

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (2): 317.doi: 10.7503/cjcu20190474
• Physical Chemistry • Previous Articles Next Articles
ZHANG Li1,QIAN Mingchao1,LIU Xueke1,Gao Shuaitao1,YU Jiang1,*( ),XIE Haishen2,WANG Hongbin2,SUN Fengjiang2,SU Xianghong3
),XIE Haishen2,WANG Hongbin2,SUN Fengjiang2,SU Xianghong3
Received:2019-09-03
Online:2020-02-10
Published:2019-12-31
Contact:
Jiang YU
E-mail:yujiang@mail.buct.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD †[J]. Chem. J. Chinese Universities, 2020, 41(2): 317.
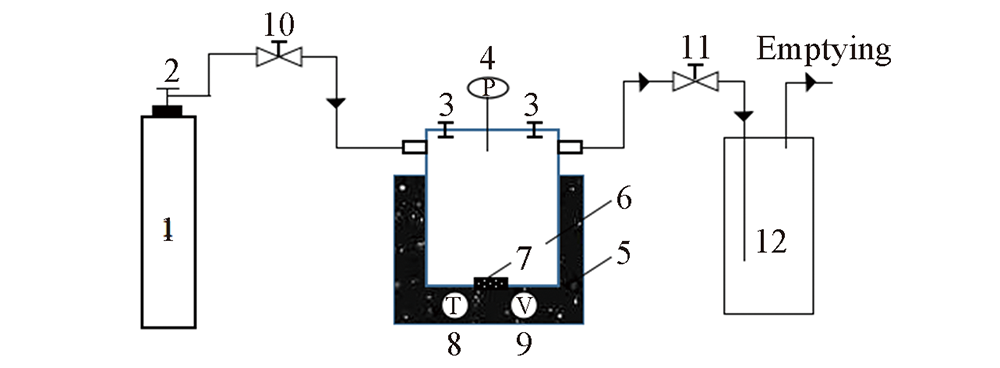
Fig.1 Static absorption reactor 1. H2S gas tank; 2. gas reducing valve; 3. pressure flange; 4. precision pressure gauge; 5. thermostat water bath; 6. high pressure absorption reactor; 7. magneto; 8. thermometer; 9. speed controller; 10. inlet valve; 11. outlet valve; 12. tail gas treatment unit.
| Variable | Dynamic region | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Concentration of Fe(Ⅲ) | + | - | + | + | ? | ? | + | + |
| Volume fraction of H2S | + | + | + | + | ? | ? | + | + |
| Phase interfacial area | + | + | + | + | + | + | + | - |
| Volume of desulfurization liquid | - | - | - | - | + | + | + | + |
| Coefficient of mass transfer in liquid phase | + | - | - | - | ? | + | + | - |
| Coefficient of mass transfer in gas phase | + | - | + | + | ? | ? | + | - |
| Second-order reaction rate constant | - | - | + | + | ? | ? | - | + |
| Variable | Dynamic region | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Concentration of Fe(Ⅲ) | + | - | + | + | ? | ? | + | + |
| Volume fraction of H2S | + | + | + | + | ? | ? | + | + |
| Phase interfacial area | + | + | + | + | + | + | + | - |
| Volume of desulfurization liquid | - | - | - | - | + | + | + | + |
| Coefficient of mass transfer in liquid phase | + | - | - | - | ? | + | + | - |
| Coefficient of mass transfer in gas phase | + | - | + | + | ? | ? | + | - |
| Second-order reaction rate constant | - | - | + | + | ? | ? | - | + |
| No. | Liquid-phase volume/mL | ||
|---|---|---|---|
| 60 | 80 | 100 | |
| 1 | 1.51 | 1.49 | 1.48 |
| 2 | 1.54 | 1.47 | 1.49 |
| 3 | 1.52 | 1.49 | 1.48 |
| No. | Liquid-phase volume/mL | ||
|---|---|---|---|
| 60 | 80 | 100 | |
| 1 | 1.51 | 1.49 | 1.48 |
| 2 | 1.54 | 1.47 | 1.49 |
| 3 | 1.52 | 1.49 | 1.48 |
| No. | KL/(r·min-1) | |
|---|---|---|
| 100 | 150 | |
| 1 | 1.48 | 1.50 |
| 2 | 1.49 | 1.52 |
| 3 | 1.48 | 1.52 |
| No. | KL/(r·min-1) | |
|---|---|---|
| 100 | 150 | |
| 1 | 1.48 | 1.50 |
| 2 | 1.49 | 1.52 |
| 3 | 1.48 | 1.52 |
| No. | Mass ratio of Fe-IL/NHD | |||
|---|---|---|---|---|
| 8:1 | 6:1 | 4:1 | 1:0 | |
| 1 | 1.35 | 1.41 | 1.48 | 1.29 |
| 2 | 1.33 | 1.42 | 1.49 | 1.28 |
| 3 | 1.32 | 1.42 | 1.48 | 1.29 |
| No. | Mass ratio of Fe-IL/NHD | |||
|---|---|---|---|---|
| 8:1 | 6:1 | 4:1 | 1:0 | |
| 1 | 1.35 | 1.41 | 1.48 | 1.29 |
| 2 | 1.33 | 1.42 | 1.49 | 1.28 |
| 3 | 1.32 | 1.42 | 1.48 | 1.29 |
| Mass ratio of Fe-IL/NHD | b | R2 |
|---|---|---|
| 1:0 | 1.07 | 0.9779 |
| 8:1 | 1.15 | 0.9771 |
| 6:1 | 1.39 | 0.9813 |
| 4:1 | 1.56 | 0.9999 |
| Mass ratio of Fe-IL/NHD | b | R2 |
