

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (7): 1342.doi: 10.7503/cjcu20160314
• Physical Chemistry • Previous Articles Next Articles
CAO Yongyong, JIANG Junhui, NI Zheming*( ), XIA Shengjie, QIAN Mengdan, XUE Jilong
), XIA Shengjie, QIAN Mengdan, XUE Jilong
Received:2016-05-05
Online:2016-07-10
Published:2016-06-27
Contact:
NI Zheming
E-mail:jchx@zjut.edu.cn
Supported by:CLC Number:
TrendMD:
CAO Yongyong, JIANG Junhui, NI Zheming, XIA Shengjie, QIAN Mengdan, XUE Jilong. Cluster Properties of Au19Pt and Selective Hydrogenation Mechanism of Cinnamaldehyde on Au19Pt Cluster Surface†[J]. Chem. J. Chinese Universities, 2016, 37(7): 1342.
| Pair | rAu-Au/(rAu+rPt) | rAu-Pt/(rAu+rPt) |
|---|---|---|
| PtE-Ausurface | 1.018 | 0.977 |
| PtE-A | 1.070 | 1.021 |
| PtE-A | 0.968 | 0.958 |
| PtV-Auedge | 0.985 | 0.946 |
| PtS-Auedge | 1.018 | 1.008 |
| PtS-Auvertex | 1.702 | 1.697 |
Table 1 Relative bond length between the Pt atom and its nearest neighbor Au atom*
| Pair | rAu-Au/(rAu+rPt) | rAu-Pt/(rAu+rPt) |
|---|---|---|
| PtE-Ausurface | 1.018 | 0.977 |
| PtE-A | 1.070 | 1.021 |
| PtE-A | 0.968 | 0.958 |
| PtV-Auedge | 0.985 | 0.946 |
| PtS-Auedge | 1.018 | 1.008 |
| PtS-Auvertex | 1.702 | 1.697 |
| System | ΔEb/eV | Ed-band center/eV |
|---|---|---|
| Au20 | 42.34 | -3.24 |
| Au19Pt-S | 43.42 | -2.45 |
| Au19Pt-V | 43.12 | -2.39 |
| Au19Pt-E | 43.40 | -2.62 |
Table 2 Binding energy and d-band center of the pure Au20 and bimetallic tetrahedral Au19Pt clusters
| System | ΔEb/eV | Ed-band center/eV |
|---|---|---|
| Au20 | 42.34 | -3.24 |
| Au19Pt-S | 43.42 | -2.45 |
| Au19Pt-V | 43.12 | -2.39 |
| Au19Pt-E | 43.40 | -2.62 |
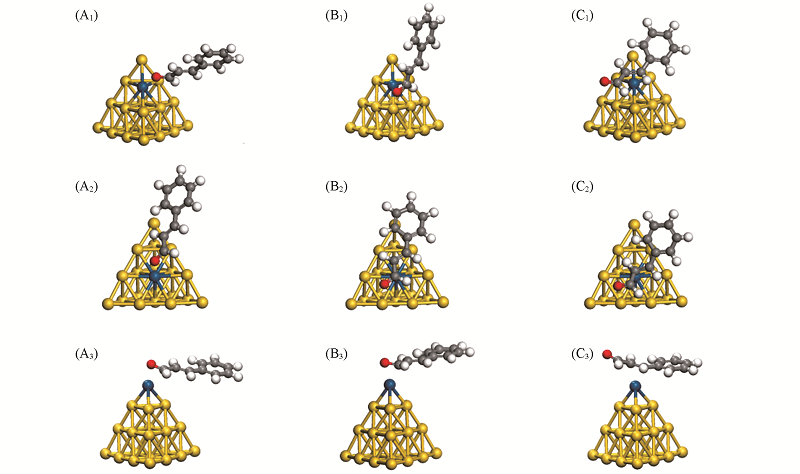
Fig.4 Optimized structure for the CAL molecule on the Au19Pt-E(A1—C1), Au19Pt-S(A2—C2) and Au19Pt-V(A3—C3) clusters(A1) PtE-σ(O); (A2) PtS-σ(O); (A3) PtV-σ(O); (B1) PtE-π(CO); (B2) PtS-π(CO); (B3) PtV-π(CO); (C1) PtE-π(CC); (C2) PtS-π(CC); (C3) PtV-π(CC).
