

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (11): 2308.doi: 10.7503/cjcu20190330
• Organic Chemistry • Previous Articles Next Articles
CAI Yanchao1,2,NIU Pengfei1,2,SHEN Zhenlu1,LI Meichao1,2,*( )
)
Received:2019-06-11
Online:2019-11-10
Published:2019-08-20
Contact:
LI Meichao
E-mail:limc@zjut.edu.cn
Supported by:CLC Number:
TrendMD:
CAI Yanchao,NIU Pengfei,SHEN Zhenlu,LI Meichao. Electrocatalytic Oxidative Coupling of Primary Amines with the Medium ABNO †[J]. Chem. J. Chinese Universities, 2019, 40(11): 2308.
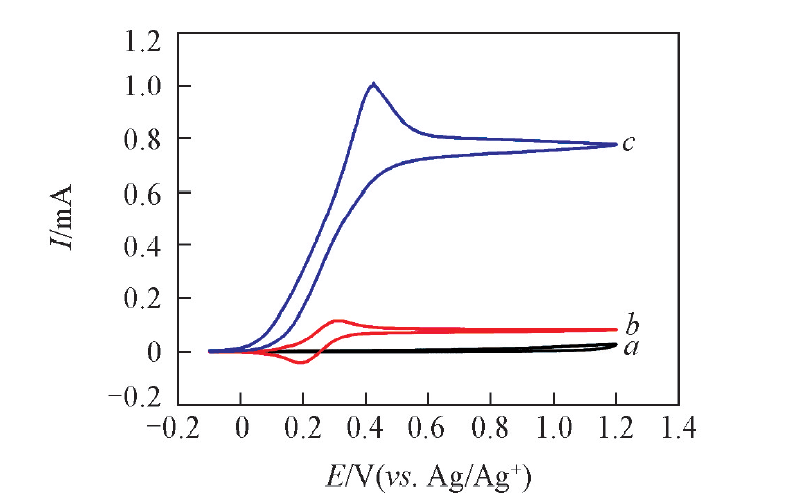
Fig.1 Cyclic voltammograms of Pt electrode in 0.1 mol/L NaClO4-MeCN solution with 1.0 mmol benzylamine(a), 0.1 mmol ABNO(b) and 0.1 mmol ABNO+1.0 mmol benzylamine(c) at the scan rate of 50 mV/s
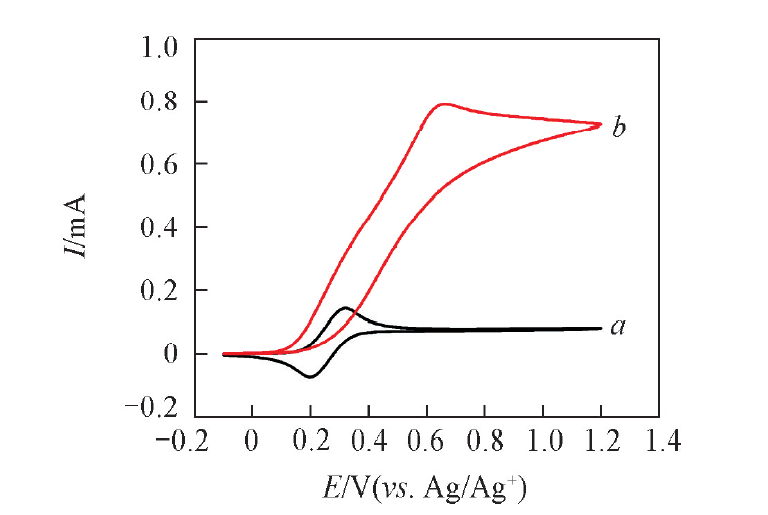
Fig.2 Cyclic voltammograms of Pt electrode in 0.1 mol/L NaClO4-MeCN solution with 0.1 mmol TEMPO(a) and 0.1 mmol TEMPO+1.0 mmol benzylamine(b) at the scan rate of 50 mV/s
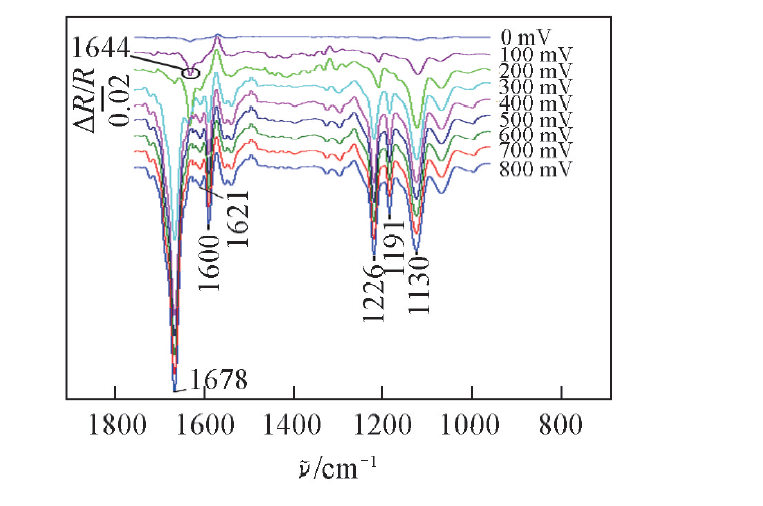
Fig.3 In situ FTIR spectra collected on Pt electrode in 0.1 mol/L NaClO4-MeCN solution with 0.1 mmol ABNO and 1.0 mmol benzylamine at different potential
| Entry | Catalyst | E/mV | Yield(%)b | Entry | Catalyst | E/mV | Yield(%)b |
|---|---|---|---|---|---|---|---|
| 1 | ABNO | 600 | 76 | 6 | ABNOc | 800 | 60 |
| 2 | ABNO | 700 | 89 | 7 | — | 800 | Trace |
| 3 | ABNO | 800 | 98 | 8 | — | 1500 | 4 |
| 4 | ABNO | 900 | 94 | 9 | TEMPO | 800 | 80 |
| 5 | ABNO | 1000 | 94 |
| Entry | Catalyst | E/mV | Yield(%)b | Entry | Catalyst | E/mV | Yield(%)b |
|---|---|---|---|---|---|---|---|
| 1 | ABNO | 600 | 76 | 6 | ABNOc | 800 | 60 |
| 2 | ABNO | 700 | 89 | 7 | — | 800 | Trace |
| 3 | ABNO | 800 | 98 | 8 | — | 1500 | 4 |
| 4 | ABNO | 900 | 94 | 9 | TEMPO | 800 | 80 |
| 5 | ABNO | 1000 | 94 |
| [1] | Galletti P., Martelli G., Prandini G., Colucci C., Giacomini D ., RSC Advances, 2018,8(18), 9723— 9730 |
| [2] | Liu D., Zhang C. H., Han N., Du M. M., Zhang X. L., Zhao P. S., Yang P., Chin. J. Org. Chem., 2018,38(6), 1350— 1363 |
