

Chem. J. Chinese Universities ›› 2024, Vol. 45 ›› Issue (10): 20240297.doi: 10.7503/cjcu20240297
• Article: Inorganic Chemistry • Previous Articles
WANG Xin, QI Jinyang, YANG Ruijie, SONG Zhiguo, WANG Min( )
)
Received:2024-06-19
Online:2024-10-10
Published:2024-08-21
Contact:
WANG Min
E-mail:wangmin@qymail.bhu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Xin, QI Jinyang, YANG Ruijie, SONG Zhiguo, WANG Min. Synthesis, Characterization and Catalytic Property of the Cu(II) Complex Based on Benzene Sulfonic Acid Ligand[J]. Chem. J. Chinese Universities, 2024, 45(10): 20240297.
| Formula | C26H30CuN8O6S2 | β/(°) | 95.787(2) |
|---|---|---|---|
| Formula weight | 678.24 | γ/(°) | 90 |
| Crystal system | Monoclinic | V/nm3 | 3.0799(5) |
| Space group | P21/n | Z | 4 |
| a/nm | 1.28841(12) | Dc/(Mg·m-3) | 1.463 |
| b/nm | 1.49801(15) | Goodness⁃of⁃fit F2 | 1.122 |
| c/nm | 1.60396(16) | R1, ωR2 | 0.0380, 0.0947 |
| α/(°) | 90 | R1, ωR2(all data) | 0.0503, 0.1075 |
Table 1 Crystallographic data of Cu(Im)4(p-CH3C6H4SO3)2(CCDC No.2111862)*
| Formula | C26H30CuN8O6S2 | β/(°) | 95.787(2) |
|---|---|---|---|
| Formula weight | 678.24 | γ/(°) | 90 |
| Crystal system | Monoclinic | V/nm3 | 3.0799(5) |
| Space group | P21/n | Z | 4 |
| a/nm | 1.28841(12) | Dc/(Mg·m-3) | 1.463 |
| b/nm | 1.49801(15) | Goodness⁃of⁃fit F2 | 1.122 |
| c/nm | 1.60396(16) | R1, ωR2 | 0.0380, 0.0947 |
| α/(°) | 90 | R1, ωR2(all data) | 0.0503, 0.1075 |
| Bond | Bond length/nm | Bond | Bond angle/(°) |
|---|---|---|---|
| Cu(1)—N(1) | 0.20131(17) | N(1)—Cu(1)—N(5) | 177.47(7)° |
| Cu(1)—N(3) | 0.19966(15) | N(3)—Cu(1)—N(7) | 177.48(7)° |
| Cu(1)—N(5) | 0.20396(17) | O(1)—Cu(1)—O(4) | 167.36(5)° |
| Cu(1)—N(7) | 0.19953(16) | N(1)—Cu(1)—N(3) | 92.06(7)° |
| Cu(1)—O(1) | 0.27392(17) | N(1)—Cu(1)—O(1) | 86.18(6)° |
| Cu(1)—O(4) | 0.26029(17) | N(1)—Cu(1)—O(4) | 81.87(6)° |
| S(1)—O(1) | 0.14557(17) | N(3)—Cu(1)—N(5) | 88.25(6)° |
| S(1)—O(2) | 0.14553(16) | N(7)—Cu(1)—N(5) | 89.85(7)° |
| S(1)—O(3) | 0.14602(15) | N(7)—Cu(1)—N(1) | 89.91(7)° |
Table 2 Partial bond lengths(nm) and bond angles(°) of Cu(Im)4(p-CH3C6H4SO3)2
| Bond | Bond length/nm | Bond | Bond angle/(°) |
|---|---|---|---|
