

Chem. J. Chinese Universities ›› 2023, Vol. 44 ›› Issue (5): 20220730.doi: 10.7503/cjcu20220730
• Review • Previous Articles Next Articles
LI Ruisong, MIAO Zhengpei( ), LI Jing, TIAN Xinlong(
), LI Jing, TIAN Xinlong( )
)
Received:2022-11-25
Online:2023-05-10
Published:2023-01-09
Contact:
MIAO Zhengpei, TIAN Xinlong
E-mail:zpmiao92@hainanu.edu.cn;tianxl@hainanu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Ruisong, MIAO Zhengpei, LI Jing, TIAN Xinlong. Research Progress on Hollow Precious Metal-based Nanostructures for Oxygen Reduction Reaction[J]. Chem. J. Chinese Universities, 2023, 44(5): 20220730.
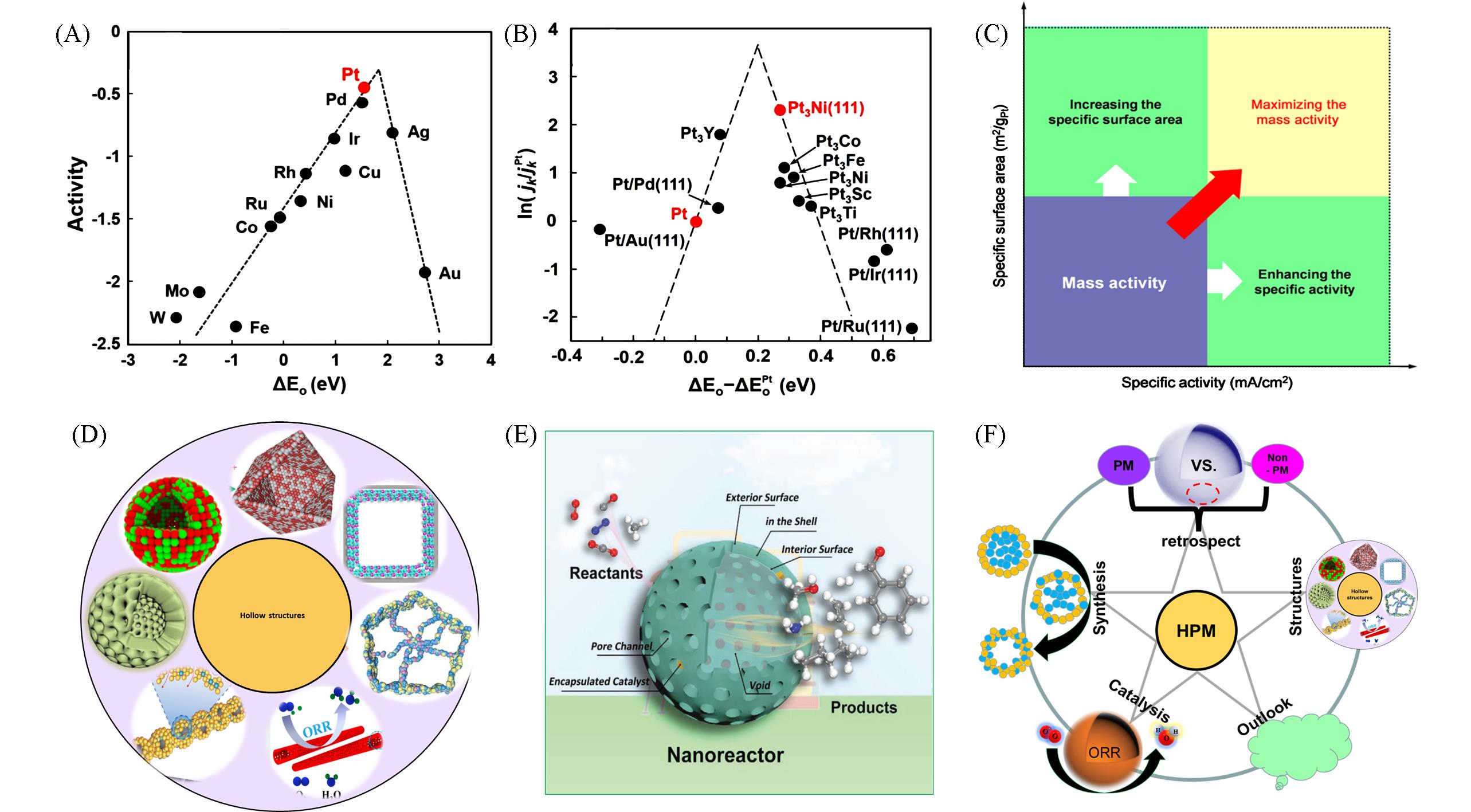
Fig.1 Activities of different metals toward ORR plotted as a function of the oxygen binding energy(A)[26], kinetic current densities(based on pure Pt) of different binary alloys as a function of the oxygen adsorption energy relative to pure Pt as(ΔEo-ΔEoPt)(B)[28], schematic illustration for maximizing the MA of PM⁃based nanostructures(C)[34], main structures of HPMs for recent progress, including nanocages, nanotubes, mesoporous nanocages, nanochains and nanoframes(D), schematic diagram of hollow nanostructure as nanoreactors(E)[36], schematic illustration of this