

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (5): 861.doi: 10.7503/cjcu20170650
Previous Articles Next Articles
ZHAO Changjiang, LIU Xin, TIAN Li, ZHAO Lun*
Received:2017-09-26
Online:2018-03-29
Published:2018-03-29
Contact:
ZHAO Lun
Supported by:TrendMD:
ZHAO Changjiang,LIU Xin,TIAN Li,ZHAO Lun. Synthesis, Structure and Electrochemical Properties of Metal Cobalt Complexes with Interpenetrating Structures†[J]. Chem. J. Chinese Universities, 2018, 39(5): 861.
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1575252 | 1575253 | 1575254 |
| Molecular formula | C32H25CoN5O5 | C51H47Co2N7O13 | C70H60Co2N12O10 |
| Fw | 618.51 | 1083.82 | 1347.16 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P2/n | P21 |
| a/nm | 2.7443(10) | 1.58338(11) | 1.1130(3) |
| b/nm | 1.0762(4) | 0.86972(6) | 2.1039(5) |
| c/nm | 2.2217(9) | 1.75432(12) | 2.6929(7) |
| α/(°) | 90.00 | 90.00 | 90.00 |
| β/(°) | 90.12 | 94.5160(10) | 90.00 |
| γ/(°) | 90.00 | 90.00 | 90.12 |
| V/nm3 | 6.562(4) | 2.4084(3) | 6.306(3) |
| Z | 8 | 2 | 4 |
| Dc/(g·cm-3) | 1.252 | 1.495 | 1.419 |
| F(000) | 2552 | 1120 | 2792 |
| GOF on F2 | 0.916 | 1.058 | 0.851 |
| R1, wR2[I>2σ(I)] | 0.0815, 0.2116 | 0.0565, 0.1562 | 0.0744, 0.1334 |
| R1, wR2(all data) | 0.1462, 0.2308 | 0.1060, 0.1971 | 0.2324, 0.1822 |
Table 1 Crystal data and structure refinement of complexes 1—3
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1575252 | 1575253 | 1575254 |
| Molecular formula | C32H25CoN5O5 | C51H47Co2N7O13 | C70H60Co2N12O10 |
| Fw | 618.51 | 1083.82 | 1347.16 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | P2/n | P21 |
| a/nm | 2.7443(10) | 1.58338(11) | 1.1130(3) |
| b/nm | 1.0762(4) | 0.86972(6) | 2.1039(5) |
| c/nm | 2.2217(9) | 1.75432(12) | 2.6929(7) |
| α/(°) | 90.00 | 90.00 | 90.00 |
| β/(°) | 90.12 | 94.5160(10) | 90.00 |
| γ/(°) | 90.00 | 90.00 | 90.12 |
| V/nm3 | 6.562(4) | 2.4084(3) | 6.306(3) |
| Z | 8 | 2 | 4 |
| Dc/(g·cm-3) | 1.252 | 1.495 | 1.419 |
| F(000) | 2552 | 1120 | 2792 |
| GOF on F2 | 0.916 | 1.058 | 0.851 |
| R1, wR2[I>2σ(I)] | 0.0815, 0.2116 | 0.0565, 0.1562 | 0.0744, 0.1334 |
| R1, wR2(all data) | 0.1462, 0.2308 | 0.1060, 0.1971 | 0.2324, 0.1822 |
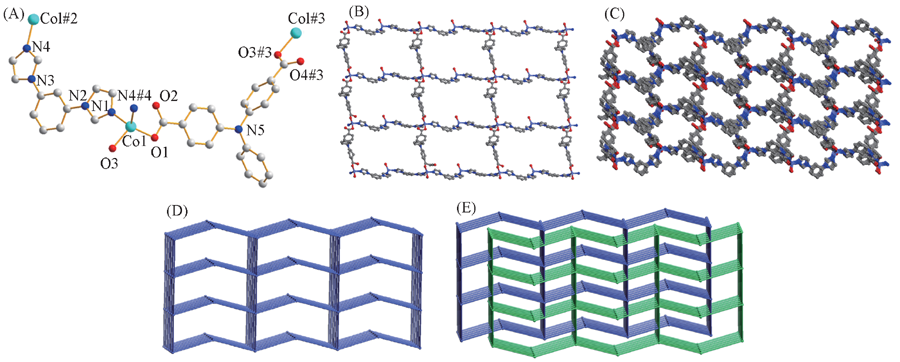
Fig.2 Coordination environment of the Co(Ⅱ) ions(A) and views of 2D sheets(B), 3D framework(C), topology network(D) and the two-fold interpenetrating framework(E) of complex 1 The hydrogen atoms are omitted for clarity. Symmetry codes: #1. x-1/2, y+1/2, z; #2. x, -y-1, z+1/2; #3. x+1/2, y-1/2, z; #4. x, -y-1, z-1/2.
