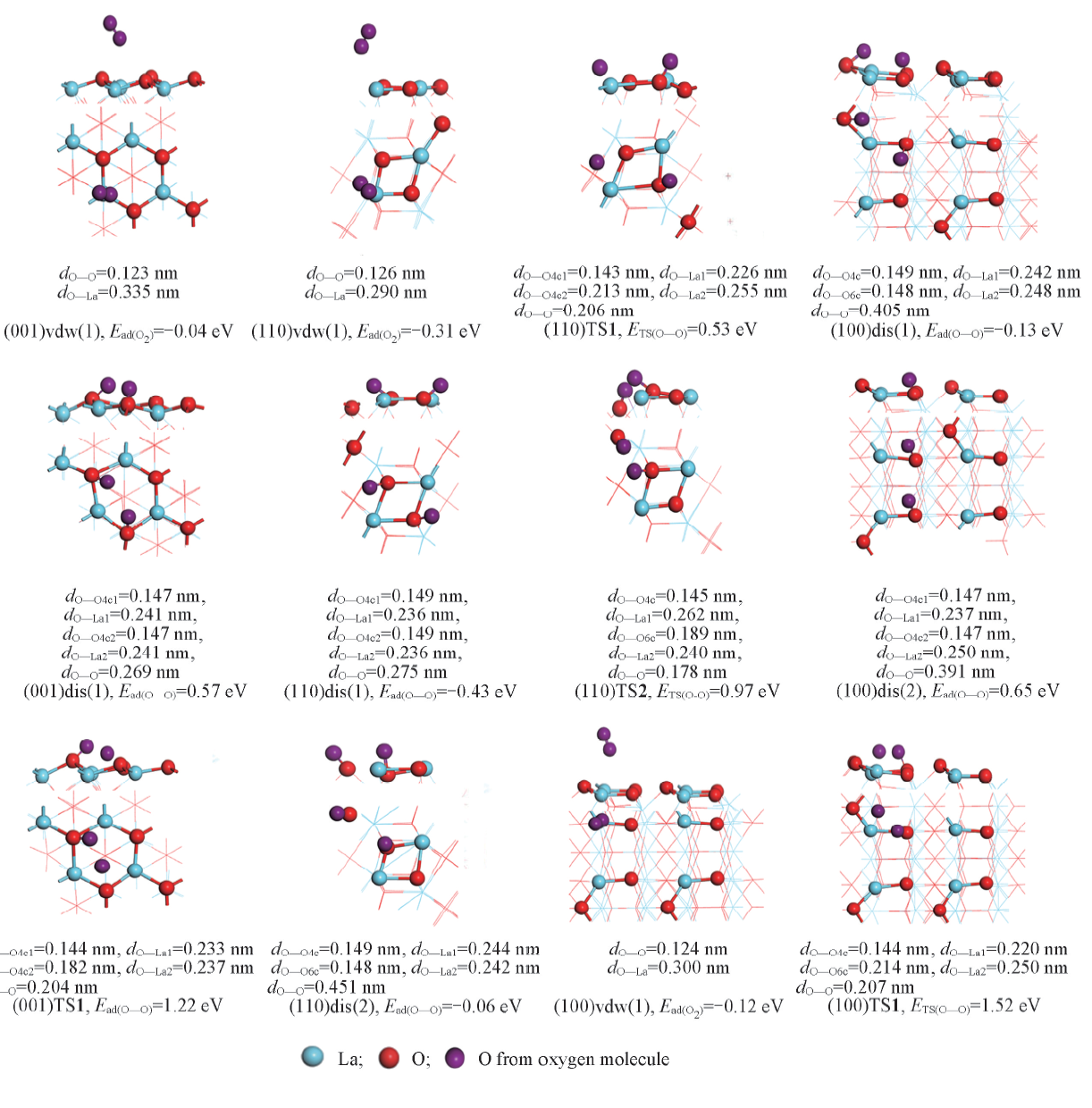
Fig.6 Structures, energies and related bond length of O2 associative adsorption(vdw), dissociative(dis) adsorption modes and corresponding transition states(TS) on La2O3 surfaces (001), (110) and (100)
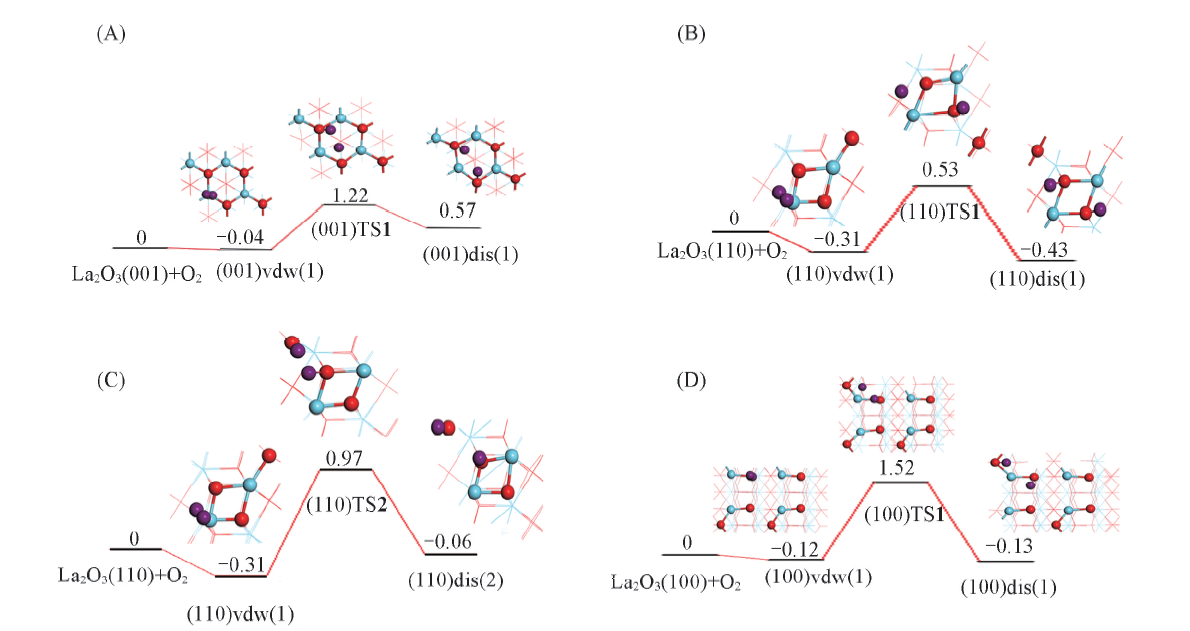
Fig.7 Diagram of potential energy surface for O—O activation on La2O3 surfaces(A) (001), O is bound to two O4c; (B) (110), O is bound to two O4c; (C) (110), O is bound to O6c/O4c;(D) (100), O is bound to O6c/O4c. The energy is in eV.
| Crystal plane of La2O3 | Ead(AB)/eV | Ead(A-B)/eV | ETS(A-B)/eV | qAB/e | qTS(A-B)/e | qA-B/e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | O2 | H—CH3 | O—O | H—CH3 | O—O | CH4 | O2 | H—CH3 | O—O | H—CH3 | O—O | |
| (001) | -0.03 | -0.04 | 1.56 | 0.57 | 2.16 | 1.22 | -0.01 | -0.02 | -0.03 | -1.02 | -0.10 | -1.19 |
| (110) | -0.04 | -0.31 | 0.45 | -0.43 | 0.68 | 0.53 | -0.02 | -0.24 | -0.10 | -1.05 | -0.10 | -1.34 |
| (100) | -0.03 | -0.12 | -0.22 | -0.13 | 0.90 | 1.52 | -0.02 | -0.10 | -0.09 | -1.04 | -0.12 | -1.30 |
Table 1 Energy of the preferred associative adsorption states, dissociative adsorption states and transition states relative to reactants(Ead(AB), Ead(A-B), ETS(A-B)) for CH4 and O2 on La2O3 surfaces, and an analysis on Bader charges(qAB, qTS(A-B), qA-B) of the corresponding species
| Crystal plane of La2O3 | Ead(AB)/eV | Ead(A-B)/eV | ETS(A-B)/eV | qAB/e | qTS(A-B)/e | qA-B/e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | O2 | H—CH3 | O—O | H—CH3 | O—O | CH4 | O2 | H—CH3 | O—O | H—CH3 | O—O | |
| (001) | -0.03 | -0.04 | 1.56 | 0.57 | 2.16 | 1.22 | -0.01 | -0.02 | -0.03 | -1.02 | -0.10 | -1.19 |
| (110) | -0.04 | -0.31 | 0.45 | -0.43 | 0.68 | 0.53 | -0.02 | -0.24 | -0.10 | -1.05 | -0.10 | -1.34 |
| (100) | -0.03 | -0.12 | -0.22 | -0.13 | 0.90 | 1.52 | -0.02 | -0.10 | -0.09 | -1.04 | -0.12 | -1.30 |
| [1] | Schwach P., Pan X., Bao X., Chem. Rev.,2017, 117(13), 8497—8520 |
| [2] | Kondratenko E. V., Peppel T., Seeburg D., Kondratenko V. A., Kalevaru N., Martin A., Wohlrab S., Catal. Sci. Technol.,2017, 7(2), 366—381 |
