

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1535.doi: 10.7503/cjcu20150895
• Physical Chemistry • Previous Articles Next Articles
QIU Chuanlong1, LI Chunfang1, LI Dongxiang1, HOU Wanguo2,*( )
)
Received:2015-11-23
Online:2016-07-14
Published:2016-07-14
Contact:
HOU Wanguo
E-mail:wghou@sdu.edu.cn
Supported by:CLC Number:
TrendMD:
QIU Chuanlong,LI Chunfang,LI Dongxiang,HOU Wanguo. Synthesis, Characterization and Aggregation Behavior of Polyethylene Glycol-conjugated Hydroxycamptothecin†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1535.
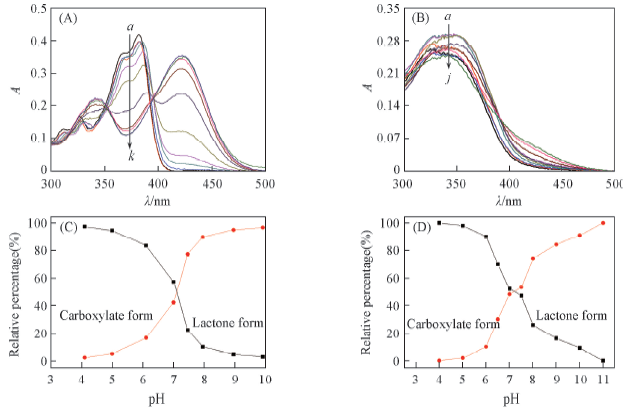
Fig.4 UV-Vis spectra of HCPT(A) and MPEG-HCPT(B) in buffer solutions with various pH values and variation of relative percentages of lactone form and carboxylate form vs. pH for HCPT(C) and MPEG-HCPT(D) pH: (A) a—k. 3.5, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9.5; (B) a—j. 4, 5, 6, 6.5, 7, 7.5, 8, 9, 10, 11.
| [1] |
Zhang, R. , Li, Y. , Cai, Q. , Liu, T. , Sun, H. , Chambless, B. , Cancer Chemother. Pharmacol., 1998, 41, 257- 267
doi: 10.1007/s002800050738 URL pmid: 9488594 |
| [2] |
Kehrer D. F., S. , Soepenberg, O. , Loos W., J. , Verweij, J. , Sparreboom, A. , Anti-Cancer Drugs, 2001, 12, 89- 105
doi: 10.1097/00001813-200102000-00002 URL pmid: 11261892 |
| [3] |
Garcia-Carbonero, R. , Supko J., G. , Clin. Cancer Res., 2002, 8, 641- 661
doi: 10.1634/theoncologist.7-6-531 URL pmid: 12490740 |
| [4] |
Burke T., G. , Mishra A., K. , Wani M., C. , Wall M., E. , Biochem., 1993, 32, 5352- 5364
doi: 10.1021/bi00071a010 URL pmid: 8499440 |
| [5] |
Tyner K., M. , Schiffman S., R. , Giannelis E., P. , J. Contr. Release, 2004, 95, 501- 514
doi: 10.1016/j.jconrel.2003.12.027 URL pmid: 15023461 |
| [6] |
Erickson-Miller C., L. , May R., D. , Tomaszewski, J. , Osborn, B. , Murphy M., J. , Page J., G. , Parchment R., E. , Cancer Chemother. Phamracol., 1997, 39, 467- 472
doi: 10.1007/s002800050600 URL pmid: 9054963 |
| [7] |
Mi, Z. , Burke T., G. , Biochem., 1994, 33, 12540- 12545
doi: 10.1021/bi00200a013 URL pmid: 8068669 |
| [8] |
Kunadharaju, S. , Savva, M. , J. Chem. Eng. Data, 2010, 55, 103- 112
doi: 10.1002/kin.20508 URL |
| [9] |
Schluep, T. , Cheng, J. , Khin K., T. , Davis M., E. , Cancer Chemother. Pharmacol., 2006, 57, 654- 662
doi: 10.1007/s00280-005-0091-7 URL pmid: 16133526 |
| [10] |
Bala, V. , Rao, S. , Boyd B., J. , Prestidge C., A. , J. Contr. Release, 2013, 172, 48- 61
doi: 10.1016/j.jconrel.2013.07.022 URL pmid: 23928356 |
| [11] | 银晓晶, 廖栩, 张瑜, 何蕊, 王淑君. 中国药剂学杂志, 2014, 12( 2), 62- 72 |
| Yin X., J. , Liao, X. , Zhang, Y. , He, R. , Wang S., J. , Chinese J. Pharmaceutics, 2014, 12( 2), 62- 72 | |
| [12] | 刘璐, 姚日升, 尤启东, 陶丽. 高分子材料科学与工程, 2011, 27( 4), 143- 150 |
| Lu, L. , Yao, R.S. , You, Q.D. , Tao, L. , J. Polym. Sci. and Eng., 2011, 27( 4), 143- 150 | |
| [13] |
皮劲松, 栾立标, 吴小涛. 中国医药工业杂志, 2009, 40( 9), 680- 683
doi: 10.3969/j.issn.1001-8255.2009.09.012 URL |
|
Pi, J.S. , Luan, L.B. , Wu, X.T. , Chinese J. Pharmaceut., 2009, 40( 9), 680- 683
doi: 10.3969/j.issn.1001-8255.2009.09.012 URL |
|
| [14] |
Cortesi, R. , Esposito, E. , Maietti, A. , Menegatti, E. , Nastruzzi, C. , Int. J. Pharm., 1997, 159, 95- 103