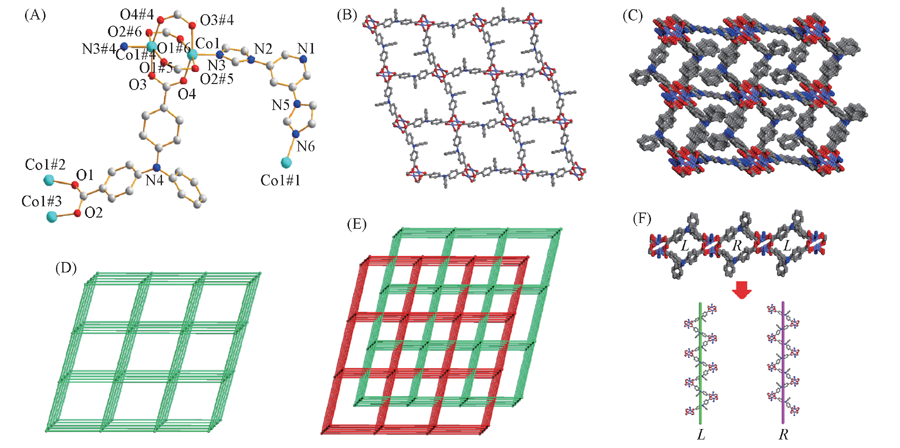
Fig.3 Coordination environment of the Co(Ⅱ) ions(A) and views of 2D sheets(B), 3D framework(C), topology of 3D framework(D), two-fold interpenetrating framework(E) and the helical chains(F) of complex 2 The hydrogen atoms are omitted for clarity. Symmetry codes: #1. -x+3/2, y, -z+3/2; #2. -x+3/2, y+1, -z+1/2; #3. x-1/2, -y+2, z-1/2; #4. -x+2, -y+1, -z+1; #5. x+1/2, -y+2, z+1/2; #6. -x+3/2, y-1, -z+1/2; #7. -x+3/2, y, -z+1/2.
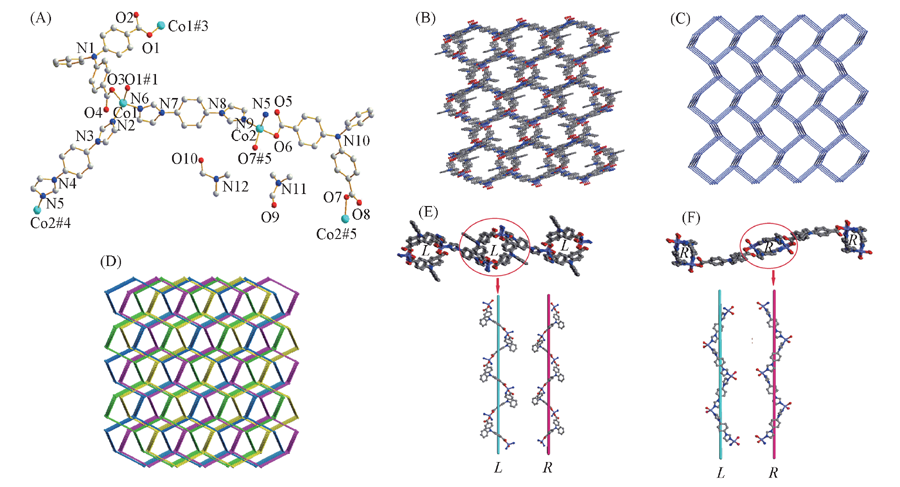
Fig.4 Coordination environment of Co(Ⅱ) ions(A) and views of 3D framework(B), topology network of 3D framework(C), four-fold interpenetrating framework(D) and the two different helical chains(E, F) of complex 3 The hydrogen atoms are omitted for clarity. Symmetry codes: #1. -x+5/2, y+1/2, -z+3/2; #2. x-2, y, z; #3. -x+5/2, y-1/2, -z+3/2; #4. x+2, y, z; #5. -x+1/2, y-1/2, -z+1/2; #6. -x+1/2, y+1/2, -z+1/2.
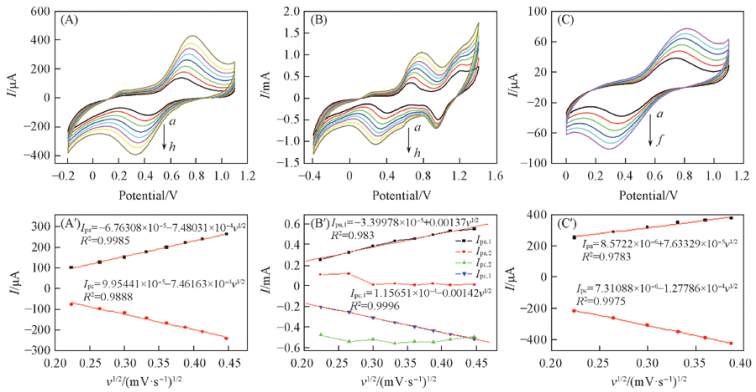
Fig.8 Cyclic voltammograms at different sweep speeds(A) and I-v1/2 plots(B) of complex 1(A, A'), complex 2(B, B') and complex 3(C, C') (A)—(C) Scan rate/(mV·s-1): a. 50; b. 70; c. 90; d. 110; e. 130; f. 150; g. 170; h. 200.