| ( 刘迪, 张成慧, 韩楠, 杜萌萌, 张效露, 赵朋杉, 杨萍 . 有机化学, 2018,38(6), 1350— 1363) | |
| [3] |
Xu Q., Li Q ., Chin. J. Org.Chem. , 2013, 33(1), 18— 35
doi: 10.6023/cjoc201208016 |
|
( 徐清, 李强 . 有机化学, 2013,33(1), 18— 35)
doi: 10.6023/cjoc201208016 |
|
| [4] | Young J. N., Chang T. C., Tsai S. C., Yang L., Yu S. J., J. Catal., 2010,272(2), 253— 261 |
| [5] | Jiang G. X., Chen J., Huang J. S., Che C. M ., Org. Lett., 2009,11(20), 4568— 4571 |
| [6] | Hu Z.Z., Kerton F. M ., Org. Biomol. Chem., 2012,10(8), 1618— 1624 |
| [7] | Li X., Xu H., Shi J. L., Hao H. M., Yuan H., Lang X. J., Appl. Catal. B-Environ., 2019,244, 758— 766 |
| [8] | Largeron M., Fleury M. B ., Angew. Chem. Int. Edit., 2012,51(22), 5409— 5412 |
| [9] | Bartelson A. L., Lambert K. M., Bobbitt J. M., Bailey W. F., ChemCatChem, 2016,8(22), 3421— 3430 |
| [10] | Ciriminna R., Pagliaro M., . Org. Process Res .Dev., 2010,14(1), 245— 251 |
| [11] | Vogler T., Studer A ., Synthesis, 2008,13, 1979— 1993 |
| [12] | Kelly C. B., Lambert K. M., Mercadante M. A ., Angew. Chem. Int. Ed., 2015,54(14), 4241— 4245 |
| [13] | Fan Z. Q., Chen C., Shen Z. L., Li M. C., Chem. J. Chinese Universities, 2018,39(1), 78— 84 |
| ( 范仲全, 陈晨, 沈振陆, 李美超 . 高等学校化学学报, 2018,39(1), 78— 84) | |
| [14] | Niu P. F., Liu X., Shen Z. L., Li M. C., Molecules, 2019,24(1), 1— 14 |
| [15] | Shibuya M., Tomizawa M., Sasano Y., Iwabuchi Y ., J. Org. Chem., 2009,74(12), 4619— 4622 |
| [16] | Lauber M. B., Stahl S. S ., ACS Catal., 2013,3(11), 2612— 2616 |
| [17] | Zultanski S. L., Zhao J. Y., Stahl S. S., J. Am. Chem. Soc., 2016,138(20), 6416— 6419 |
| [18] | Nicholson R. S., Shain L ., Anal. Chem., 1964,36, 706— 723 |
| [19] | Christensen P. A., Mashhadani Z. T. A. W., Ali A. B ., Phys. Chem. Chem. Phys., 2018,20, 9053— 9062 |
| [20] | Egawa T., Ito M., Konaka S ., J. Mol. Struct., 2001,560(1—3), 337— 344 |
| [21] | Berkesi O., Kortvelyesi T., Hetenyi C., Nemeth T., Palinko I., Phys. Chem. Chem. Phys., 2003,5(10), 2009— 2014 |
| [22] | Egawa T., Konaka S ., J. Phys. Chem. A, 2001,105(10), 2085— 2090 |