review including retrospect, synthesis, structure, catalysis and outlook(F)(A) Copyright 2004, American Chemical Society; (B) Copyright 2018, Wiley-VCH; (C) Copyright 2017, American Chemical Society; (E) Copyright 2019, Wiley-VCH.
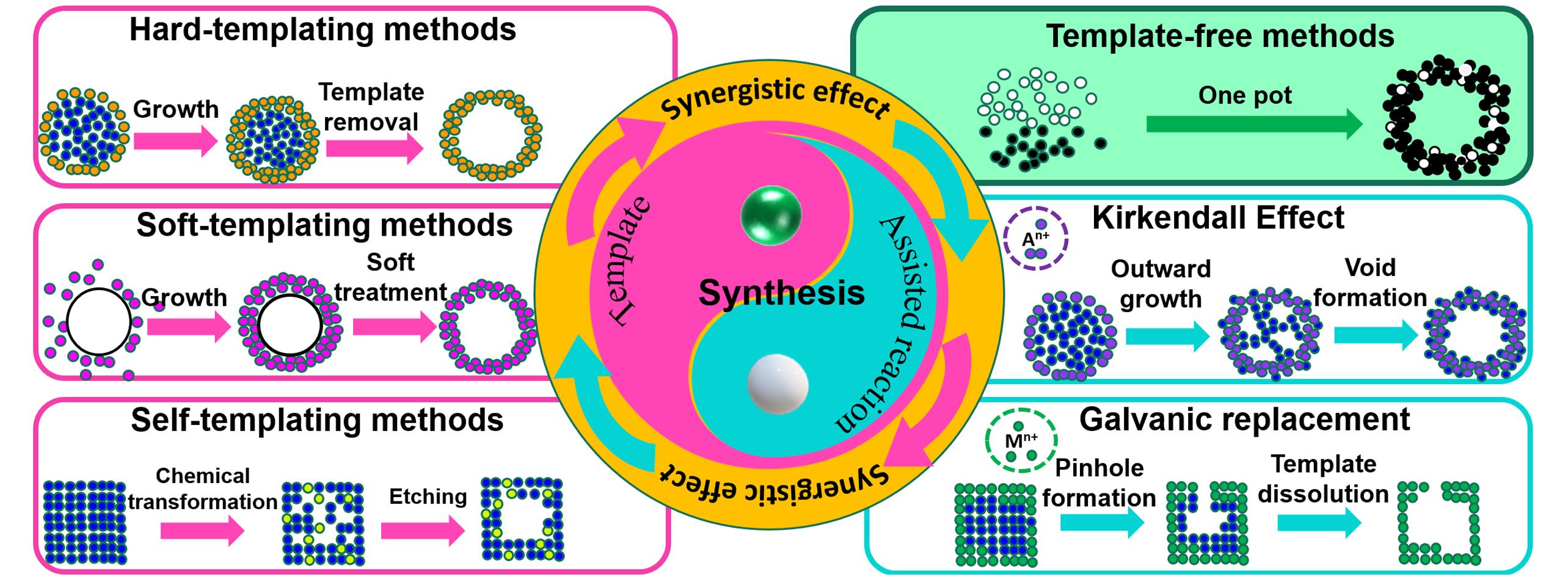
Fig.2 Essential and schematic plots of synthetic methodologies for hollow structures concluding hard⁃, soft⁃, and self⁃templating methods, as well as assisted kirkendall effect and galvanic replacement reaction
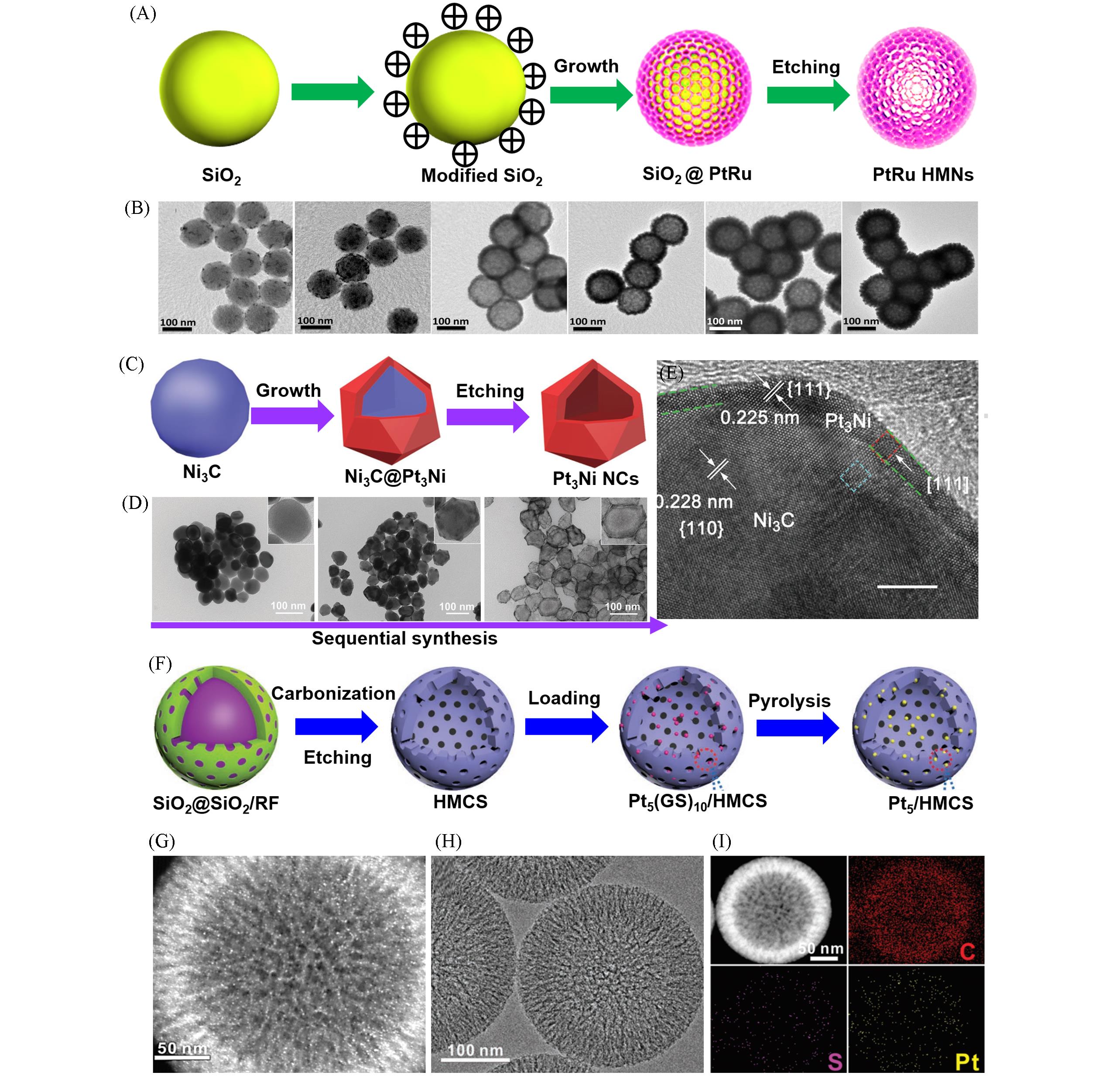
Fig.3 Hard⁃templating method for synthesizing HPMs: schematic illustration for synthesizing hollow Pt⁃Ru nanostructures(A), TEM images during the preparation process(B)[50], schematic illustration for synthesizing hollow Pt3Ni nanostructures(C), corresponding TEM images(D), high⁃ resolution TEM image of Ni3C@Pt3Ni particle(E)[56], schematic illustration for synthesizing Pt5/HMCS(F), SEM image(G), TEM image(H) and EDS mappings(I) of Pt5/HMCS[57](A, B) Copyright 2013, Wiley-VCH; (C—E) Copyright 2022, Wiley-VCH; (F—I) Copyright 2020, Wiley-VCH.
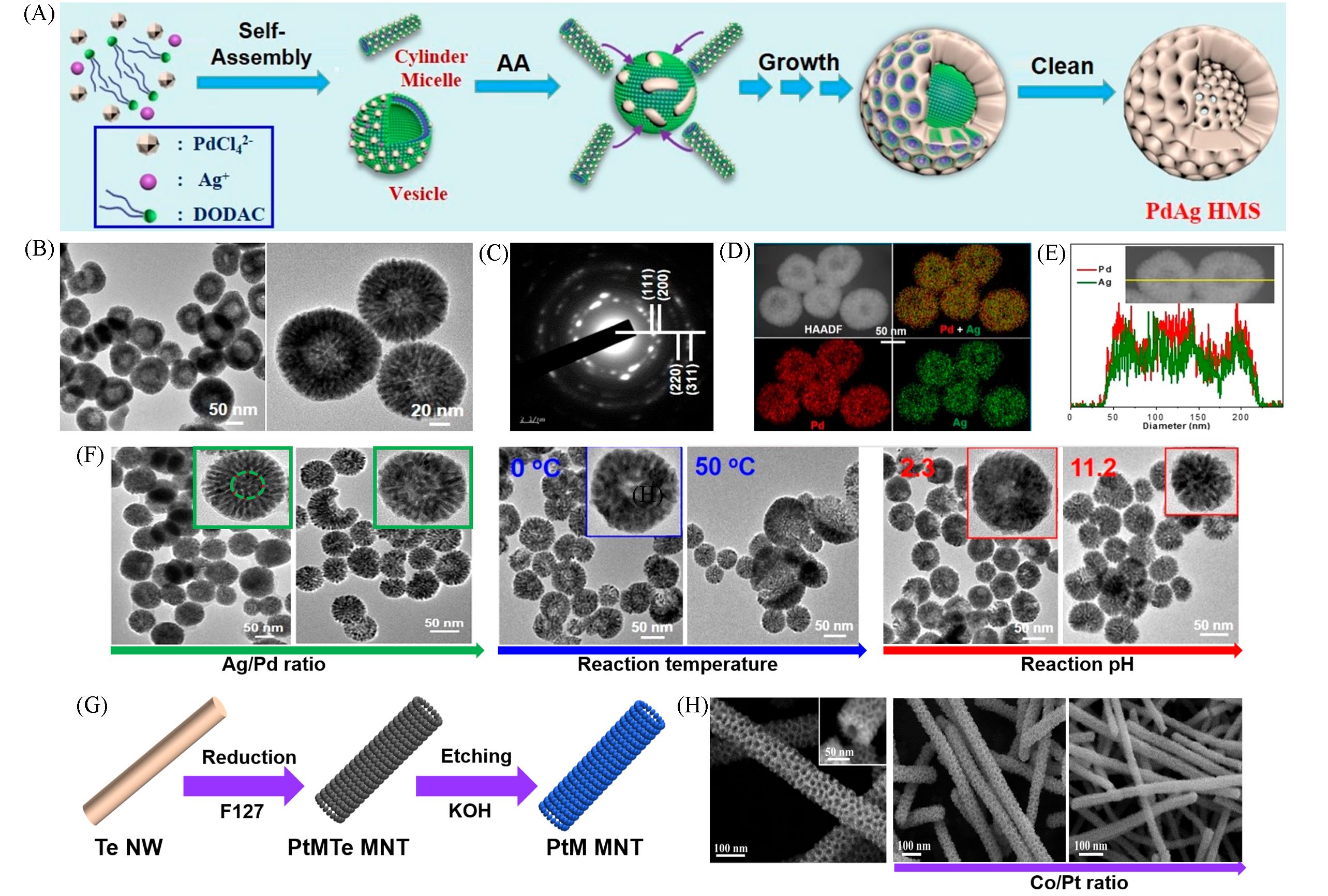