| System | Final adsorption mode | Eads /eV | QCAL/e |
|---|---|---|---|
| CAL/Au19Pt-E | PtE-σ(O) | 1.01 | +0.185 |
| PtE-π(CO) | 0.89 | +0.138 | |
| PtE-π(CC) | 1.61 | +0.220 | |
| CAL/Au19Pt-S | PtS-σ(O) | 0.66 | +0.005 |
| PtS-π(CO) | 0.82 | +0.019 | |
| PtS-π(CC) | 0.74 | +0.221 | |
| CAL/Au19Pt-V | PtV-σ(O) | 1.28 | +0.113 |
| PtV-π(CO) | 1.31 | +0.270 | |
| PtV-π(CC) | 1.87 | +0.272 |
Table 3 Adsorption energies(Eads) and Mulliken atomic charges(Q) of CAL on Au19Pt clusters
| System | Final adsorption mode | Eads /eV | QCAL/e |
|---|---|---|---|
| CAL/Au19Pt-E | PtE-σ(O) | 1.01 | +0.185 |
| PtE-π(CO) | 0.89 | +0.138 | |
| PtE-π(CC) | 1.61 | +0.220 | |
| CAL/Au19Pt-S | PtS-σ(O) | 0.66 | +0.005 |
| PtS-π(CO) | 0.82 | +0.019 | |
| PtS-π(CC) | 0.74 | +0.221 | |
| CAL/Au19Pt-V | PtV-σ(O) | 1.28 | +0.113 |
| PtV-π(CO) | 1.31 | +0.270 | |
| PtV-π(CC) | 1.87 | +0.272 |
| Mechanism | Step | Reaction | Ea/eV | ΔE/eV |
|---|---|---|---|---|
| H2 dissociation | H2→H*+H* | 0.99 | -0.55 | |
| A | A(1) | CAL*+H*→MS1* | 0.51 | 0.17 |
| A(2) | MS1*+H*→COL*+ * | 1.75 | 0.15 | |
| B | B(1) | CAL*+H*→MS2* | 3.97 | 1.12 |
| B(2) | MS2*+H*→COL*+* | 0.48 | 0.05 | |
| C | C(1) | CAL*+H*→MS3* | 1.08 | 0.33 |
| C(2) | MS3*+H*→HCAL*+* | 0.65 | 0.71 | |
| D | D(1) | CAL*+H*→MS4* | 1.38 | 1.03 |
| D(2) | MS4*+H*→HCAL*+* | 0.36 | 0.73 | |
| E | E(1) | CAL*+H*→MS1* | 0.51 | 0.17 |
| E(2) | MS1*+H*→ENOL*+ * | 2.09 | 1.28 | |
| F | F(1) | CAL*+H*→MS4* | 1.38 | 1.03 |
| F(2) | MS4*+H*→ENOL*+ * | 4.20 | 1.41 |
Table 4 Activation energy(Ea) and reaction energy(ΔE) of possible six main reactions of CAL on Au19Pt cluster
| Mechanism | Step | Reaction | Ea/eV | ΔE/eV |
|---|---|---|---|---|
| H2 dissociation | H2→H*+H* | 0.99 | -0.55 | |
| A | A(1) | CAL*+H*→MS1* | 0.51 | 0.17 |
| A(2) | MS1*+H*→COL*+ * | 1.75 | 0.15 | |
| B | B(1) | CAL*+H*→MS2* | 3.97 | 1.12 |
| B(2) | MS2*+H*→COL*+* | 0.48 | 0.05 | |
| C | C(1) | CAL*+H*→MS3* | 1.08 | 0.33 |
| C(2) | MS3*+H*→HCAL*+* | 0.65 | 0.71 | |
| D | D(1) | CAL*+H*→MS4* | 1.38 | 1.03 |
| D(2) | MS4*+H*→HCAL*+* | 0.36 | 0.73 | |
| E | E(1) | CAL*+H*→MS1* | 0.51 | 0.17 |
| E(2) | MS1*+H*→ENOL*+ * | 2.09 | 1.28 | |
| F | F(1) | CAL*+H*→MS4* | 1.38 | 1.03 |
| F(2) | MS4*+H*→ENOL*+ * | 4.20 | 1.41 |
| [1] | Lee J. D., Christopher M. A., Parlett1 N. S., Scientific Reports,2015, 5(9426), 1—7 |
| [2] | Kolodziej M., Drelinkiewicz A., Lalik E., Gurgul J., Duraczynska D., Kosydar R., Applied Catalysis A: General,2016, 515, 60—71 |
| [3] | Karl R., Kahsar D. K., Schwartz J., Will M., J. Am. Chem. Soc., 2014, 136, 520—526 |
| [4] | Fang C., Chen Y. J., Mao B., Zhao J., Jiang Y. F., Zhao S. L., Ma J., Chem. J. Chinese Universities,2015, 36(1), 124—130(方超, 陈亚君, 毛卉, 赵俊, 蒋云福, 赵仕林, 马骏. 高等学校化学学报, 2015, 36(1), 124—130) |