| [23] | Timmiati S. N., Jalil A. A., Triwahyono S., Setiabudi H. D., Annuar N. H. R., Appl. Catal. A Gen., 2013,459, 8— 16 |
| [24] | Duncan W., Soine W. H., J. Chromatogr. Sci., 1988,26(10), 521— 526 |
| [25] | Olgun U., Gulfen M ., Dyes Pigments, 2013,99(3), 1004— 1009 |
| [26] | Richards S., Ropic M., Blackmond D., Walmsley A., Anal. Chim. Acta, 2004,519(1), 1— 9 |
| [27] | Gerken J. B., Pang Y. T. Q., Lauber M. B., Stahl S. S., J. Org. Chem., 2018,83(14), 7323— 7330 |
| [28] | Erkabaev A. M., Yaroslavtseva T. V., Popov S. E., Bushkova O. V ., Vib. Spectrosc., 2014,79, 19— 25 |
| [29] | Lennox A. J. J., Goes S. L., Webster M. P., Koolman H. F., Djuric S. W., Stahl S. S., J. Am. Chem. Soc., 2018,140(36), 11227— 11231 |
| [1] | ZHANG Jiayi,JIA Mengyang,JIANG Xiaohui,ZHANG Zhiming,YU Liangmin,WANG Xuan. Antifouling Properties of Dodecyl Benzene Sulfonic Acid Doped Polypyrrole Under Alternating Anodic-cathodic Polarization † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2396. |
| [2] | ZHAO Bangtun, TAO Jingjing, CHEN Xiaoji, FU Huimin, ZHU Weimin. Synthesis, Structure and Electrochemistry of Tetrathiafulvalene Vinylogues Bearing Thienyl and Pyridyl Groups† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1449. |
| [3] | SUN Jie, MING Tingyun, QIAN Huixuan, ZHANG Manke, TAN Yong. Electrochemical Behavior of Copper Electrodeposition in BMIMPF6 Ionic Liquid [J]. Chem. J. Chinese Universities, 2018, 39(7): 1497. |
| [4] | ZHAO Changjiang,LIU Xin,TIAN Li,ZHAO Lun. Synthesis, Structure and Electrochemical Properties of Metal Cobalt Complexes with Interpenetrating Structures† [J]. Chem. J. Chinese Universities, 2018, 39(5): 861. |
| [5] | BAI Yan, XIA Wensheng, WENG Weizheng, LIAN Mengshui, ZHAO Mingquan, WAN Huilin. Influence of Phosphate on La-based Catalysts for Oxidative Coupling of Methane† [J]. Chem. J. Chinese Universities, 2018, 39(2): 247. |