|---|---|---|
| 1:0 | 1.07 | 0.9779 |
| 8:1 | 1.15 | 0.9771 |
| 6:1 | 1.39 | 0.9813 |
| 4:1 | 1.56 | 0.9999 |
| [1] | Zhang Q., Hou Y. C., Ren S. H., Zhang K., Wu W. Z., Chem. Eng., 2019,7(12), 10931— 10936 |
| [2] | Debski B., Hanel A., Aranowski R., Stolte S., Markiewicz M., Veltzke T., Cichowska-Kopczynska I ., J. Mol. Lid., 2019,291, 110477 |
| [3] | Zheng W. T., Wu D. S., Feng X., Hu J. L., Zhang F., Wu Y. T., Hu X. B., J. Mol. Lid., 2018,263, 209— 217 |
| [4] |
Paramanik M., Singh R., Mukhopadhyay S., Ghosh S. K., J. Fluorine Chem., 2015,178, 47— 55
doi: 10.1016/j.jfluchem.2015.06.022 URL |
| [5] |
Ruan C. X., Sun Z. L., Lu S. S., Li L. F., Lou J., Sun W., Russ. J. Electrochem., 2014,50(2), 129— 135
doi: 10.1134/S1023193513020158 URL |
| [6] |
Ma Y. Q., Liu X. P., Wang R., J. Hazard. Mater., 2017,331, 109— 116
doi: 10.1016/j.jhazmat.2017.02.036 URL |
| [7] |
Liu X. P., Qang R., Fuel Process. Technol., 2017,160, 78— 85
doi: 10.1016/j.fuproc.2017.02.024 URL |
| [8] |
Lv B. H., Jing G. H., Qian Y. H., Zhou Z. M., Chem. Eng. J., 2016,289, 212— 218
doi: 10.1016/j.cej.2015.12.096 URL |
| [9] | Kazmi B., Haider J., Qyyum M. A., Saeed S., Kazmi M. R., Int. J. Greenhouse Gas Control, 2019,87, 88— 99 |
| [10] |
Dai F., Chen X. J., He B., Liu R. X., Zhang S. J., Cat. Sci. Technol., 2018,8(17), 4515— 4525
doi: 10.1039/C8CY01023D URL |
| [11] |
Ding W. J., Zhu W. S., Xiong J., Yang L., Wei A. M., Zhang M., Li H. M., Chem. Eng. J., 2015,266, 213— 221
doi: 10.1016/j.cej.2014.12.040 URL |
| [12] | Xie M. Y., Li P. P., Guo H. F., Gao L. X., Yu J., Chinese J. Chem. Eng., 2012,1, 140— 145 |
| [13] |
Lee S. H., Hu S. H., You C. Y., Koo Y. M., Korean J. Chem. Eng., 2007,24, 436— 437
doi: 10.1007/s11814-007-0075-x URL |
| [14] | He Y., Yu J., Chen L. B., CIESC J., 2010,61(4), 963— 968 |
| ( 何义, 余江, 陈灵波 . 化工学报, 2010,61(4), 963— 968) | |
| [15] | Wang J. H., Chen J. Q., Yan H. Z., Chem. Res., 2012,23(1), 9— 13 |
| ( 王建宏, 陈家庆, 阎红昭 . 化学研究, 2012,23(1), 9— 13) | |
| [16] |
Wang J. H., Zhang W. D., Energy Fuels, 2014,28(9), 5930— 5935
doi: 10.1021/ef500527w URL |
| [17] | Guo Z. H., Zhang T. T., Sun L. L., He Y., Yu J., Fresenius Environ. Bulletin, 2015,24(8A), 2587— 2592 |
| [18] |
Guo Z. H., Zhang T. T., Liu T. T., Du J., Jia B., Gao S. J., Yu J., Environ. Sci. Technol., 2015,49(9), 5697— 5703
doi: 10.1021/es505728f URL |
| [19] | Hu J. C., Gao L. X., Liu W. H., Zhao Y. L., Gao S., Pan X. P., Guo Z. H., Yu J., CIESC J., 2016,67(S1), 347— 352 |
| ( 胡锦超, 高丽霞, 刘伟海, 赵永禄, 高尚, 潘兴朋, 郭智慧, 余江 . 化工学报, 2016,67(S1), 347— 352) | |
| [20] | Liu K., Liu H. J., Guo Q., Chem. Enterprise Management, 2019, ( 11), 185— 186 |
| ( 刘凯, 刘海菊, 郭琦 . 化工管理, 2019, ( 11), 185— 186) | |
| [21] |
Rayer A. V., Henni A., Tontiwachwuthikul P., Can. J. Chem. Eng., 2012,90(3), 576— 583
doi: 10.1002/cjce.20615 URL |
| [22] |
Schmidt K. A. G., Mather A. E., Can. J. Chem. Eng., 2001,79(6), 946— 960
doi: 10.1002/cjce.v79:6 URL |
| [23] | Lin M. H., Guo S. C., Chem. Eng., 2000, ( 3), 58— 61 |
| ( 林民鸿, 郭淑翠 . 化学工程, 2000, ( 3), 58— 61) | |
| [24] | Xu B ., Chem. Enterprise Management, 2016, ( 18), 87 |