| Cu(1)—N(1) | 0.20131(17) | N(1)—Cu(1)—N(5) | 177.47(7)° |
| Cu(1)—N(3) | 0.19966(15) | N(3)—Cu(1)—N(7) | 177.48(7)° |
| Cu(1)—N(5) | 0.20396(17) | O(1)—Cu(1)—O(4) | 167.36(5)° |
| Cu(1)—N(7) | 0.19953(16) | N(1)—Cu(1)—N(3) | 92.06(7)° |
| Cu(1)—O(1) | 0.27392(17) | N(1)—Cu(1)—O(1) | 86.18(6)° |
| Cu(1)—O(4) | 0.26029(17) | N(1)—Cu(1)—O(4) | 81.87(6)° |
| S(1)—O(1) | 0.14557(17) | N(3)—Cu(1)—N(5) | 88.25(6)° |
| S(1)—O(2) | 0.14553(16) | N(7)—Cu(1)—N(5) | 89.85(7)° |
| S(1)—O(3) | 0.14602(15) | N(7)—Cu(1)—N(1) | 89.91(7)° |
| D—H···A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| N(2)—H(2)···O(3)#3 | 0.086 | 0.196 | 0.2806(2) | 166.9 |
| N(4)—H(4)···O(6)#1 | 0.086 | 0.195 | 0.2795(2) | 168.4 |
| N(6)—H(6)···O(5)#4 | 0.086 | 0.189 | 0.2740(3) | 171.8 |
| N(8)—H(8)···O(2)#2 | 0.086 | 0.196 | 0.2822(2) | 175.9 |
Table 3 Hydrogen bond lengths(nm) and bond angles(°) of Cu(Im)4(p-CH3C6H4SO3)2*
| D—H···A | d(D—H)/nm | d(H…A)/nm | d(D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| N(2)—H(2)···O(3)#3 | 0.086 | 0.196 | 0.2806(2) | 166.9 |
| N(4)—H(4)···O(6)#1 | 0.086 | 0.195 | 0.2795(2) | 168.4 |
| N(6)—H(6)···O(5)#4 | 0.086 | 0.189 | 0.2740(3) | 171.8 |
| N(8)—H(8)···O(2)#2 | 0.086 | 0.196 | 0.2822(2) | 175.9 |
| Entry | R | Product | Time/min | Yield(%) | m. p./℃ | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 4a | H | 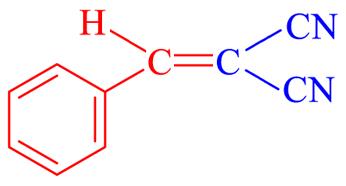 | 10 | 93.6 | 81—83 | 82—84[ |
| 4b | 2⁃Cl | 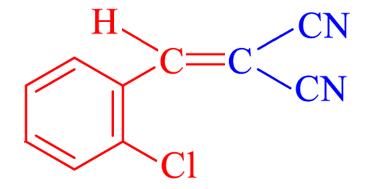 | 7 | 93.4 | 95—96 | 95—96[ |
| 4c | 4⁃Cl | 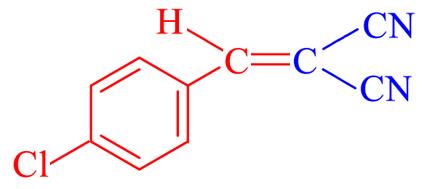 | 8 | 94.3 | 161—163 | 161—163[ |
| 4d | 2,4⁃Cl2 | 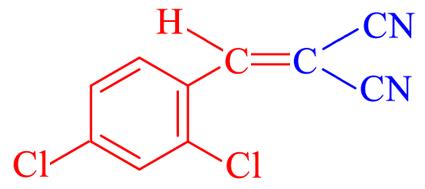 | 9 | 93.8 | 154—156 | 154—155[ |
| 4e | 2⁃NO2 | 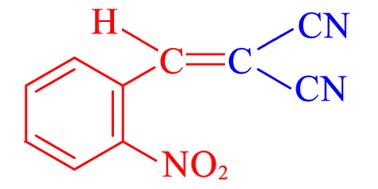 | 2 | 96.4 | 137—138 | 137—138[ |
| 4f | 3⁃NO2 | 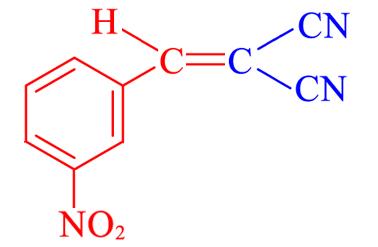 | 10 | 97.9 | 103—105 | 102—104[ |
| 4g | 4⁃NO2 | 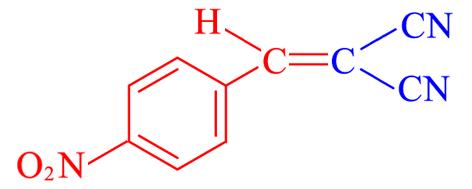 | 2 | 99.2 | 159—161 | 159—160[ |
| 4h | 3⁃OH | 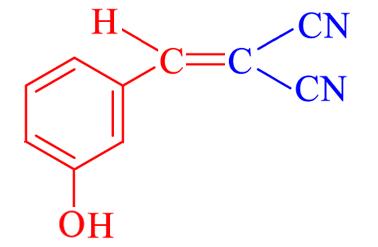 | 23 | 88.8 | 154—155 | — |
| 4i | 4⁃OH | 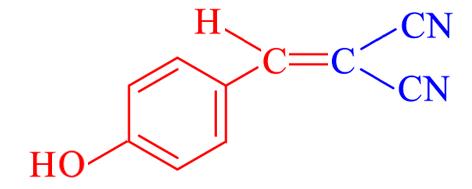 | 10 | 93.9 | 188—189 | 187—188[ |
| 4j | 4⁃CH3O | 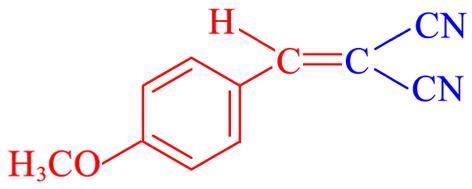 | 19 | 90.6 | 115—116 | 115—116[ |
