

Chem. J. Chinese Universities ›› 2025, Vol. 46 ›› Issue (5): 20240570.doi: 10.7503/cjcu20240570
• Physical Chemistry • Previous Articles Next Articles
HONG Yang, LI Dandan, ZHANG Jingshun, ZHANG Ziwang, GAO Guohua( )
)
Received:2024-12-30
Online:2025-05-10
Published:2025-03-24
Contact:
GAO Guohua
E-mail:ghgao@chem.ecnu.edu.cn
Supported by:CLC Number:
TrendMD:
HONG Yang, LI Dandan, ZHANG Jingshun, ZHANG Ziwang, GAO Guohua. Porous Poly(ionic liquid)s-catalyzed Carbon Dioxide-promoted Hydration of Ethylene Oxide[J]. Chem. J. Chinese Universities, 2025, 46(5): 20240570.
| Catalyst | Surface area a /(m2‧g‒1) | Pore volume b /(cm3‧g‒1) | Number of basic sites c /(mmol‧ g‒1) |
|---|---|---|---|
| P(VBVImI) | 374.7 | 0.86 | — |
| P(VBVImI⁃VIm)⁃0.5 | 145.5 | 0.57 | 1.26(1.30) |
| P(VBVImI⁃VIm)⁃1.0 | 106.4 | 0.61 | 2.24(2.31) |
| P(VBVImI⁃VIm)⁃1.5 | 100.9 | 0.59 | 3.13(3.13) |
| P(VIm) | 6.0 | 0.01 | 10.63(10.63) |
| P(VBVImBr⁃VIm)⁃1.0 | 107.9 | 0.41 | 2.44(2.60) |
| P(VBVImCl⁃VIm)⁃1.0 | 107.3 | 0.46 | 2.60(2.93) |
| P(VBVImI⁃4⁃VP)⁃1.0 | 127.9 | 0.41 | 2.18(2.26) |
Table 1 Surface area, pore volume and number of basic sites of poly(ionic liquid)s
| Catalyst | Surface area a /(m2‧g‒1) | Pore volume b /(cm3‧g‒1) | Number of basic sites c /(mmol‧ g‒1) |
|---|---|---|---|
| P(VBVImI) | 374.7 | 0.86 | — |
| P(VBVImI⁃VIm)⁃0.5 | 145.5 | 0.57 | 1.26(1.30) |
| P(VBVImI⁃VIm)⁃1.0 | 106.4 | 0.61 | 2.24(2.31) |
| P(VBVImI⁃VIm)⁃1.5 | 100.9 | 0.59 | 3.13(3.13) |
| P(VIm) | 6.0 | 0.01 | 10.63(10.63) |
| P(VBVImBr⁃VIm)⁃1.0 | 107.9 | 0.41 | 2.44(2.60) |
| P(VBVImCl⁃VIm)⁃1.0 | 107.3 | 0.46 | 2.60(2.93) |
| P(VBVImI⁃4⁃VP)⁃1.0 | 127.9 | 0.41 | 2.18(2.26) |
| Entry | Catalyst | Reaction atmosphere | Yield(%) | MEG Select.(%) | |||
|---|---|---|---|---|---|---|---|
| MEG | EC | DEG | TEG | ||||
| 1 | P(VBVImI⁃VIm)⁃1.0 | CO2 | 96.5 | 0.5 | 1.5 | 0 | 96.5 |
| 2 | N2 | 42.4 | 0 | 25.1 | 2.0 | 43.0 | |
| 3 | P(VBVImI⁃VIm)⁃0.5 | CO2 | 95.3 | 1.2 | 1.7 | 0 | 95.4 |
| 4 | P(VBVImI⁃VIm)⁃1.5 | CO2 | 95.0 | 0.4 | 2.0 | 0 | 95.6 |
| 5 | P(VBVImBr⁃VIm)⁃1.0 | CO2 | 88.1 | 4.9 | 2.8 | 0 | 89.4 |
| 6 | P(VBVImCl⁃VIm)⁃1.0 | CO2 | 74.2 | 1.2 | 12.0 | 0 | 74.6 |
| 7 | P(VBVImI⁃4⁃VP)⁃1.0 | CO2 | 95.4 | 0.6 | 1.0 | 0 | 97.3 |
| 8 | P(VBVImI) | CO2 | 75.0 | 21.6 | 0.7 | 0 | 76.5 |
| 9 | P(VIm) | CO2 | 58.6 | 0.1 | 18.9 | 0 | 60.7 |
| 10 | VBVImI + VIm | CO2 | 96.9 | 0.1 | 1.2 | 0 | 97.5 |
Table 2 Hydration of EO under CO2/N2 atmosphere catalyzed by poly(ionic liquid)s*
| Entry | Catalyst | Reaction atmosphere | Yield(%) | MEG Select.(%) | |||
|---|---|---|---|---|---|---|---|
| MEG | EC | DEG | TEG | ||||
| 1 | P(VBVImI⁃VIm)⁃1.0 | CO2 | 96.5 | 0.5 | 1.5 | 0 | 96.5 |
| 2 | N2 | 42.4 | 0 | 25.1 | 2.0 | 43.0 | |
| 3 | P(VBVImI⁃VIm)⁃0.5 | CO2 | 95.3 | 1.2 | 1.7 | 0 | 95.4 |
| 4 | P(VBVImI⁃VIm)⁃1.5 | CO2 | 95.0 | 0.4 | 2.0 | 0 | 95.6 |
| 5 | P(VBVImBr⁃VIm)⁃1.0 | CO2 | 88.1 | 4.9 | 2.8 | 0 | 89.4 |