Fig.4 Soft⁃templating method for synthesizing HPMs: schematic illustration for synthesizing hollow PdAg nanostructure(A), TEM image(B), selective area electron diffraction pattern(C), dark⁃field scanning transmission electron microscopy and EDS mapping(D), line scan profiles(E), the morphology change based on different Ag/Pd ration, temperature and pH of PdAg HMNs(F)[62], schematic illustration for synthesizing hollow PtCo and PtNi nanostructures(G), different morphologies of PtCo MNT with mesoporous nanotube structures at different metallic ratio(H)[64]
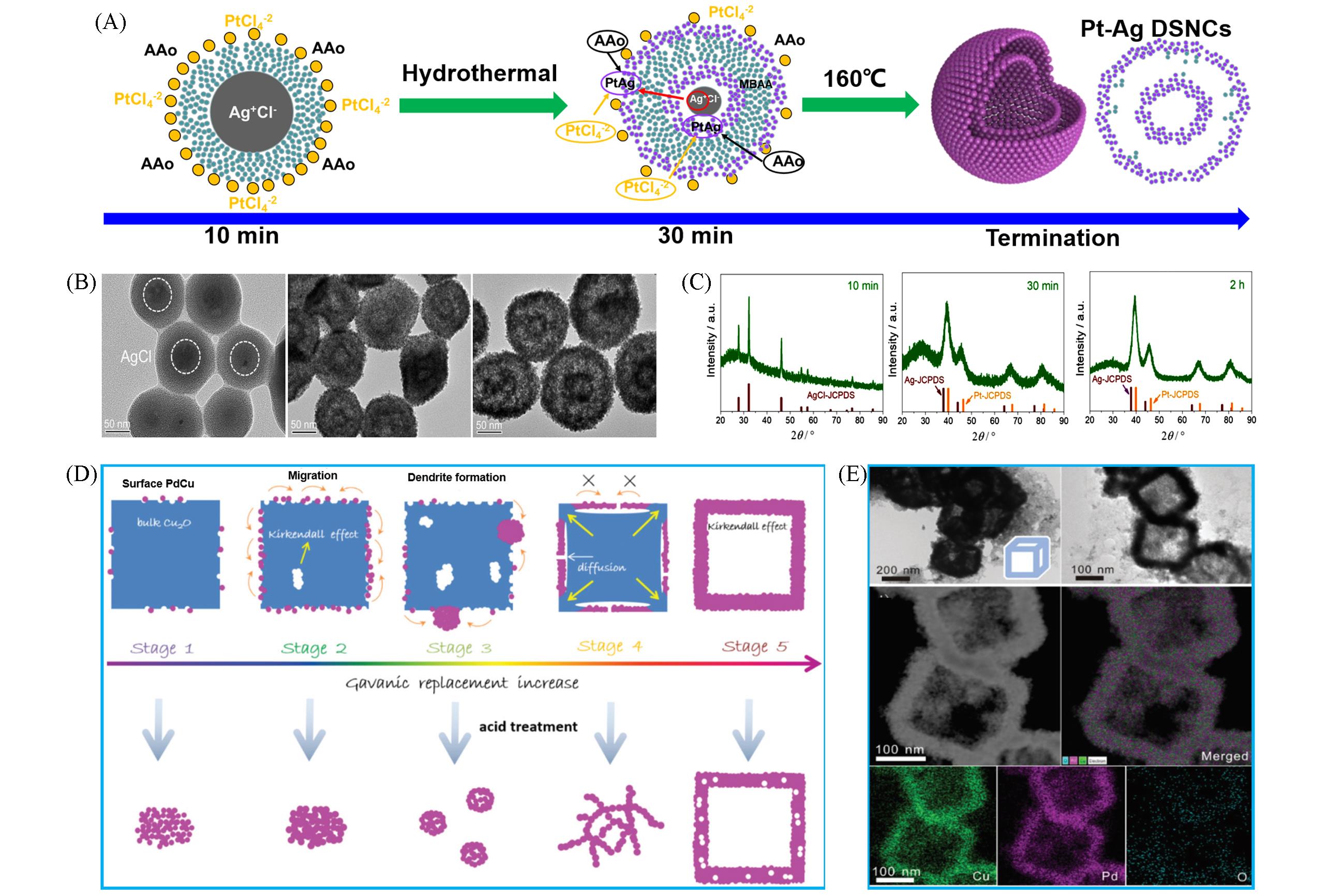
Fig.5 Self⁃templating method for synthesizing HPMs: schematic illustration for synthesizing(A), TEM images(B), X⁃ray diffraction patterns with different crystals during preparation process(C) of hollow Pt⁃Ag nanostructures[25], schematic illustration for synthesizing hollow PdCu nanoboxes(D), corresponding TEM images and EDS mapping(E)[67]
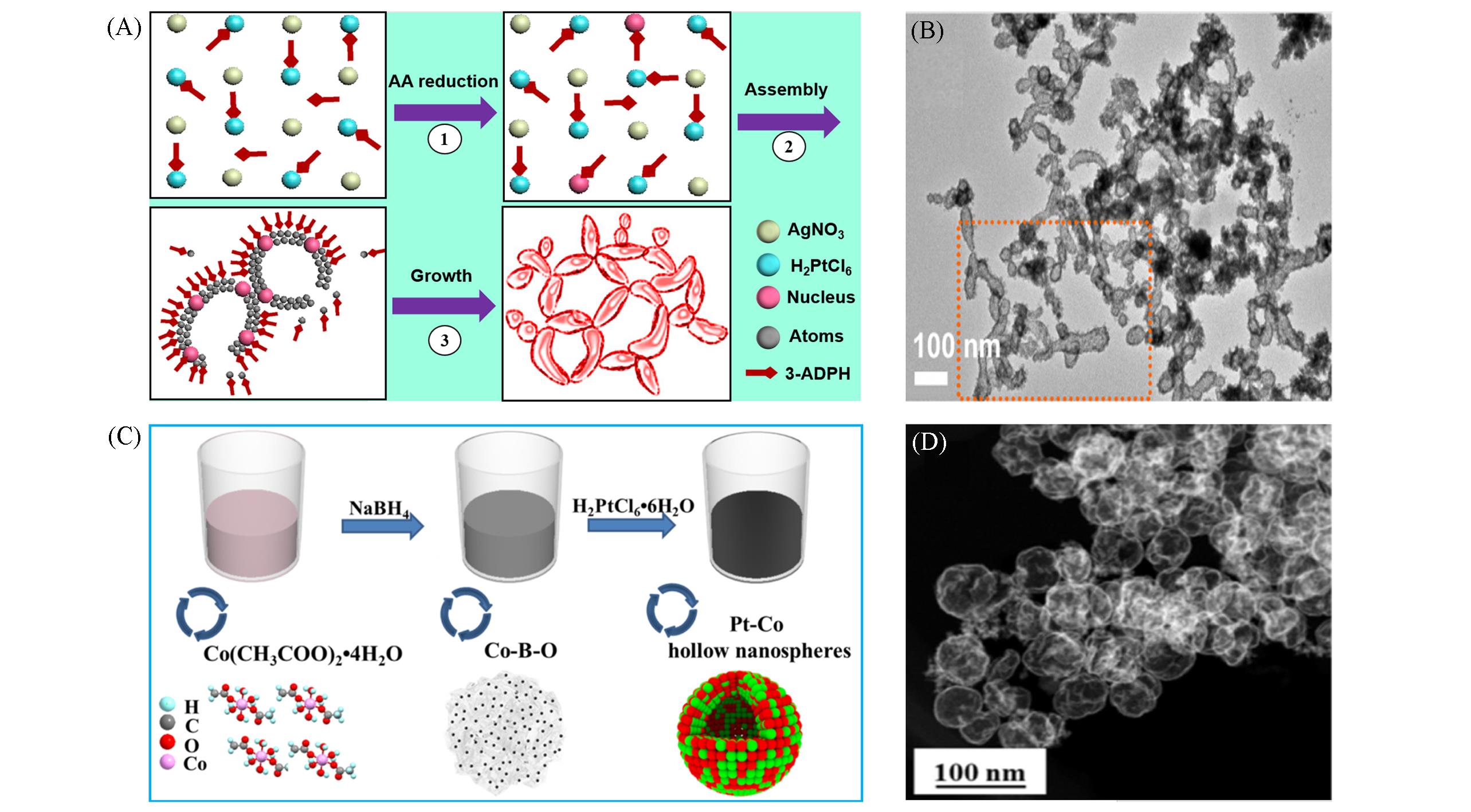
Fig.6 Template⁃free method for synthesizing HPMs: schematic illustration for synthesizing hollow Pt⁃Ag nanochains by one⁃pot strategy(A), TEM image for nanochains(B)[68], schematic illustration for synthesizing hollow PdCo nanospheres with Co⁃B⁃O as intermedium(C), TEM image for nanospheres(D)[71](A, B) Copyright 2017, Elsevier; (C, D) Copyright 2020, Elsevier.