| 4k | 2⁃CH3O | 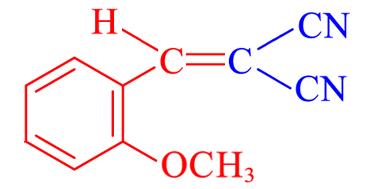 | 10 | 87.6 | 84—86 | 84—86[ |
| 4l | 4⁃CH3 | 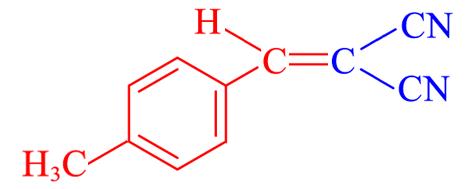 | 10 | 91.1 | 136—138 | 136—138[ |
Table 4 Synthesis of arylidene compounds catalyzed by Cu(Im)4(p⁃CH3C6H4SO3)2
| Entry | R | Product | Time/min | Yield(%) | m. p./℃ | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| 4a | H |  | 10 | 93.6 | 81—83 | 82—84[ |
| 4b | 2⁃Cl |  | 7 | 93.4 | 95—96 | 95—96[ |
| 4c | 4⁃Cl |  | 8 | 94.3 | 161—163 | 161—163[ |
| 4d | 2,4⁃Cl2 |  | 9 | 93.8 | 154—156 | 154—155[ |
| 4e | 2⁃NO2 |  | 2 | 96.4 | 137—138 | 137—138[ |
| 4f | 3⁃NO2 |  | 10 | 97.9 | 103—105 | 102—104[ |
| 4g | 4⁃NO2 |  | 2 | 99.2 | 159—161 | 159—160[ |
| 4h | 3⁃OH |  | 23 | 88.8 | 154—155 | — |
| 4i | 4⁃OH |  | 10 | 93.9 | 188—189 | 187—188[ |
| 4j | 4⁃CH3O |  | 19 | 90.6 | 115—116 | 115—116[ |
| 4k | 2⁃CH3O |  | 10 | 87.6 | 84—86 | 84—86[ |
| 4l | 4⁃CH3 |  | 10 | 91.1 | 136—138 | 136—138[ |
| 1 | Yu N. F., Aramini J. M., Germann M. W., Huang Z., Tetrahedron Lett., 2000, 41(36), 6993—6996 |
| 2 | Tietze L. F., Rackelmann N., Pure Appl. Chem., 2004, 76(11), 1967—1983 |
| 3 | Jones G., The Knoevenagel Condensation, John Wiley & Sons, New York, 1967, 204—273 |
| 4 | Prajapati D., Lekhok K. C., Sandhu J. S., Ghosh A. C., J. Chem. Soc. Perk. T. 1, 1996, 9, 959—956 |
| 5 | Rao P. S., Venkataratnam R. V., Tetrahedron Lett., 1991, 32(41), 5821—5822 |
| 6 | Balalaie S., Sheikh⁃Ahmadi M., Bararjanian M., Catal. Commun., 2007, 8(11), 1724—1728 |
| 7 | Liu Y. T., Li R., Xing Y. J., Chin. J. Org. Chem., 2015, 35(7), 1520—1525 |
| 刘玉婷, 李戎, 邢彦军. 有机化学, 2015, 35(7), 1520—1525 | |
| 8 | Li J. T., Wang S. X., Chen G. F., Li T. S., Curr. Org. Synth., 2005, 2(3), 415—436 |
| 9 | Chandrasekhar V., Thirumoorthi R., Metre R. K., Mahanti B., J. Organomet. Chem., 2011, 696(2), 600—606 |
| 10 | Xie Z. L., Xie Y. R., Xu G. H., Du Z. Y., Zhou Z. G., Lai W. L., Inorg. Chim. Acta, 2012, 384, 117—124 |
| 11 | Jianrattanasawat S., Mezei G., Inorg. Chim. Acta, 2012, 384, 318—323 |
| 12 | Zhou Y. H., Huang R. J., Yan J. Y., Li Y. J., Qiu H. H., Yang J. X., Zheng Y. X., Chem. J. Chinese Univerties, 2022, 43(1), 20210415 |
| 周永慧, 黄如军, 严健洋, 李亚军, 邱欢欢, 杨进轩, 郑佑轩. 高等学校化学学报, 2022, 43(1), 20210415 | |