| ( 徐斌 . 化工管理, 2016, ( 18), 87) | |
| [25] | Feng S., Anhui Chem. Industry, 2018,44(1), 49— 52 |
| ( 冯升 . 安徽化工, 2018,44(1), 49— 52) | |
| [26] |
Karamanev D. G., Nikolov L. N., Mamatarkova V., Minerals Eng., 2002,15(5), 341— 345
doi: 10.1016/S0892-6875(02)00026-2 URL |
| [27] | Chen J., Luo W. L., Li H., CIESC J., 2014,65(1), 12— 21 |
| ( 陈健, 罗伟亮, 李晗 . 化工学报, 2014,65(1), 12— 21) | |
| [28] | Shen Y. T., Zhang H. T., Fang D. Y., Li T., J. East China University Sci. Technol.(Nat. Sci. Ed.), 2016,42(4), 446— 453 |
| ( 沈叶婷, 张海涛, 房鼎业, 李涛 . 华东理工大学学报(自然科学版), 2016,42(4), 446— 453) |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [3] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| [4] | ZENG Xianyang, ZHAO Xi, HUANG Xuri. Mechanism of Inhibition of Glucose and Proton Cotransport Protein GlcPSe by Cytochalasin B [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210822. |
| [5] | LIU Jiaxin, MIN Jie, XU Huajie, REN Haisheng, TAN Ningxin. Interaction Between Produced Radicals During Ethylene Combustion and Nitrogen Molecules Based on Reaxff Molecular Dynamics Simulation [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210834. |
| [6] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [7] | CHEN Hanxiang, BIAN Shaoju, HU Bin, LI Wu. Molecular Simulation of the Osmotic Pressures for LiCl-NaCl-KCl-H2O Solution System [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210727. |
| [8] | HU Bo, ZHU Haochen. Dielectric Constant of Confined Water in a Bilayer Graphene Oxide Nanosystem [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210614. |
| [9] | WANG Kun, ZOU Xingli, CAO Zhanmin, LI Chonghe, LU Xionggang. Modified Quasichemical Model for Manifold Short-range Orders in Binary Solutions: Unity of Opposites for the Ordered Pairs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220391. |
| [10] | ZHANG Mi, TIAN Yafeng, GAO Keli, HOU Hua, WANG Baoshan. Molecular Dynamics Simulation of the Physicochemical Properties of Trifluoromethanesulfonyl Fluoride Dielectrics [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220424. |
| [11] | YANG Weiming, XI Aoqian, YANG Bin, ZENG Yanning. Fabrication and Properties of Epoxy Vitrimer Based on Multiply Dynamic Covalent Bonds [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220308. |
| [12] | ZHANG Lingyu, ZHANG Jilong, QU Zexing. Dynamics Study of Intramolecular Vibrational Energy Redistribution in RDX Molecule [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220393. |
| [13] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [14] | TANG Yuanhui, LI Chunyu, LIN Yakai, ZHANG Chunhui, LIU Ze, YU Lixin, WANG Haihui, WANG Xiaolin. Dissipative Particle Dynamics Simulation of the Effect of Polymer Chain Rigidity on Membranes Formation by Nonsolvent Induced Phase Separation Process [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220169. |
| [15] | ZHANG Xiang, XIE Xulan, XIONG Likun, PENG Yang. Urchin-like Gold Nanoneedle for Efficient Electrocatalytic CO2 Reduction [J]. Chem. J. Chinese Universities, 2021, 42(9): 2824. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||