| Structure | Composition | Synthesis strategy | Mass activity/(A·mg | Ref. |
|---|---|---|---|---|
| Mesoporous nanocages | PtPdNi | Pd cores, selective etching | 1.140 | [ |
| Nano⁃hollow spheres | PdCuMoNiCo | One⁃pot solvothermal method | 0.882 | [ |
| Nanocages | Pt⁃Ag | Ag nanocubes, galvanic replacement | 0.640 | [ |
| Nanoframes | Pt3Ni | PtNi3 polyhedra, erosion | — | [ |
| Hollow tesseracts | Pd⁃Pt | Pd⁃Pt alloy nanocubes, Oxygen reaction | 1.860 | [ |
| Double⁃shelled nanocages | Pt⁃Ag | One⁃pot self⁃templated strategy | — | [ |
| Hollow nanostructures | Pt/Ag | AgCl nanoparticles, galvanic replacement | — | [ |
| Nanoframes | PtNi | PtNi rhombic dodecahedron nanoparticles, oxidative etching | 0.920 a | [ |
| Hollow nanoparticles | Pt⁃Ni | Sacrificial SiO2 template | 0.490 | [ |
| Nanocages | Pt | Pd icosahedral seeds, HNO3 oxygen | 1.120 | [ |
| Mesoporous nanocages | S, P⁃PtPd | SiO2 as template | 0.560 | [ |
| Hollow nanospheres | PdCu | Soft template method | 0.370 | [ |
| Hollow nanospheres | Pd4S | A self⁃templating process | 0.160 | [ |
| Holey nanotubes | Pt | PtII ⁃dimethylglyoxime complex, Ostwald ripening | — | [ |
| Hollow nanochain | PtAg | One⁃pot co⁃reduction method | 0.370 | [ |
| Hollow nanocubes | PtNi | Eutectic salt⁃mediated pyrolysis strategy, Kirkendall effect | 2.020 | [ |
| Hollow nanochains | PtNi | Ni nanosponges, galvanic replacement | 0.340 a,b | [ |
| Nanorings | PtPdCo | Colloidal chemistry method | 3.580 b | [ |
| Hollow tetrapods | Pt | Pd cores, chemical etching | 0.800 | [ |
| Superlong nanotubes | Pt | Pd nanowire, etching | 2.090 | [ |
| Nanocages | Pt⁃Ni⁃P | SiO2 spheres as template | 1.210 | [ |
| Ultrathin nanoring | PdPtCu | A sequential reduction method | 1.970 | [ |
| Core/shell⁃nanotubes | Pd/PtFe | Pd nanowire, galvanic dissolution | 2.710 | [ |
| Hollow nanochains | PtNi | Pt⁃Ni bunched nanospheres, etching | 3.520 | [ |
| Hollow nanochains | Pd⁃Au | Co nanochains, galvanic replacement | 0.287 a,b,c | [ |
| Hollow nanospheres | PdAu | Cobalt nanoparticles as templates | 0.153 b,c | [ |
| Nanoframes | Pt⁃Cu⁃Mn | A simple wet⁃chemical approach | 1.450 b | [ |
Table 1 Synthetic strategies and corresponding catalytic applications of HPMs
| Structure | Composition | Synthesis strategy | Mass activity/(A·mg | Ref. |
|---|---|---|---|---|
| Mesoporous nanocages | PtPdNi | Pd cores, selective etching | 1.140 | [ |
| Nano⁃hollow spheres | PdCuMoNiCo | One⁃pot solvothermal method | 0.882 | [ |
| Nanocages | Pt⁃Ag | Ag nanocubes, galvanic replacement | 0.640 | [ |
| Nanoframes | Pt3Ni | PtNi3 polyhedra, erosion | — | [ |
| Hollow tesseracts | Pd⁃Pt | Pd⁃Pt alloy nanocubes, Oxygen reaction | 1.860 | [ |
| Double⁃shelled nanocages | Pt⁃Ag | One⁃pot self⁃templated strategy | — | [ |
| Hollow nanostructures | Pt/Ag | AgCl nanoparticles, galvanic replacement | — | [ |
| Nanoframes | PtNi | PtNi rhombic dodecahedron nanoparticles, oxidative etching | 0.920 a | [ |
| Hollow nanoparticles | Pt⁃Ni | Sacrificial SiO2 template | 0.490 | [ |
| Nanocages | Pt | Pd icosahedral seeds, HNO3 oxygen | 1.120 | [ |