| [3] | Wang B., Albarracin-Suazo S., Pagan-Torres Y., Nikolla E., Catal. Today,2017, 285, 147—158 |
| [4] | Narsimhan K., Iyoki K., Dinh K., Roman-Leshkov Y., ACS Cent. Sci.,2016, 2(6), 424—429 |
| [5] | Ravi M., Ranocchiari M., van Bokhoven J. A., Angew. Chem. Int. Ed.,2017, 56(52), 16464—16483 |
| [6] | Zhu W., Shen M., Fan G., Yang A., Meyer J. R., Ou Y., Yin B., Fortner J., Foston M., Li Z., Zou Z., Sadtler B., ACS Appl. Nano Mater.,2018, 1(12), 6683—6691 |
| [7] | Farrell B. L., Igenegbai V. O., Linic S., ACS Catal.,2016, 6(7), 4340—4346 |
| [8] | Olivos-Suarez A. I., Szecsenyi A., Hensen E. J. M., Ruiz-Martinez J., Pidko E. A., Gascon J., ACS Catal.,2016, 6(5), 2965—2981 |
| [9] | Taifan W., Baltrusaitis J., Appl. Catal. B,2016, 198, 525—547 |
| [10] | Noon D., Seubsai A., Senkan S., ChemCatChem,2013, 5(1), 146—149 |
| [11] | Zuo Z., Ramirez P. J., Senanayake S. D., Liu P., Rodriguez J. A., J. Am. Chem. Soc.,2016, 138(42), 13810—13813 |
| [12] | Cui X., Li H., Wang Y., Hu Y., Hua L., Li H., Han X., Liu Q., Yang F., He L., Chen X., Li Q., Xiao J., Deng D., Bao X., Chem.,2018, 4(8), 1902—1910 |
| [13] | Hou Y. H., Han W. C., Xia W. S., Wan H. L., ACS Catal.,2015, 5(3), 1663—1674 |
| [14] | Greis O., J. Solid State Chem., 1980, 34(1), 39—44 |
| [15] | Molinari M., Parker S. C., Sayle D. C., Islam M. S., J. Phys. Chem. C,2012, 116(12), 7073—7082 |
| [16] | Kresse G., Furthmuller J., Comput. Mater. Sci.,1996, 6(1), 15—50 |
| [17] | Kresse G., Joubert D., Phys. Rev. B: Condens. Matter Mater. Phys.,1999, 59(3), 1758—1775 |
| [18] | Li B., Metiu H., J. Phys. Chem. C,2010, 114(28), 12234—12244 |
| [19] | Anisimov V. I., Aryasetiawan F., Lichtenstein A. I., J. Phys.: Condens. Matter,1997, 9(4), 767—808 |
| [20] | Perdew J. P., Zunger A., Phys. Rev. B: Condens. Matter,1981, 23(10), 5048—5079 |
| [21] | Zhou W., Jefferson D. A., Liang W. Y., Surf. Sci.,1989, 209(3), 444—454 |
| [22] | Islam M. S., Ilett D. J., Catal. Today,1994, 21(2/3), 417—422 |