| 13 | Qi J. Y., Wang M., Yang R. J., Zhang Y. C., Song Z. G., Chem. Res. Appl., 2024, 53(2), 300—306 |
| 祁金阳, 王敏, 杨瑞杰, 张迎春, 宋志国. 人工晶体学报, 2024, 53(2), 300—306 | |
| 14 | Guo T. T., Yin S. W., Wang Y., Theor. Chem. Acc., 2017, 136(10), 126 |
| 15 | Sizova O. V., Ivanova N. V., Lyubimova O. O., Nikol’ski A. B., Russ. J. Gen. Chem., 2004, 74(2), 155—163 |
| 16 | Türkyilmaz M., Dönmez M., Altun Ö., Chem. Res. Chinese Universities, 2023, 39(6), 968—975 |
| 17 | He J. Y., Cui L., Qi Y. L., Dai Q. Q., Bai C. X., Chin. J. Polym. Sci., 2019, 37(3), 208—215 |
| 18 | Leng Y., Wang J., Zhu D. R., Wu Y. J., Zhao P. P., J. Mol. Catal. A⁃Chem., 2009, 313(1/2), 1—6 |
| 19 | Burd S. D., Ma S. Q., Perman J. A., Sikora B. J., Snurr R. L. Q., Thallapally P. K., Tian J., Wojtas L., Zaworotko M. J., J. Am. Chem. Soc., 2012, 134(8), 3663—3666 |
| 20 | Yang Y. T., Tu C. Z., Shi J. Y., Zhao T. X., Liu Z. N., Cheng F. X., Luo F., J. Solid State Chem., 2021, 302, 122439 |
| 21 | Dai J. Y., Tao Y. X., Gu X. G., Liu Z., Kong Y., Liu W. J., Ma J. F., Wei Y., J. Appl. Polym. Sci., 2015, 132(17), 41895 |
| 22 | Liao Q. S., Hou H. Y., Duan J. X., Liu S., Yao Y., Dai Z. P., Yu C. Y., Li D. D., J. Appl. Polym. Sci., 2017, 134(24), 44935 |
| 23 | Martyak N. M., Seefeldt R., Electrochim. Acta, 2004, 49(25), 4303—4311 |
| 24 | Danilov F. I., Sknar I. V., Sknar Y. E., Russ. J. Electrochem., 2014, 50(3), 293—330 |
| 25 | Qian B. H., Ma W. X., Lu L. D., Yang X. J., Wang X., Acta Phys.⁃Chim. Sin., 2010, 26(3), 610—616 |
| 钱保华, 马卫兴, 陆路德, 杨绪杰, 汪信. 物理化学学报, 2010, 26(3), 610—616 | |
| 26 | Bruker S., SMART(Version 5.628), SAINT(Version 6.45), SADABS, Bruker AXS Inc., Madison, WI, 2001 |
| 27 | Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., J. Appl. Crystallogr., 2009, 42, 339—341 |
| 28 | Sheldrick G. M., SHELXL⁃97, Program for Crystal Structure Solution, University of G9ttingen, G9ttingen, 1997 |
| 29 | Guo X. L., Ding Z. Y., Deng S. M., Wen C. C., Shen X. C., Jiang B. P., Liang H., Carbon, 2018, 134, 519—530 |
| 30 | Liu X. L., Wang C. J., Mao K. L., Zhang Y. J., Zhou X. L., Huang S., Shan L. H., Wang X. Y., J. Inorg. Chem., 2014, 30(8), 1938—1946 |