| Mesoporous nanocages | S, P⁃PtPd | SiO2 as template | 0.560 | [ |
| Hollow nanospheres | PdCu | Soft template method | 0.370 | [ |
| Hollow nanospheres | Pd4S | A self⁃templating process | 0.160 | [ |
| Holey nanotubes | Pt | PtII ⁃dimethylglyoxime complex, Ostwald ripening | — | [ |
| Hollow nanochain | PtAg | One⁃pot co⁃reduction method | 0.370 | [ |
| Hollow nanocubes | PtNi | Eutectic salt⁃mediated pyrolysis strategy, Kirkendall effect | 2.020 | [ |
| Hollow nanochains | PtNi | Ni nanosponges, galvanic replacement | 0.340 a,b | [ |
| Nanorings | PtPdCo | Colloidal chemistry method | 3.580 b | [ |
| Hollow tetrapods | Pt | Pd cores, chemical etching | 0.800 | [ |
| Superlong nanotubes | Pt | Pd nanowire, etching | 2.090 | [ |
| Nanocages | Pt⁃Ni⁃P | SiO2 spheres as template | 1.210 | [ |
| Ultrathin nanoring | PdPtCu | A sequential reduction method | 1.970 | [ |
| Core/shell⁃nanotubes | Pd/PtFe | Pd nanowire, galvanic dissolution | 2.710 | [ |
| Hollow nanochains | PtNi | Pt⁃Ni bunched nanospheres, etching | 3.520 | [ |
| Hollow nanochains | Pd⁃Au | Co nanochains, galvanic replacement | 0.287 a,b,c | [ |
| Hollow nanospheres | PdAu | Cobalt nanoparticles as templates | 0.153 b,c | [ |
| Nanoframes | Pt⁃Cu⁃Mn | A simple wet⁃chemical approach | 1.450 b | [ |
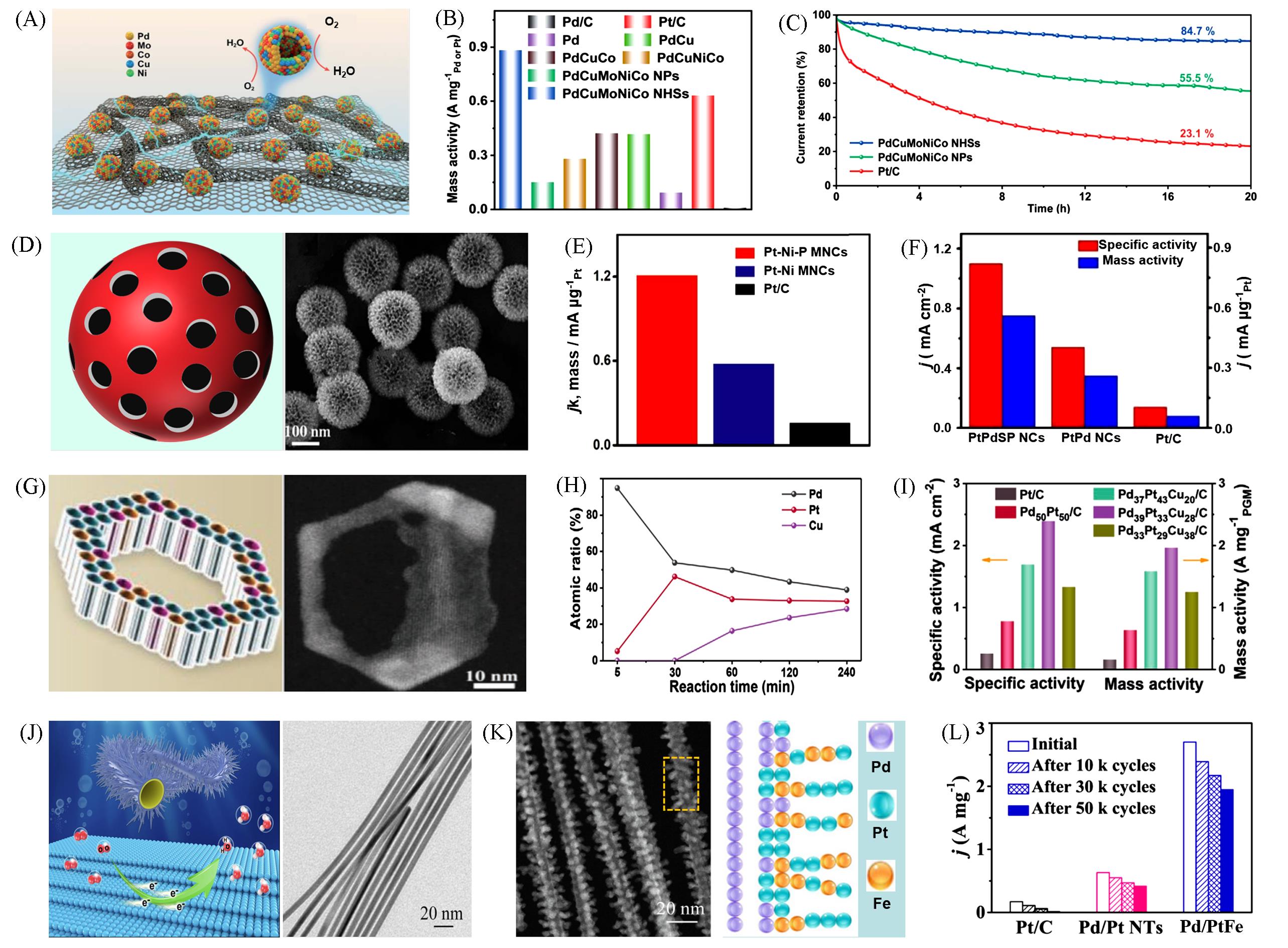
Fig.7 PdCuMoNiCo with hollow nanosphere structures(A), corresponding MA of hollow PdCu, PdCuCo, PdCuNiCo, and PdCuMoNiCo nanospheres for ORR in 0.1 mol/L KOH(B), long⁃term stability of PdCuMoNiCo hollow nanospheres and nanoparticles(C)[15], Pt⁃Ni⁃P with hollow nanocage structures(D), corresponding MA of Pt⁃Ni and Pt⁃Ni⁃P in 0.1 mol/L HClO4(E)[80], MA of S, P co⁃ doing PtPd nanocage in 0.1 mol/L HClO4(F)[51], PdPtCu with nanoring structures(G), controlling compositions for Pd, Pt and Cu in PdPtCu by reaction times(H), corresponding MA in 0.1 mol/L KOH(I)[81], spiny Pd/PtFe with core/shell nanotube structures(J), corresponding TEM images(K) and MA(L) in 0.1 mol/L HClO4[82](A—C) Copyright 2021, the Royal Society of Chemistry; (D, E) Copyright 2019, the Royal Society of Chemistry; (F) Copyright 2020, the Royal Society of Chemistry; (G—I) Copyright 2021, Wiley-VCH; (J—L) Copyright 2021, Elsevier.
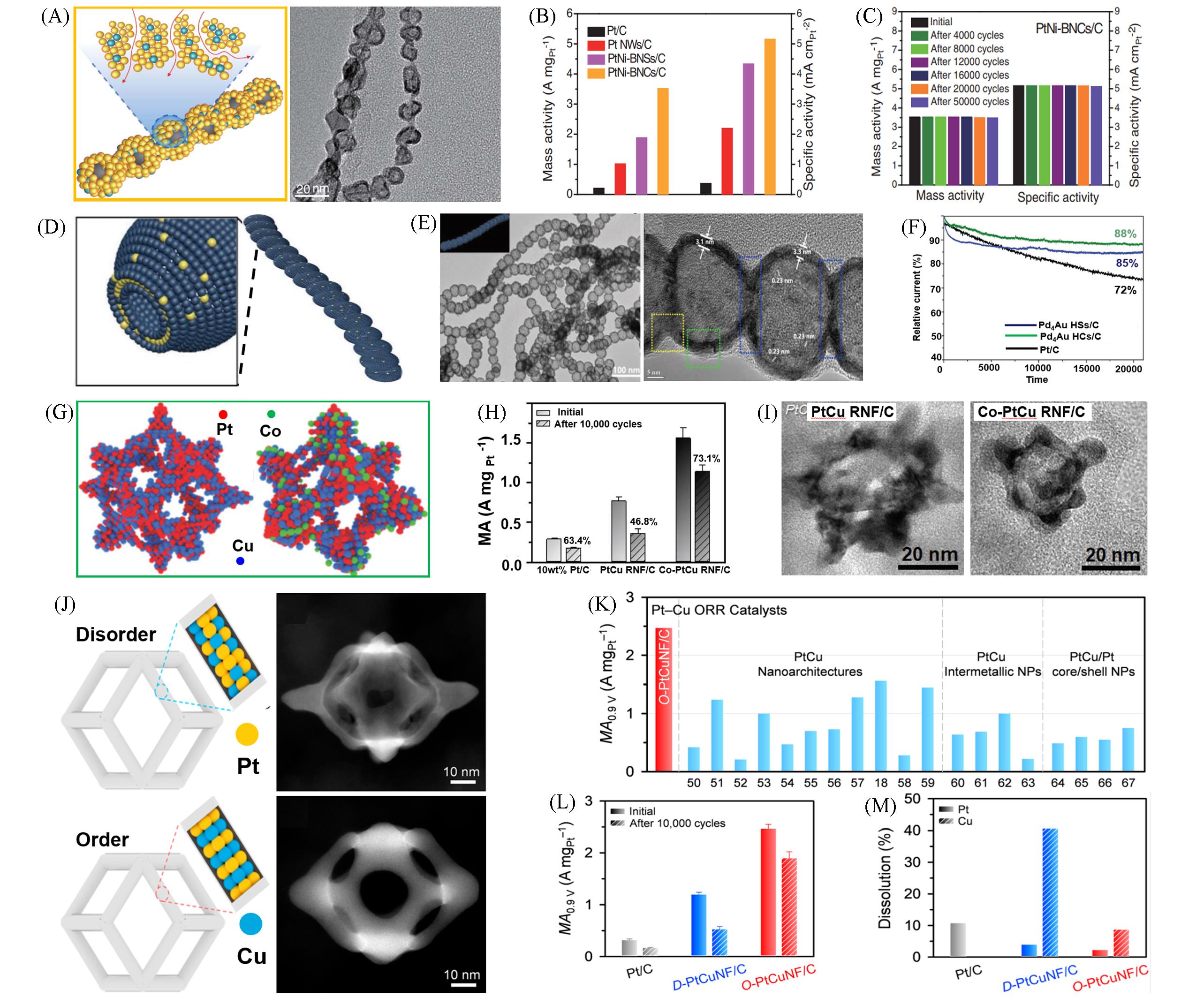
Fig.8 Pt⁃Ni bunched nanocages with 1D form(A), corresponding ORR activity in 0.1 mol/L HClO4(B), ORR stability of the optimized PtNi nanochains in 0.1 mol/L HClO4(C)[83], hollow Pd4Au nanochains(D), TEM images(E), ORR stability in 0.1 mol/L KOH(F)[84], Co⁃PtCu nanoframe(G), corresponding ORR activity and stability(H), TEM images of PtCu NF and Co⁃PtCu NF after running stability test(I)[101], disorder and order PtCu nanoframe(J), ORR activity compared with recent PtCu catalysts(K), ORR activity and stability in 0.1 mol/L HClO4(L), dissolution of Pt and Cu in D⁃PtCu NF and O⁃PtCu NF after running stability test(M)[105](A—C) Copyright 2019, AAAS; (D—F) Copyright 2020, Wiley-VCH; (G—I) Copyright 2018, Wiley-VCH; (J—M) Copyright 2020, American Chemical Society.