| [5] | Li H., Ma C.J., Li H. X., Acta Chimica Sinica, 2006, 24, 1947—1953(李辉, 马春景, 李和兴. 化学学报, 2006, 24, 1947—1953) |
| [6] | Xue Q. Q., Karine P., Pierre L., Philippe S., Dalton Trans., 2014, 43, 9283—9295 |
| [7] | Haeck J.D., Veldeman N., J. Phys.Chem. A, 2011, 115, 2103—2109 |
| [8] | Qian H., Jiang D., Gayathri C., J. Am. Chem. Soc., 2012, 134, 16159—16162 |
| [9] | Cheng L., Xiao Y. K., Zhi W. L., J. Phys. Chem. A,2011, 115, 9273—9281 |
| [10] | Zhang X. D., Gao M. L., Wu D., Int. J. Mol. Sci., 2011, 12, 2972—2981 |
| [11] | Yuan D. W., Wang Y., Zenga Z., J. Chem. Phys., 2005, 122, 114310 |
| [12] | Mondal K., Ghanty T. K., Banerjee A., Mol. Phys., 2013, 111, 725—734 |
| [13] | Sinfelt J. H., Accounts Chem. Res., 1987, 20, 134—136 |
| [14] | Gholizadeh R., Yu Y. X., Appl. Surf. Sci., 2015, 357, 1187—1195 |
| [15] | Liu T. T., Lu X., Zhang M. T., Chem. Res. Chinese Universities,2014, 30(4), 656—660 |
| [16] | Pan W., Ma G. W., Yang X. D., Chem. J. Chinese Universities,2015, 36(2), 325—329(潘威, 马广文, 杨小东. 高等学校化学学报, 2015, 36(2), 325—329) |
| [17] | Tapan K. G., J. Phys. Chem. C, 2014, 118, 11935—11945 |
| [18] | Anna V., Beletskaya D. P., Alexander F. S., J. Phys. Chem. A,2013, 117, 6817—6826 |
| [19] | Ke Q. S., Yong C. H., Gui R. Z., ACS Catal., 2011, 1, 1336—1346 |
| [20] | Yong C. H., Ke Q. S., Gui R. Z., Chem. Commun., 2011, 47, 1300—1302 |
| [21] | Delley B., J. Phys. Chem. A, 2006, 110, 13632—13639 |
| [22] | Chen W. K., Cao M. J., Liu S. H., Acta Phys-Chim Sinica,2005, 21, 903—908(陈文凯, 曹梅娟, 刘书红. 物理化学学报, 2005,21, 903—908) |
| [23] | Xiao X. C., Shi W., Ni Z. M., Zhang L. Y., Acta Phys-Chim Sinica,2015, 31, 885—892(肖雪春, 施炜, 倪哲明, 张连阳. 物理化学学报, 2015,31, 885—892) |
| [24] | Tao J., Yao Z.J., Xue F., Fundamentals of Material Science, Chemical Industry Press, 2006, 50—51 |
| (陶杰, 姚正军, 薛烽. 化学工业出版社, 2006, 50—51) | |
| [25] | Ham H. C., Hwang G. S., Han J., Nam S. W., Lim T. H., J. Phys. Chem. C,2010, 114, 14922—14928 |
| [26] | Morrow B. H., Resasco D. E., Striolo A., Nardelli M. B., J. Phys. Chem. C,2011, 115, 5637—5647 |
| [27] | Diego C. A., Maria P. O., Luis S., J. Phys. Chem. A,2015, 119, 6909—6918 |
| [28] | Delbecq D., Sautet P., J. Catal., 1995, 152, 217—236 |
| [29] | Mulliken R. S., J. Chem. Phys., 1955, 23, 1833—1840 |
| [30] | Loffreda D., Delbecq F., Vigne F., Sautet P., J. Am. Chem. Soc., 2006, 128, 1316—1323 |
| [31] | Shi W., Zhang L. Y., Ni Z. M., Xiao X. C., Xia S. J., RSC Advances,2014, 4, 27003—27012 |
| [32] | Zhang L.Y., Jiang J. H., Shi W., Xia S. J., Ni Z. M., RSC Advances,2015, 5, 34319—34326 |
| [33] | Jiang J. H., Xia S. J., Ni Z. M., Zhang L.Y., Chem. J. Chinese Universities,2016, 37(4), 693—700(蒋军辉, 夏盛杰, 倪哲明, 张连阳. 高等学校化学学报, 2016, 37(4), 693—700) |