| [6] | JIN Yanxian, JIA Wenping, LIANG Danxia, LI Fang, LI Rongrong, ZHENG Mengmeng, GAO Weiyi, NI Jiamin, HU Jiajie, WU Tinghua. Performance of WO3 Modified Graphene Supported Pd Nanocatalysts for Formic Acid Electro-oxidation† [J]. Chem. J. Chinese Universities, 2017, 38(4): 653. |
| [7] | ZHAO Bangtun, MA Shuxiu, TAO Jingjing, ZHU Weimin. Synthesis, Structures and Electrochemical Properties of Pyridine-based Tetrathiafulvalene Derivatives† [J]. Chem. J. Chinese Universities, 2017, 38(2): 193. |
| [8] | GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo. Influence of Solvent on Structure of Ni(Ⅱ) Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1589. |
| [9] | YANG Yanling, DONG Lingyu, XIA Wensheng, WAN Huilin. Ca, Sr co-Doped Ceria and Its Application in Oxidative Coupling of Methane† [J]. Chem. J. Chinese Universities, 2016, 37(12): 2206. |
| [10] | ZHANG Xuena, ZHONG Xinwen, ZHONG Yan, LU Haiyan. Optimization of Conditions of the Electrochemical Detection of Methamphetamine† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1799. |
| [11] | LÜ Jiangwei, QU Youpeng, FENG Yujie, LIU Junfeng. Electrochemical Impedance Spectroscopy of Dichlorophenols at Boron-doped Diamond Electrodes† [J]. Chem. J. Chinese Universities, 2016, 37(1): 142. |
| [12] | WU Ranran, TIAN Xiaochun, WU Shenjian, LIU Yuangang, JIANG Yanxia, ZHAO Feng. Research on the Electrochemical Activity of Magnetospirillum Magneticum AMB-1† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1730. |
| [13] | JING Weixuan, ZHOU Fan, CHEN Lujia, QI Han, JIANG Zhuangde, WANG Bing, NIU Lingling. Glucose Sensor of Spirally Hierarchical Structure with ZnO Nanowires Synthesized on a Spiralled Au Fiber† [J]. Chem. J. Chinese Universities, 2014, 35(3): 493. |
| [14] | LIANG Fangyuan, WU Ranran, CAO Changli, ZHENG Yue, YANG Zhaohui, ZHAO Feng. Research on Extracellular Electron Transfer of Acidithiobacillus Ferrooxidans† [J]. Chem. J. Chinese Universities, 2014, 35(2): 372. |
| [15] |
HASIMU Yushanjiang, LIU Ruiquan, MI Hongyu.
Electrodeposition Behavior of Chromium in Ionic Liquid [BMIM]P |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||