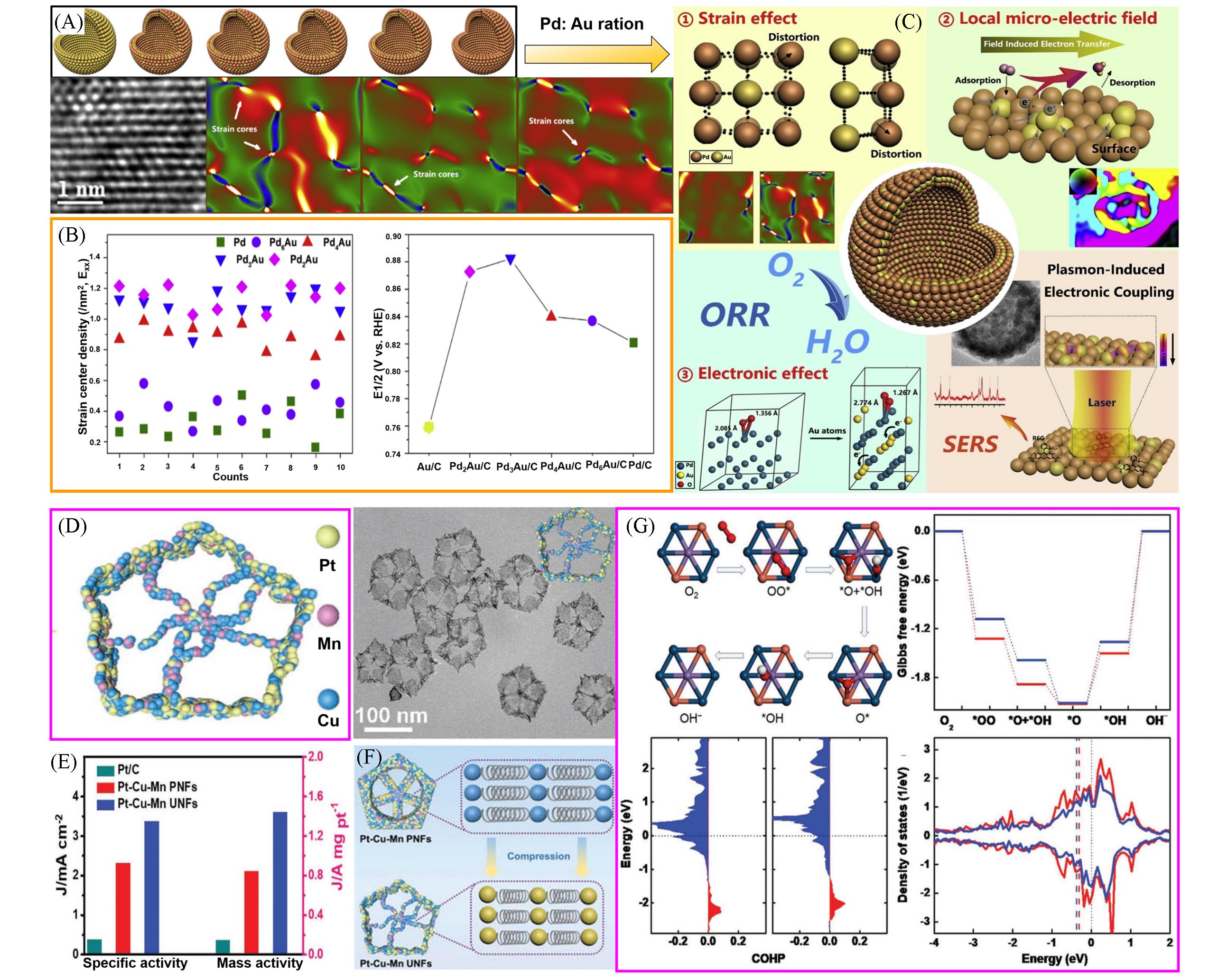
Fig.9 A series of hollow PdAu nanospheres with different ratios of Pd and Au, and corresponding high⁃ resolution TEM images(A), strain center density for hollow PdAu nanospheres and corresponding ORR activity in 0.1 mol/L KOH(B), the mechanisms for ORR performance enhancement(C)[85], ultrafine Pt⁃Cu⁃Mn nanoframes(D), corresponding ORR activities in 0.1 mol/L KOH solution(E), compression strain for the two nanoframes of Pt⁃Cu⁃Mn(F), density functional theory(DFT) based on d⁃band center for strain effect toward ORR enhancement(G)[86](A—C) Copyright 2020, Elsevier; (D—G) Copyright 2020, Wiley-VCH.
| 1 | Stamenkovic V., Mun B. S., Mayrhofer K. J. J., Ross P. N., Markovic N. M., Rossmeisl J., Greeley J., Nørskov J. K., Angew. Chem. Int. Ed., 2006, 118(18), 2963—2967 |
| 2 | Han Y., Wang Y., Xu R., Chen W., Zheng L., Han A., Zhu Y., Zhang J., Zhang H., Luo J., Chen C., Peng Q., Wang D., Li Y., Energ. Environ. Sci., 2018, 11(9), 2348—2352 |
| 3 | Chen J., Qian G., Chu B., Jiang Z., Tan K., Luo L., Li B., Yin S., Small, 2022, 18(12), 2106773 |
| 4 | Yoshida T., Kojima K., Electrochem. Soc. Interface, 2015, 24(2), 45 |
| 5 | Koenigsmann C., Wong S. S., Energ. Environ. Sci., 2011, 4(4), 1161—1176 |
| 6 | Li Y., Sun Y., Qin Y., Zhang W., Wang L., Luo M., Yang H., Guo S., Adv. Energy Mater., 2020, 10(11), 1903120 |
| 7 | Tian X., Lu X. F., Xia B. Y., Lou X. W., Joule, 2020, 4(1), 45—68 |
| 8 | Huang L., Zaman S., Tian X., Wang Z., Fang W., Xia B. Y., Acc. Chem. Res., 2021, 54(2), 311—322 |
| 9 | Liu M., Zhao Z., Duan X., Huang Y., Adv. Mater., 2019, 31(6), 1802234 |
| 10 | Wu T., Sun M., Huang B., InfoMat, 2020, 2(4), 715—734 |
| 11 | Zeng X., Zhao Y., Hu X., Stucky G. D., Moskovits M., Small Structures, 2021, 2(4), 2000138 |
| 12 | Zhao M., Wang X., Yang X., Gilroy K. D., Qin D., Xia Y., Adv. Mater., 2018, 30(48), 1801956 |
| 13 | Dubau L., Asset T., Chattot R., Bonnaud C., Vanpeene V., Nelayah J., Maillard, F., ACS Catal., 2015, 5(9), 5333—5341 |
| 14 | Wang H., Li Y., Deng K., Li C., Xue H., Wang Z., Li X., Xu, Y., Wang L., ACS Appl. Mater. Interfaces, 2019, 11(4), 4252— 4257 |
| 15 | Zuo X., Yan R., Zhao L., Long Y., Shi L., Cheng Q., Liu, D., Hu C., J. Mater. Chem. A, 2022, 10(28), 14857—14865 |
| 16 | Yang X., Roling L. T., Vara M., Elnabawy A. O., Zhao M., Hood Z. D., Bao S., Mavrikakis M., Xia Y., Nano Lett., 2016, 16(10), 6644—6649 |
| 17 | Chen C., Kang Y., Huo Z., Zhu Z., Huang W., Xin H. L., Snyder J. D., Li D., Herron J. A., Mavrikakis M., Chi M., More K. L., Li Y., Markovic N. M., Somorjai G. A., Yang P., Stamenkovic V. R., Science, 2014, 343(6177), 1339—1343 |
| 18 | Ouyang Y., Cao H., Wu H., Wu D., Wang F., Fan X., Yuan W., He M., Zhang L. Y., Li C. M., Appl. Catal. B: Environ., 2020, 265, 118606 |
| 19 | Chen S., Zhao J., Su H., Li H., Wang H., Hu Z., Bao J., Zeng J., J. Am. Chem. Soc., 2021, 143(1), 496—503 |
| 20 | Zhu J., Wei M., Meng Q., Chen Z., Fan Y., Hasan S. W., Zhang X., Lyu D., Tian Z. Q., Shen, P. K., Nanoscale, 2020, 12(47), 24070—24078 |
| 21 | Li C., Wang M., Ren L., Sun H., Inorg. Chem. Front., 2022, 9(7), 1467—1473 |
| 22 | Li H. H., Yu S. H., Adv. Mater., 2019, 31(38), 1803503 |
| 23 | Sun L., Lv H., Feng J., Guselnikova O., Wang Y., Yamauchi Y., Liu B., Adv. Mater., 2022, 34(31), 2201954 |
| 24 | Yang T. H., Ahn J., Shi S., Wang P., Gao R., Qin D., Chem. Rev., 2021, 121(2), 796—833 |
| 25 | Yao W., Jiang X., Li M., Li Y., Liu Y., Zhan X., Fu G., Tang Y., Appl. Catal. B: Environ., 2021, 282, 119595 |
| 26 | Nørskov J. K., Rossmeisl J., Logadottir A., Lindqvist L., Kitchin J. R., Bligaard T., Jónsson H., J. Phys. Chem. B, 2004, 108(46), 17886—17892 |
| 27 | Strasser P., Koh S., Anniyev T., Greeley J., More K., Yu C., Liu Z., Kaya S., Nordlund D., Ogasawara H., Toney M. F., Nilsson A., Nat. Chem., 2010, 2(6), 454—460 |
| 28 | Greeley J., Stephens I. E. L., Bondarenko A. S., Johansson T. P., Hansen H. A., Jaramillo T. F., Rossmeisl J., Chorkendorff I., Nørskov J. K., Nat. Chem., 2009, 1(7), 552—556 |
| 29 | Cao L., Zhao Z., Liu Z., Gao W., Dai S., Gha J., Xue W., Sun H., Duan X., Pan X., Mueller T., Huang Y., Matter, 2019, 1(6), 1567—1580 |
| 30 | Li X., Huang Y., Chen Z., Hu S., Zhu J., Tsiakaras P., Kang S. P., Chem. Eng. J., 2023, 454, 140131 |
| 31 | Mo Y., Feng S., Yu T., Chen J., Qian G., Luo L., Yin S., J. Colloid Interface Sci., 2022, 607, 1928—1935 |
| 32 | Feng Q., Wang X., Klingenhof M., Heggen M., Strasser P., Angew. Chem. Int. Ed., 2022, 61(36), e202203728 |
| 33 | Polani S., MacArthur K. E., Klingenhof M., Wang X., Paciok P., Pan L., Feng Q., Kormányos A., Cherevko S., Heggen M., Strasser P., ACS Catal., 2021, 11(18), 11407—11415 |
| 34 | Xia Y., Yang X., Acc. Chem. Res., 2017, 50(3), 450—454 |
| 35 | Chen J., Li H., Fan C., Meng Q., Tang Y., Qiu X., Fu G., Ma T., Adv. Mater., 2020, 32(30), 2003134 |
| 36 | Zhu W., Chen Z., Pan Y., Dai R., Wu Y., Zhuang Z., Wang D., Peng Q., Chen C., Li Y., Adv. Mater., 2019, 31(38), 1800426 |
| 37 | Wang J., Cui Y., Wang D., Adv. Mater., 2019, 31(38), 1801993 |
| 38 | Zhang W., Yang J., Lu X., ACS Nano, 2012, 6(8), 7397—7405 |
| 39 | Zhao M., Lyu Z., Xie M., Hood Z. D., Cao Z., Chi M., Xia Y., Small Methods, 2020, 4(5), 1900843 |