| [23] | Chretien S., Metiu H., J. Phys. Chem. C,2014, 118(47), 27336—27342 |
| [24] | Palmer M. S., Neurock M., Olken M. M., J. Am. Chem. Soc.,2002, 124(28), 8452—8461 |
| [25] | Wang S., Cong L., Zhao C., Li Y., Pang Y., Zhao Y., Li S., Sun Y., Phys. Chem. Chem. Phys.,2017, 19(39), 26799—26811 |
| [26] | Xing B., Pang X. Y., Wang G. C., J. Catal.,2011, 282(1), 74—82 |
| [27] | Xing B., Wang G. C., Phys. Chem. Chem. Phys.,2014, 16(6), 2621—2629 |
| [28] | Wang J., Wang G. C., J. Phys. Chem. C,2018, 122(30), 17338—17346 |
| [29] | Wang G. C., J. Xinyang Normal University (Nat. Sci. Ed.), 2018, 31(2), 333—338 |
| (王贵昌. 信阳师范学院学报(自然科学版), 2018, 31(2), 333—338) |
| [1] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [2] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [3] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [4] | CHEN Jiamin, QU Xiaozhang, QI Guohua, XU Weiqing, JIN Yongdong, XU Shuping. SERS Nanoprobe for the Detection of Reactive Oxygen Species in Cells Produced by Electrostimulus [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220033. |
| [5] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| [6] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [7] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [8] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [9] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [10] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [11] | XU Huan, KE Lyu, TANG Mengke, SHANG Han, XU Wenxuan, ZHANG Zilin, FU Yanan, HAN Guangdong, CUI Jinsheng, YANG Haoran, GAO Jiefeng, ZHANG Shenghui, HE Xinjian. In⁃situ Liquid Exfoliation of Montmorillonite Nanosheets in Poly(lactic acid) to Resist Oxygen Permeation [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220316. |
| [12] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [13] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [14] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [15] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||