doi: 10.1016/S0378-5173(97)00275-5 URL |
| [15] |
Koo O., M. , Rubinstein, I. , Onyuksel, H. , Nanomedicine: Nanotechnol. Biol. Med., 2005, 1, 77- 84
doi: 10.1016/j.nano.2004.11.002 URL pmid: 17292061 |
| [16] | Çirpanli Y. , Bilensoy E. , A., Çaliʂ S., Eur. J. Pharm. Biopharm ., 2009, 73, 82—89 |
| [17] |
Marier J., F. , Pheng, L. , Trinh M., M. , Burris H., A. , Jones, S. , Anderson, K. , Warner, S. , Porubek, D. , J. Pharm. Sci., 2011, 100, 4536- 4545
doi: 10.1002/jps.22645 URL pmid: 21630281 |
| [18] |
Dong, L. , Li, Y. , Hou W., G. , Liu S., J. , J. Solid State Chem., 2010, 183, 1811- 1816
doi: 10.1016/j.jssc.2010.05.035 URL |
| [19] |
马秀明, 庞秀江, 全贞兰, 侯万国. 高等学校化学学报, 2013, 34( 4), 913- 918
doi: 10.7503/cjcu20120878 |
|
Ma X., M. , Pang X., J. , Quan Z., L. , Hou W., G. , Chem. ., J. , Chinese Universities, 2013, 34( 4), 913- 918
doi: 10.7503/cjcu20120878 |
|
| [20] |
Wu, X. , Li, H. , Song, S. , Zhang R., J. , Hou W., G. , Int. J. Pharmaceut., 2013, 454, 453- 461
doi: 10.1016/j.pharma.2012.12.005 URL pmid: 23537412 |
| [21] |
兀晓文, 杜娜, 李海平, 张人杰, 侯万国, 化学学报, 2014, 72, 963- 969 Wu X., W. , Du, N. , Li H., P. , Zhang R., J. , Hou W., G. , Acta Chemica Sinica, 2014, 72, 963- 969
doi: 10.6023/A14030146 URL |
| [22] |
Kohler, N. , Fryxell G., E. , Zhang, M. , J. Am. Chem. Soc., 2004, 126( 23), 7206- 7211
doi: 10.1021/ja049195r URL pmid: 15186157 |
| [23] |
Zhang, M. , Ferran, M. , Biomed. Microdevices, 1998, 1, 81- 89
doi: 10.1023/A:1009938507935 URL |
| [24] | Yu, D. , Peng, P. , Dharap S., S. , Wang, Y. , Mehlig, M. , Chandna, P. , Zhao, H. , Filpula, D. , Yang, K. , Borowski, V. , Borchard, G. , Zhang, Z. , Minko, T. , J. Control Release, 2005, 110, 90- 102 |
| [25] | Zalipsky, S. , Bioconj. Chem., 1995, 6( 2), 150- 165 |
| [26] | Sun C. J. Wang X. J. Sun F. Y. Handbook of Organic Synthesis, Chemical Industry Press, Beijing, 2011, 154— 155 |
| 孙昌俊, 王秀菊, 孙风云. 有机化合物合成手册, 北京: 化学工业出版社, 2011, 154— 155 | |
| [27] |
Liu, Z. , Robinson J., T. , Sun, X. , Dai, H. , J. Am. Chem. Soc., 2008, 13, 10876- 10877
doi: 10.1021/ja806242t URL pmid: 2617744 |
| [28] |
徐春燕, 黄明智, 薛传薪. 光谱学与光谱分析, 2005, 25( 11), 1772- 1774
doi: 10.3321/j.issn:1000-0593.2005.11.008 URL |
|
Xu C., Y. , Huang M., Z. , Xue C., X. , Spectroscopy and Spectral Analysis, 2005, 25( 11), 1772- 1774
doi: 10.3321/j.issn:1000-0593.2005.11.008 URL |
|
| [29] |
Chourpa, I. , Millot J., M. , Sockalingum G., D. , Riou J., F. , Manfait, M. , Biophy. Acta, 1998, 1379, 353- 366
doi: 10.1016/S0304-4165(97)00115-3 URL pmid: 9545598 |
| [30] | Barreiro-Iglesias, R. , Bromberg, L. , Temchenko, M. , Hatton T., A. , Concheiro, A. , Alvarez-Lorenzo, C. , J. Control Release, 2004, 97, 537- 549 |
| [31] |
Akimoto, K. , Kawai, A. , Ohya, K. , Chem. Pharm. Bull., 1994, 42, 2135- 2138
doi: 10.1248/cpb.42.2135 URL |
| [32] | 任学贞, 李干佐, 王弘立, 翟立民, 隋卫平, 徐欣艳. 高等学校化学学报, 1995, 16( 8), 1295- 1297 |
| Ren X., Z. , Li G., Z. , Wang H., L. , Zhai L., M. , Sui W., P. , Xu X., Y. , Chem., J. , Chinese Universities, 1995, 16( 8), 1295- 1297 |
| [1] | WANG Shoubai, WU Xiuming, WU Jinming, TANG Yanfeng, SHU Chen, ZHONG Min, HUANG Wei, YAN Deyue. Synthesis and Properties of Soluble Transparent Polyimides Containing tert-Butyl and Isobutyl Groups [J]. Chem. J. Chinese Universities, 2021, 42(9): 2944. |