| 40 | Wang Y., Chen S., Wang X., Rosen A., Beatrez W., Sztaberek L., Tan H., Zhang L., Koenigsmann C., Zhao J., ACS Appl. Energ. Mater., 2020, 3(1), 768—776 |
| 41 | Au L., Chen Y., Zhou F., Camargo P. H. C., Lim B., Li Z. Y., Ginger D. S., Xia Y., Nano Res., 2008, 1(6), 441—449 |
| 42 | Wang J. X., Ma C., Choi Y., Su D., Zhu Y., Liu P., Si R., Vukmirovic M. B., Zhang Y., Adzic R. R., J. Am. Chem. Soc., 2011, 133(34), 13551—13557 |
| 43 | Han L., Liu H., Cui P., Peng Z., Zhang S., Yang J., Scientific Reports, 2014, 4(1), 6414 |
| 44 | González E., Arbiol J., Puntes V. F., Science, 2011, 334(6061), 1377—1380 |
| 45 | Wang X., Feng J. I., Bai Y., Zhang Q., Yin Y., Chem. Rev., 2016, 116(18), 10983—11060 |
| 46 | Wang Q., Mi B., Zhou J., Qin Z., Chen Z., Wang H., Molecules, 2022, 27(8), 2524 |
| 47 | He D. S., He D., Wang J., Lin Y., Yin P., Hong X., Wu Y., Li Y., J. Am. Chem. Soc., 2016, 138(5), 1494—1497 |
| 48 | Choi S., Oh M., Angew. Chem. Int. Ed., 2019, 58(3), 866—871 |
| 49 | Ataee⁃Esfahani H., Nemoto Y., Wang L., Yamauchi Y., Chem. Commun., 2011, 47(13), 3885—3887 |
| 50 | Ataee⁃Esfahani H., Liu J., Hu M., Miyamoto N., Tominaka S., Wu K. C. W., Yamauchi Y., Small, 2013, 9(7), 1047—1051 |
| 51 | Yin S., Xu Y., Liu S., Yu H., Wang Z., Li X., Wang L., Wang H., Nanoscale, 2020, 12(27), 14863—14869 |
| 52 | Yin S., Kumar R. D., Yu H., Li C., Wang Z., Xu Y., Li X., Wang L., Wang H., ACS Sustain. Chem. Eng., 2019, 7(17), 14867—14873 |
| 53 | Wang H., Qian X., Liu S., Yin S., Yu H., Xu Y., Li X., Wang Z., Wang L., Chem⁃Asian J., 2019, 14(17), 3019—3024 |
| 54 | Wang H., Lin G., Li X., Lu W., Peng Z., J. Colloid Interface Sci., 2019, 554, 396—403 |
| 55 | Fu Q. Q. Li H. H., Ma S. Y., Hu B. C., Yu S. H., Sci. China Mater., 2016, 59(2), 112—121 |
| 56 | Ding H., Wang P., Su C., Liu H., Tai X., Zhang N., Lv H., Lin Y., Chu W., Wu X., Wu C., Xie Y., Adv. Mater., 2022, 34(12), 2109188 |
| 57 | Wan X. K., Wu H. B., Guan B. Y., Luan D., Lou X. W., Adv. Mater., 2020, 32(7), 1901349 |
| 58 | Yan X., Hu X., Fu G., Xu L., Lee J. M., Tang Y., Small, 2018, 14(13), 1703940 |
| 59 | Bai Z., Xu P., Chao S., Yan H., Cui Q., Niu L., Yang L., Qiao J., Catal. Sci. Technol., 2013, 3(10), 2843—2848 |
| 60 | An L., Zhu M., Dai B., Yu F., Electrochimica Acta, 2015, 176, 222—229 |
| 61 | Liu Y., Goebl J., Yin Y., Chem. Soc. Rev., 2013, 42(7), 2610—2653 |
| 62 | Lv H., Sun L., Lopes A., Xu D., Liu B., J. Phys. Chem. Lett., 2019, 10(18), 5490—5498 |
| 63 | Lv H., Lopes A., Xu D., Liu B., ACS Central Sci., 2018, 4(10), 1412—1419 |
| 64 | Yin S., Wang Z., Qian X., Yang D., Xu Y., Li X., Wang L., Wang H., ACS Sustain. Chem. Eng., 2019, 7(8), 7960—7968 |
| 65 | Wang Q., Zhao D., Yu J., Shi L., Wang Y., Chen H., Nano Res., 2022,1—7 |
| 66 | Wang T. J., Sun H. Y., Xue Q., Zhong M. J., Li F. M., Tian X., Chen P., Yin S. B., Chen Y., Sci. Bull., 2021, 66(20), 2079— 2089 |
| 67 | Lu L., Wang B., Wu D., Zou S., Fang B., Nanoscale, 2021, 13(6), 3709—3722 |
| 68 | Wang A. J., Liu L., Lin X. X., Yuan J., Feng J. J., Electrochimica Acta, 2017, 245, 883—892 |
| 69 | Wang X., Sun M., Xiang S., Waqas M., Fan Y., Zhong J., Huang K., Chen W., Liu L., Yang J., Electrochimica Acta, 2020, 337, 135742 |
| 70 | Zhou S., Liao W., Wang Z., Chen M., Long J., Zhou Q., Wang Q., ACS Appl. Energ. Mater., 2022, 5(5), 6472—6480 |
| 71 | Wei M., Huang L., Huang S., Chen Z., Lyu D., Zhang X., Wang S., Tian Z. Q., Shen P. K., J. Catal., 2020, 381, 385—394 |
| 72 | Fu S., Zhu C., Song J., Engelhard M. H., He Y., Du D., Wang C., Lin Y., J. Mater. Chem. A, 2016, 4(22), 8755—8761 |
| 73 | Sun Y., Zhang X., Luo M., Chen X., Wang L., Li Y., Li M., Qin Y., Li C., Xu N., Lu G., Gao P., Guo S., Adv. Mater., 2018, 30(38), 1802136 |
| 74 | Li M., Yang A., Wang S., Wang Y., Huang Q., Cai B., Qiu X., Tang Y., J. Mater. Chem. A, 2021, 9(19), 11537—11544 |
| 75 | Tao L., Yu D., Zhou J., Lu X., Yang Y., Gao F., Small, 2018, 14(22), 1704503 |
| 76 | Zhou K., Li Y., Angew. Chem. Int. Ed., 2012, 51(3), 602—613 |
| 77 | Sun H., Xu X., Yan Z., Chen X., Cheng F., Weiss P. S., Chen J., Chem. Mater., 2017, 29(19), 8539—8547 |
| 78 | Wu J., Shan S., Cronk H., Chang F., Kareem H., Zhao Y., Luo J., Petkov V., Zhong C. J., J. Phys. Chem. C, 2017, 121(26), 14128—14136 |
| 79 | Balkan T., Küçükkeçeci H., Zarenezhad H., Kaya S., Metin Ö., J. Alloy. Compd., 2020, 831, 154787 |
| 80 | Deng K., Xu Y., Yang D., Qian X., Dai Z., Wang Z., Li X., Wang L., Wang H., J. Mater. Chem. A, 2019, 7(16), 9791—9797 |
| 81 | Li M., Tian F., Lin T., Tao L., Guo X., Chao Y., Guo Z., Zhang Q., Gu L., Yang W., Yu Y., Guo S., Small Methods, 2021, 5(6), 2100154 |
| 82 | Tao L., Xia Z., Zhang Q., Sun Y., Li M., Yin K., Gu L., Guo S., Sci. Bull., 2021, 66(1), 44—51 |
| 83 | Tian X., Zhao X., Su Y. Q., Wang L., Wang H., Dang D., Chi B., Liu H., Hensen E. J. M., Lou X. W., Xia B. Y., Science, 2019, 366(6467), 850—856 |
| 84 | Jiao W., Chen C., You W., Zhao X., Zhang J., Feng Y., Wang P., Che R., Adv. Energy Mater., 2020, 10(18), 1904072 |
| 85 | Jiao W., Chen C., You W., Chen G., Xue S., Zhang J., Liu J., Feng Y., Wang P., Wang Y.,Wen H., Che R., Appl. Catal. B: Environ., 2020, 262, 118298 |
| 86 | Qin Y., Zhang W., Guo K., Liu X., Liu J., Liang X., Wang X., Gao D., Gan L., Zhu Y., Zhang Z., Hu W., Adv. Funct. Mater., 2020, 30(11), 1910107 |