| 6 | P(VBVImCl⁃VIm)⁃1.0 | CO2 | 74.2 | 1.2 | 12.0 | 0 | 74.6 |
| 7 | P(VBVImI⁃4⁃VP)⁃1.0 | CO2 | 95.4 | 0.6 | 1.0 | 0 | 97.3 |
| 8 | P(VBVImI) | CO2 | 75.0 | 21.6 | 0.7 | 0 | 76.5 |
| 9 | P(VIm) | CO2 | 58.6 | 0.1 | 18.9 | 0 | 60.7 |
| 10 | VBVImI + VIm | CO2 | 96.9 | 0.1 | 1.2 | 0 | 97.5 |
| Entry | Epoxide | Conv.(%) | Yield(%) | Select.(%) | |
|---|---|---|---|---|---|
| Diol | Carbonate | ||||
| 1 b | 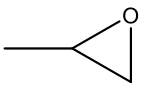 | 99.3 | 97.1 | 1.2 | 97.8 |
| 2 c | 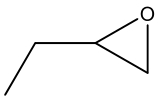 | 98.3 | 98.1 | 0.2 | 99.8 |
| 3 c | 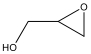 | 100 | 93.0 | 7.0 | 93.0 |
| 4 d | 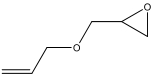 | 100 | 94.4 | 5.6 | 94.4 |
| 5 d | 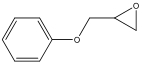 | 100 | 95.5 | 4.5 | 95.5 |
Table 3 CO2-promoted hydration of epoxides catalyzed by P(VBVImI-VIm)-1.0 a
| Entry | Epoxide | Conv.(%) | Yield(%) | Select.(%) | |
|---|---|---|---|---|---|
| Diol | Carbonate | ||||
| 1 b |  | 99.3 | 97.1 | 1.2 | 97.8 |
| 2 c |  | 98.3 | 98.1 | 0.2 | 99.8 |
| 3 c |  | 100 | 93.0 | 7.0 | 93.0 |
| 4 d |  | 100 | 94.4 | 5.6 | 94.4 |
| 5 d |  | 100 | 95.5 | 4.5 | 95.5 |
| Entry | CO2/N2(%, volume fraction) | Yield(%) | Select.(%) | |
|---|---|---|---|---|
| MEG | DEG | |||
| 1 | 20 | 91.0 | 3.4 | 93.0 |
| 2 | 30 | 92.8 | 3.0 | 93.6 |
| 3 | 40 | 93.9 | 2.6 | 94.7 |
| 4 | 50 | 93.2 | 2.7 | 94.2 |
| 5 | 70 | 94.2 | 1.9 | 96.0 |
Table 4 CO2-promoted hydration of ethylene oxide under simulated flue gas *
| Entry | CO2/N2(%, volume fraction) | Yield(%) | Select.(%) | |
|---|---|---|---|---|
| MEG | DEG | |||
| 1 | 20 | 91.0 | 3.4 | 93.0 |
| 2 | 30 | 92.8 | 3.0 | 93.6 |
| 3 | 40 | 93.9 | 2.6 | 94.7 |
| 4 | 50 | 93.2 | 2.7 | 94.2 |
| 5 | 70 | 94.2 | 1.9 | 96.0 |
| 1 | Yue H., Zhao Y., Ma X., Gong J., Chem. Soc. Rev., 2012, 41, 4218—4244 |
| 2 | Liang W. B., Chen Y., Hou X. W., Ding G. R., Chem. Ind., 2024, 42(1), 58—63, 68 |
| 梁文博, 陈颖, 侯雪微, 丁国荣. 化学工业, 2024, 42(1), 58—63, 68 | |
| 3 | Marchesan A. N., Oncken M. P., Filho R. M., Maciel M. R. W., Green Chem., 2019, 21(19), 5168—5194 |
| 4 | Van Hal J. W., Ledford J. S., Zhang X., Catal. Today, 2007, 123(1), 310—315 |
| 5 | Yu F. P., Cai H., He W. J., Yang W. M., Xie Z. K., J. Appl. Polym. Sci., 2010, 115(5), 2946—2954 |
| 6 | Li Y. C., Yan S. R., Qian L. P., Yang W. M., Xie Z. K., Chen Q. L., Yue B., He H. Y., J. Catal., 2006, 241(1), 173—179 |
| 7 | Li B., Bai S., Wang X. F., Zhong M. M., Yang Q. H., Angew. Chem. Int. Ed., 2012, 51(46), 11517—11521 |
| 8 | Dai W. L., Wang C. M., Tang B., Wu G. J., Guan N. J., Xie Z. K., Hunger M., Li L. D., ACS Catal., 2016, 6(5), 2955—2964 |
| 9 | Xu H., Tian W. W., Xu L. P., Jin X., Xue T., Chen L., He M. Y., Wu P., Chinese J. Catal., 2020, 41(7), 1109—1117 |
| 10 | Zhang Z., Zheng Y., Qian L. T., Luo D., Dou H. Z., Wen G. B., Yu A. P., Chen Z. W., Adv. Mater., 2022, 34(29), 2201547 |