| 刘晓雷, 王萃娟, 毛凯力, 张亚军, 周先礼, 黄帅, 单连海, 王尧宇. 无机化学学报, 2014, 30(8), 1938—1946 | |
| 31 | Wu G. Z., Wang P. F., Li S. Q., Fang X. L., Inorg. Chem., 2020, 36(2), 226—232 |
| 吴国志, 汪鹏飞, 李善青, 方霄龙. 无机化学学报, 2020, 36(2), 226—232 | |
| 32 | Li C. C., Qiao X. C., Chem. Eng. J., 2016, 302, 388—394 |
| 33 | Li Y. Q., Ye H. O., Chin. J. Org. Chem., 2002, 22(9), 678—680 |
| 李毅群, 叶海鸿. 有机化学, 2002, 22(9), 678—680 | |
| 34 | Wang X. S., Zeng Z. S., Li Y. L., Shi D. Q., Tu S. J., Wei X. Y., Zong Z. M., Synth. Commun., 2005, 35(14), 1915—1920 |
| 35 | Vassileva P., Krastev V., Lakov L., Peshev O., J. Mater. Sci., 2004, 39(9), 3201—3202 |
| 36 | Neuvonen H., Neuvonen K., Koch A., Kleinpeter E., Pasanen P., J. Org. Chem., 2002, 67(20), 6995—7003 |
| 37 | Xiao J., Wu Z. Y., Chen Z. Y., Zhao P. F., Liu C. Y., Chin. J. Org. Chem., 2022, 42(4), 1179—1187 |
| 肖剑, 武志英, 陈姿依, 赵朋飞, 刘春艳. 有机化学, 2022, 42(4), 1179—1187 | |
| 38 | Takakura R., Koyama K., Kuwata M., Yamada T., Sajiki H., Sawama Y., Org. Biomol. Chem., 2020, 18(34), 6594—6597 |
| [1] | LAN Xing, HE Yuting, ZHANG Qing, FANG Yanfen, DENG Anping, ZHAO Haixia, ZHANG Zhaonian. Mechanism of Hydrolysis of Microcystins Enhanced by Lewis Acid Sites on the Surface of Pyrite-Fe(Ⅲ) [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240086. |
| [2] | HE Jun, ZHU Aoyang, WEI Yuchen, ZHU Yiquan, JIANG Li, HE Xiaojun. Preparation and Zinc Storage Properties of Three-dimensional Nitrogen-doped Hierarchical Porous Carbon Nanosheets [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240099. |
| [3] | CHEN Junjie, ZHANG Ruidan, CHEN Yue. First-principles Study of Potential Applications of Monolayer GeTe in Lithium/sodium/potassium Ion Batteries [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240148. |
| [4] | ZHANG Shuo, ZHAO Liuyang, HUANG Hao, WU Aimin, LI Aikui. Oxygen Framework Mechanism of Layered Lithium-rich Manganese-based Materials Stabilized by High-valent Element Mo Based on First-principles Calculations [J]. Chem. J. Chinese Universities, 2024, 45(5): 20240035. |
| [5] | CHEN Rong, WEN Liangying, YUE Dong, YANG Zhongqing. Density Functional Theory Analysis of Coadsorption Behavior of Cl2 and O2 on TiC(100) Surface [J]. Chem. J. Chinese Universities, 2024, 45(4): 20230497. |