| 87 | Ma Z., Cano Z. P., Yu A., Chen Z., Jiang G., Fu X., Yang L., Wu T., Bai Z., Lu J., Angew. Chem. Int. Ed., 2020, 59(42), 18334—18348 |
| 88 | Wang Y., Wang D., Li Y., SmartMat, 2021, 2(1), 56—75 |
| 89 | Guo S., Dong S., Wang E., Chem⁃Eur. J., 2008, 14(15), 4689—4695 |
| 90 | Zhu Z., Zhai Y., Dong S., ACS Appl. Mater. Interfaces, 2014, 6(19), 16721—16726 |
| 91 | Ou L., Comput. Theor. Chem., 2014, 10(48), 69—76 |
| 92 | Bing Y., Liu H., Zhang L., Ghosh D., Zhang J., Chem. Soc. Rev., 2010, 39(6), 2184—2202 |
| 93 | Li J., Yin H. M., Li X. B., Okunishi E., Shen Y. L., He J., Tang Z. K., Wang W. X., Yücelen E., Li C., Gong Y., Gu L., Miao S., Liu L. M., Luo J., Ding Y., Nat. Energy, 2017, 2(8), 17111 |
| 94 | Suo Y., Zhuang L., Lu J., Angew. Chem., 2007, 119(16), 2920—2922 |
| 95 | Zamora Zeledón J. A., Stevens M. B., Gunasooriya G. T. K. K., Gallo A., Landers A. T., Kreider M. E., Hahn C., Nørskov J. K., Jaramillo T. F., Nat. Commun., 2021, 12(1), 620 |
| 96 | Li H. H., Cui C. H., Zhao S., Yao H. B., Gao M. R., Fan F. J., Yu S. H., Adv. Energy Mater., 2012, 2(10), 1182—1187 |
| 97 | Kang Y. S., Jung J. Y., Choi D., Sohn Y., Lee S. H., Lee K. S., Kim N. D., Kim P., Yoo S. J., ACS Appl. Mater. Interfaces, 2020, 12(14), 16286—16297 |
| 98 | Lin R., Cai X., Zeng H., Yu Z., Adv. Mater., 2018, 30(17), 1705332 |
| 99 | Rao P., Luo J., Li J., Huang W., Sun W., Chen Q., Jia C. M., Liu Z. X., Deng P. L., Shen Y. J., Tian X. L., Carbon Energy, 2022, 4(6), 1003—1010 |
| 100 | Niu H., Xia C., Huang L., Zaman S., Maiyalagan T., Guo W., You B., Xia B. Y., Chinese J. Catal., 2022, 43(6), 1459—1472 |
| 101 | Kwon T., Jun M., Kim H. Y., Oh A., Park J., Baik H., Joo S. H., Lee K., Adv. Funct. Mater., 2018, 28(13), 1706440 |
| 102 | Dhavale V. M., Kurungot S., ACS Catal., 2015, 5(3), 1445—1452 |
| 103 | Chen H. S., Benedetti T. M., Gonçales V. R., Bedford N. M., Scott R. W. J., Webster R. F., Cheong S., Gooding J. J., Tilley R. D., J. Am. Chem. Soc., 2020, 142(6), 3231—3239 |
| 104 | Zhou M., Li C., Fang J., Chem. Rev., 2021, 121(2), 736—795 |
| 105 | Kim H. Y., Kwon T., Ha Y., Jun M., Baik H., Jeong H. Y., Kim H., Lee K., Joo S. H., Nano Lett., 2020, 20(10), 7413—7421 |
| 106 | Xia Z., Guo S., Chem. Soc. Rev., 2019, 48(12), 3265—3278 |
| 107 | Eid K., Wang H., Malgras V., Alothman Z. A., Yamauchi Y., Wang L., J. Phys. Chem. C, 2015, 119(34), 19947—19953 |
| 108 | Ren Y., Li C., Li B., Gao F., Zhang X., Yang X., Li L., Lu Z., Yu X., Inorg. Chem. Front., 2021, 8(9), 2280—2287 |
| [1] | WANG Jun, DU Shiqian, TAO Li. Recent Progress of Catalysts in the High Temperature Polymer Electrolyte Membrane Fuel Cells [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220722. |
| [2] | ZHANG Xiaoyu, QU Gan, XUE Dongping, YAN Wenfu, ZHANG Jianan. Recent Process of Carbon-based Catalysts for the Production of H2O2 by Electrocatalytic Oxygen Reduction: Strategies, Calculation and Practical Applications [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220775. |
| [3] | LI Xuan, QI Shuai, ZHOU Weiliang, LI Xiaojie, JING Lingyan, FENG Chao, JIANG Xingxing, YANG Hengpan, HU Qi, HE Chuanxin. Advances in Nanofiber-based Electrocatalysts for Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2023, 44(5): 316. |
| [4] | BAO Chunzhu, XIANG Zhonghua. Pyrolysis-free Strategy of Covalent Organic Polymers-based Oxygen Reduction Electrocatalytic Materials [J]. Chem. J. Chinese Universities, 2023, 44(5): 20220715. |
| [5] | WANG Sijia, HOU Lu, LI Chenglong, LI Wencui, LU Anhui. Recent Advances in Synthesis and Applications of Hollow Nano-carbons [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220637. |
| [6] | YANG Qingfeng, LYU Liang, LAI Xiaoyong. Progress on Preparation and Electrocatalytic Application of Hollow MOFs [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220666. |
| [7] | SHEN Xinyi, ZHANG Sen, WANG Shutao, SONG Yongyang. Synthesis Strategies for Polymer Hollow Particles [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220627. |
| [8] | ZHU Kerun, REN Wenxuan, ZHANG Wei, LI Wei. Salt-templated Synthesis and Morphological Control of Monodisperse Hollow Mesoporous Structures [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220607. |
| [9] | WANG Hui, ZHAO Decai, YANG Nailiang, WANG Dan. Gate Keeper in the Smart Hollow Drug Carrier [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220237. |
| [10] | LI Ziruo, ZHANG Hongjuan, ZHU Guoxun, XIA Wei, TANG Jing. Iron Phthalocyanine Coated Nitrogen-doped Hollow Carbon Spheres for Efficient Catalysis of Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220677. |
| [11] | WU Yucai, DU Huan, ZHU Jiexin, XU Nuo, ZHOU Liang, MAI Liqiang. Intricate Hollow Structured Materials: Synthesis and Energy Applications [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220689. |
| [12] | LIU Shuanghong, XIA Siyu, LIU Shiqi, LI Min, SUN Jiajie, ZHONG Yong, ZHANG Feng, BAI Feng. Current Advances of Hollow All-solid-state Z-Scheme Photocatalysts [J]. Chem. J. Chinese Universities, 2023, 44(1): 20220512. |
| [13] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [14] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [15] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||