| [1] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [2] | SONG Youwei, AN Jiangwei, WANG Zheng, WANG Xuhui, QUAN Yanhong, REN Jun, ZHAO Jinxian. Effects of Ag,Zn,Pd-doping on Catalytic Performance of Copper Catalyst for Selective Hydrogenation of Dimethyl Oxalate [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210842. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | LIU Yang, LI Wangchang, ZHANG Zhuxia, WANG Fang, YANG Wenjing, GUO Zhen, CUI Peng. Theoretical Exploration of Noncovalent Interactions Between Sc3C2@C80 and [12]Cycloparaphenylene Nanoring [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220457. |
| [5] | WANG Yuanyue, AN Suosuo, ZHENG Xuming, ZHAO Yanying. Spectroscopic and Theoretical Studies on 5-Mercapto-1,3,4-thiadiazole-2-thione Microsolvation Clusters [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220354. |
| [6] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [7] | LI Xueyu, WANG Zhao, CHEN Ya, LI Keke, LI Jianquan, JIN Shunjing, CHEN Lihua, SU Baolian. Enhanced Catalytic Performance of Supported Nano-gold by the Localized Surface Plasmon Resonance for Selective Hydrogenation of Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220174. |
| [8] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [9] | MA Lijuan, GAO Shengqi, RONG Yifei, JIA Jianfeng, WU Haishun. Theoretical Investigation of Hydrogen Storage Properties of Sc, Ti, V-decorated and B/N-doped Monovacancy Graphene [J]. Chem. J. Chinese Universities, 2021, 42(9): 2842. |
| [10] | HUANG Luoyi, WENG Yueyue, HUANG Xuhui, WANG Chaojie. Theoretical Study on the Structures and Properties of Flavonoids in Plantain [J]. Chem. J. Chinese Universities, 2021, 42(9): 2752. |
| [11] | ZHONG Shengguang, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Theoretical Study on Direct Conversion of CH4 and CO2 into Acetic Acid over MCu2Ox(M = Cu2+, Ce4+, Zr4+) Clusters [J]. Chem. J. Chinese Universities, 2021, 42(9): 2878. |
| [12] | ZHENG Ruoxin, ZHANG Igor Ying, XU Xin. Development and Benchmark of Lower Scaling Doubly Hybrid Density Functional XYG3 [J]. Chem. J. Chinese Universities, 2021, 42(7): 2210. |
| [13] | LIU Changhui, LIANG Guojun, LI Yanlu, CHENG Xiufeng, ZHAO Xian. Density Functional Theory Study of NH3 Adsorption on Boron Nanotubes [J]. Chem. J. Chinese Universities, 2021, 42(7): 2263. |
| [14] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [15] | HU Wei, LIU Xiaofeng, LI Zhenyu, YANG Jinlong. Surface and Size Effects of Nitrogen-vacancy Centers in Diamond Nanowires [J]. Chem. J. Chinese Universities, 2021, 42(7): 2178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||