| 11 | Zhang M. J., Zou N., Luo J. M., Zhong X. H., Li L., Chem. J. Chinese Universities, 2024, 45(10), 20240113 |
| 张孟佳, 邹南, 罗佳美, 钟雄辉, 李玲. 高等学校化学学报, 2024, 45(10), 20240113 | |
| 12 | He M. Y., Sun Y. H., Han B. X., Angew. Chem. Int. Ed., 2013, 52(37), 9620—9633 |
| 13 | Ding T., Zha J. Y., Zhang D. W., Yuan H. X., Xia F., Gao G. H., Green Chem., 2021, 23(9), 3386—3391 |
| 14 | Li M. R., Gu G. G., Yue T. J., Ren W. M., Lu X. B., J. CO2 Util., 2024, 80, 102684 |
| 15 | Zhao Z. Y., Zhang W. D., Jiang L. L., Tao H., Wang S. Y., Wang K. L., Lin W. J., Shi J. L., Li H. R., Wang C. M., Green Chem., 2023, 25(9), 3437—3442 |
| 16 | Zhao Z. Y., Lu J. W., Lin W. J., Wang X. M., Bai J. Y., Zheng X. Q., Xie R. X., Shi J. L., Li H. R., Wang C. M., Ind. Eng. Chem. Res., 2022, 61(44), 16402—16407 |
| 17 | Zhang J. S., Wang X. K., Hu H., Wang Z. Y., Wang L., Zhang J. L., Gao G. H., Fuel, 2025, 381, 133308 |
| 18 | Yuan H. X., Dang L. L., Tang H. R., Wang B. S., Zhang J. S., Lai T., Gao G. H., Fuel, 2023, 345, 128210 |
| 19 | Zhang J. S., Dang L. L., Lai T., Yuan H. X., Zhang D. W., Gao G. H., Fuel, 2024, 355, 129521 |
| 20 | Dang L. L., Yuan H. X., Wang B. S., Zhang J. S., Wang Z. Y., Gao G. H., ACS Appl. Mater. Interfaces, 2023, 15(12), 16017—16025 |
| 21 | Guo M. Z., Zhang L. Y., Du Y., Du W. C., Liu D. T., Guo C., Pan Y. J., Tang D. Q., Anal. Chem., 2018, 90(6), 3906—3913 |
| 22 | Kai A. T., Sheng Y. J., Chen Q. B., Zhang J. S., Li S. W., Wu Y. M., Liu H. L., Chem. Lett., 2018, 47(7), 913—915 |
| 23 | Zhang Y. Y., Wang B. S., Elageed E. H. M., Qin L., Ni B., Liu X. L., Gao G. H., ACS Macro Lett., 2016, 5(4), 435—438 |
| 24 | Chi W. S., Jeon H., Kim S. J., Kim D. J., Kim J. H., Macromol. Res., 2013, 21(3), 315—320 |
| 25 | Wang M., Wang X., Zhou J., Gao G. H., Chem. J. Chinese Universities, 2021, 42(12), 3701—3708 |
| 王蔓, 王鑫, 周静, 高国华. 高等学校化学学报, 2021, 42(12), 3701—3708 | |
| 26 | Sun J., Yao X. Q., Cheng W. G., Zhang S. J., Green Chem., 2014, 16(6), 3297—3304 |
| 27 | Yang Z. J., Ren N., Zhang Y. H., Tang Y., Catal. Commun., 2010, 11(5), 447—450 |
| 28 | Li J., Han Y. L., Lin H., Wu N. H., Li Q. K., Jiang J., Zhu J. H., ACS Appl. Mater. Interfaces, 2020, 12(1), 609—618 |
| 29 | Lu X. Q., Xu H., Yan J. Y., Zhou W. J., Liebens A., Wu P., J. Catal., 2018, 358, 89—99 |
| [1] | AN Yaolong, LI Zi⁃Heng, WU Fu⁃Gen. A Self-assembled Nanomicelle for Realizing Tumor Photodynamic Therapy via Increasing Drug Accumulation and Prolonging Retention Time [J]. Chem. J. Chinese Universities, 2025, 46(1): 20240331. |
| [2] | LIU Shijia, LI Qian, CUI Kun, MA Zhi, ZHANG Danwei, WANG Hui, LI Zhanting. Synthesis and Rheological Properties of Pillar[5]arene-polyethylene Glycol-conjugated Poly(pseudo)rotaxanes as Slide-ring Materials [J]. Chem. J. Chinese Universities, 2023, 44(10): 20230211. |
| [3] | ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD † [J]. Chem. J. Chinese Universities, 2020, 41(2): 317. |