| [6] | CHEN Qingqing, LI Jiangtao, HUANG Xinrong, GU Fang, WANG Haijun. Excess Entropy of Janus Particles Immersed in Hydrogen Bonding Fluids [J]. Chem. J. Chinese Universities, 2024, 45(2): 20230443. |
| [7] | ZHANG Xiaoran, ZHENG Jianyun, LYU Yanhong, WANG Shuangyin. Recent Advances in Green C-N Coupling for Urea Synthesis [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220717. |
| [8] | BAO Chunzhu, XIANG Zhonghua. Pyrolysis-free Strategy of Covalent Organic Polymers-based Oxygen Reduction Electrocatalytic Materials [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220715. |
| [9] | FU Zhongheng, CHEN Xiang, YAO Nan, YU Legeng, SHEN Xin, ZHANG Rui, ZHANG Qiang. Research Advances in Transport Mechanism of Lithium Ions in Solid Electrolytes [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220703. |
| [10] | PENG Xinzhe, GE Jiaoyang, WANG Fangli, YU Guojing, RAN Xueqin, ZHOU Dong, YANG Lei, XIE Linghai. Theoretical Study on the Strain Energy and Reorganization Energy Based on Planar Grid Benzothiophene [J]. Chem. J. Chinese Universities, 2023, 44(2): 20220313. |
| [11] | ZHANG Haiping, KONG Xue, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Methane C—H Activation by Cyclo[18] Carbon-based Single-atom Transition Metal(Os, Ir) [J]. Chem. J. Chinese Universities, 2023, 44(11): 20230259. |
| [12] | FENG Linyan, HU Xiaobo, YAN Miao, MIAO Changqing, CHEN Rui, GUO Jinchang, WANG Yingjin. Theoretical Study of MB8C4(M=Ca, Sr, Ba) Molecular Wheels Clusters with Dodeca-coordination Number in Plane [J]. Chem. J. Chinese Universities, 2023, 44(10): 20230281. |
| [13] | HE Hongrui, XIA Wensheng, ZHANG Qinghong, WAN Huilin. Density-functional Theoretical Study on the Interaction of Indium Oxyhydroxide Clusters with Carbon Dioxide and Methane [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220196. |
| [14] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [15] | GAO Wenxiu, LYU Jieqiong, GAO Yongping, KONG Changjian, WANG Xueping, GUO Shengnan, LOU Dawei. Preparation of Ethyl α⁃Cyanocinnamate Catalyzed by Nitrogen-rich Porous Organic Polymers [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220078. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||