| [2] | WANG Xianwei, KE Hongjun, YUAN Hang, LU Gewu, LI Liying, MENG Xiangsheng, SONG Shulin, WANG Zhen. High Temperature Resistant and Soluble Polyimide Resins and Their Composites [J]. Chem. J. Chinese Universities, 2021, 42(6): 2041. |
| [3] | ZHANG Danwei, WANG Hui, LI Zhanting. Water-soluble Regular Three-dimensional Supramolecular and Covalent Organic Polymers † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1139. |
| [4] | ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD † [J]. Chem. J. Chinese Universities, 2020, 41(2): 317. |
| [5] | GUO Zhaopei,LIN Lin,CHEN Jie,TIAN Huayu,CHEN Xuesi. Polyglutamic Acid Grafted Polyethylene Glycol@Calcium Carbonate Based Shielding System for Improving Polyethyleneimine Gene Transfection Efficiency † [J]. Chem. J. Chinese Universities, 2020, 41(2): 235. |
| [6] | LI Lin, XU Xinru, LI Yingqi, ZHANG Caifeng. Preparation of Targeting Nanodiamond-metaminopterone Drug System and Its Interaction with MCF-7 Cells † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1998. |
| [7] | ZHAO Yuxuan,CHEN Yanjun,PAN Guxin,WANG Chang,PENG Zhenbo,SUN Zongxu,LIANG Yongri,SHI Qisong. Preparation and Performance of Novel Tb-PEG+Eu-PEG/PANI/PAN Luminescent-electrical-phase Change Composite Fibers by Electrospinning† [J]. Chem. J. Chinese Universities, 2019, 40(4): 824. |
| [8] | JIANG Jing,HUANG Yali,ZHANG Qilong,XU Hong,SUN Xiaohong. Effects of Cucurbit[8]uril on the Solubility, Stability and Antioxidation of Cyanidin† [J]. Chem. J. Chinese Universities, 2019, 40(1): 76. |
| [9] | QIAN Yihao, ZHANG Dongjie, CHENG Zhongjun, KANG Hongjun, LIU Yuyan. Preparation of Hydrophilic Epoxy Resin and Its Wettability Regulation† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1823. |
| [10] | YU Kaile,PAN Xinyu,ZHANG Zhengfang,WANG Qiang. Determination of Solubility Parameters of Imidazolyl Acetate Ionic Liquid by Inverse Gas Chromatography and Hansen Solubility Parameters† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1048. |
| [11] | LIU Jie, ZHOU Hao, HUANG Yufang, CHEN Xin. Soy Protein Isolate/Agar Composite Hydrogel† [J]. Chem. J. Chinese Universities, 2018, 39(3): 591. |
| [12] | GAO Yan, ZHANG Hua, ZHANG Wen, LI Xiangpeng, YUE Pan, LI Wei. Preparation of Micro/Nano-fibers Membranes Encapsulated with Dual Drugs by Emulsion Electrospun and Controlled Release of Hydrophilic and Hydrophobic Drugs† [J]. Chem. J. Chinese Universities, 2018, 39(3): 575. |
| [13] | LIU Jie, ZHOU Hao, HUANG Yufang, CHEN Xin. Polyethylene Glycol Chemically Modified Soy Protein Isolate Hydrogel† [J]. Chem. J. Chinese Universities, 2018, 39(2): 390. |
| [14] | YANG Tong, PENG Rong, HUANG Xinyu, SUN Yansong, CHEN Xiaonong. Relative Hydrophobicity of Drug Molecules and Retention Behavior in Hydrophobic Interaction Chromatography† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2405. |
| [15] | ZHANG Hanyu, LUO Wu, LIU Zhenchao, LI Xiaobai, Guan Shaowei, LIU Baijun. Preparation of the Biphenyl-containing Polyarylethers with Highly Fluorinated Contents [J]. Chem. J. Chinese Universities, 2017, 38(7): 1107. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||