| [4] | GUO Zhaopei,LIN Lin,CHEN Jie,TIAN Huayu,CHEN Xuesi. Polyglutamic Acid Grafted Polyethylene Glycol@Calcium Carbonate Based Shielding System for Improving Polyethyleneimine Gene Transfection Efficiency † [J]. Chem. J. Chinese Universities, 2020, 41(2): 235. |
| [5] | LI Lin, XU Xinru, LI Yingqi, ZHANG Caifeng. Preparation of Targeting Nanodiamond-metaminopterone Drug System and Its Interaction with MCF-7 Cells † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1998. |
| [6] | ZHAO Yuxuan,CHEN Yanjun,PAN Guxin,WANG Chang,PENG Zhenbo,SUN Zongxu,LIANG Yongri,SHI Qisong. Preparation and Performance of Novel Tb-PEG+Eu-PEG/PANI/PAN Luminescent-electrical-phase Change Composite Fibers by Electrospinning† [J]. Chem. J. Chinese Universities, 2019, 40(4): 824. |
| [7] | QIAN Yihao, ZHANG Dongjie, CHENG Zhongjun, KANG Hongjun, LIU Yuyan. Preparation of Hydrophilic Epoxy Resin and Its Wettability Regulation† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1823. |
| [8] | GAO Yan, ZHANG Hua, ZHANG Wen, LI Xiangpeng, YUE Pan, LI Wei. Preparation of Micro/Nano-fibers Membranes Encapsulated with Dual Drugs by Emulsion Electrospun and Controlled Release of Hydrophilic and Hydrophobic Drugs† [J]. Chem. J. Chinese Universities, 2018, 39(3): 575. |
| [9] | LIU Jie, ZHOU Hao, HUANG Yufang, CHEN Xin. Soy Protein Isolate/Agar Composite Hydrogel† [J]. Chem. J. Chinese Universities, 2018, 39(3): 591. |
| [10] | LIU Jie, ZHOU Hao, HUANG Yufang, CHEN Xin. Polyethylene Glycol Chemically Modified Soy Protein Isolate Hydrogel† [J]. Chem. J. Chinese Universities, 2018, 39(2): 390. |
| [11] | WANG Zhengguang, HU Duo, WU Dongwei, LU Lu, ZHOU Changren. Preparation and Properties of Double Network Hydrogels Based on Gellan Gum and Polyethylene Glycol Acrylate† [J]. Chem. J. Chinese Universities, 2017, 38(2): 275. |
| [12] | QIU Chuanlong,LI Chunfang,LI Dongxiang,HOU Wanguo. Synthesis, Characterization and Aggregation Behavior of Polyethylene Glycol-conjugated Hydroxycamptothecin† [J]. Chem. J. Chinese Universities, 2016, 37(8): 1535. |
| [13] | LI Qi, ZHAO Wenjie, ZHAO Ziliang, JI Xiangling, BO Shuqin, LIU Yonggang. Band Broadening and Chain Conformation of Polyethylene Oxide by Gel Permeation Chromatography Coupled with Multi-angle Laser Light Scattering† [J]. Chem. J. Chinese Universities, 2016, 37(4): 761. |
| [14] | YU Tao, HAN Yu, WANG Hui, XIONG Shizhao, XIE Kai, GUO Qingpeng. Preparation and Lithium Ion Transport Behavior for Li1.5Al0.5Ge1.5(PO4)3 Based Solid Composite Electrolyte [J]. Chem. J. Chinese Universities, 2016, 37(2): 306. |
| [15] | LI Xingjian, WU Ruiqing, LAI Jingjuan, PAN Yi, ZHENG Zhaohui, DING Xiaobin. Shape-memory Properties and Molecular Mechanism of Poly(methyl methacrylate)/Star-shaped Poly(ethylene glycol) Semi-interpenetrating Polymer Network† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1932. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||