| [1] | Furukawa H., Cordova K.E., O’Keeffe M., Yaghi O. M., Science, 2013, 341(6149), 1230444 |
| [2] | Huang C.T., Wu X. F., Li G. H., Gao L., Feng S. H., Chem. [J]. Chinese Universities, 2015, 36(9), 1661—1666 |
| (黄楚婷, 吴小峰, 李光华, 高路, 冯守华. 高等学校化学学报, 2015, 36(9), 1661—1666) | |
| [3] | Shustova N.B., McCarthy B. D., Dinca M., [J]. Am. Chem. Soc., 2011, 133, 20126—20129 |
| [4] | Zhao C.J., Zhao L., Zhang M., Inorg. Chim. Acta, 2018, 468, 136—143 |
| [5] | Cao Y., Zhu Z., Xu J., Wang L., Sun J., Chen X., Fan Y., Dalton Trans., 2015, 44, 1942—1947 |
| [6] | Zhao L., Guo H.D., Tang D., Zhang M., CrystEngComm, 2015, 17, 5451—5467 |
| [7] | Hoskins B.F., Robson R. J., [J]. Am. Chem. Soc., 1989, 111(15), 5962—5964 |
| [8] | Wang X.Y., Wang L., Wang Z. M., Gao S., [J]. Am. Chem. Soc., 2006, 128(3), 674—675 |
| [9] | Guo D., Pang K.L., Duan C. Y., He C., Meng Q. [J]., Inorg. Chem., 2002, 41(23), 5978—5985 |
| [10] | Li J.R., Ma Y., McCarthy M. C., Sculley J., Yu J., Jeong H. K., Balbuena P. B., Zhou H. C., Coord. Chem. Rev., 2011, 255, 1791—1823 |
| [11] | He Y., Furukawa H., Wu C., O’Keeffe M., Krishna R., Chen B., Chem. Commun., 2013, 49, 6773—6775 |
| [12] | Li D.S., Zhao J., Wu Y. P., Liu B., Bai L., Zou K., Du M., Inorg. Chem., 2013, 52, 8091—8098 |
| [13] | Lee J., Farha O.K., Roberts J., Scheidt K. A., Nguyen S. T., Hupp J. T., Chem. Soc. Rev., 2009, 38, 1450—1459 |
| [14] | Liu Y., Xuan W.M., Cui Y., Adv. Mater., 2010, 22(37), 4112—4135 |
| [15] | Wei N., Zhang M.Y., Zhang X. N., Li G. M., Zhang X. D., Han Z. B., Cryst. Growth Des., 2014, 14, 3002—3009 |
| [16] | Liu Y., Mo K., Cui Y., Inorg. Chem., 2013, 52, 10286—10291 |
| [17] | Heine J., Muller-Buschbaum K., Chem. Soc. Rev., 2013, 42, 9232—9242 |
| [18] | Jiang H.L., Feng D., Wang K., Gu Z. Y., Wei Z., Chen Y. P., Zhou H. C., [J]. Am. Chem. Soc., 2013, 135, 13934—13938 |
| [19] | Zhu M., Hao Z.M., Song X. Z., Meng X., Zhao S. N., Song S. Y., Zhang H. [J]., Chem. Commun., 2014, 50, 1912—1914 |
| [20] | Kreno L.E., Leong K., Farha O. K., Allendorf M., van Duyne R. P., Hupp J. T., Chem. Soc. Rev., 2012, 112(2), 1105—1125 |
| [21] | Yamada T., Otsubo K., Makiura R., Kitagawa H., Chem. Soc. Rev., 2013, 42, 6655—6669 |
| [22] | Yoon M., Suh K., Natarajan S., Kim K., Angew. Chem. Int.Ed., 2013, 52(10), 2688—2700 |
| [23] | Ma L.F., Wang L. Y., Luand D. H., Batten S. R., Cryst. Growth Des., 2009, 9, 1741—1749 |
| [24] | Liu D., Ren Z.G., Li H. X., Chen Y., Wang J., Zhang Y., Lang J. P., CrystEngComm, 2010, 12, 1912—1919 |
| [25] | Long L.S., CrystEngComm, 2010, 12, 1354—1365 |
| [26] | Gao L.J., Wang L., Wang S. Y., Jing S. B., Chem. [J]. Chinese Universities, 2016, 37(9), 1589—1595 |
| (高丽娟, 王莉, 王圣燕, 井淑波. 高等学校化学学报, 2016, 37(9), 1589—1595) | |
| [27] | Hu F.L., Wang S. L., Wu B., Yu H., Wang F., Lang J. P., CrystEngComm, 2014, 16, 6354—6363 |
| [28] | Sun Y.X., Sun W. Y., Chin. Chem. Lett., 2014, 25, 823—828 |
| [29] | Liu L.L., Ren Z. G., Zhu L. W., Wang H. F., Yan W. Y., Lang J. P., Cryst. Growth Des., 2011, 11, 3479—3488 |
| [30] | Cui G.H., Li J. R., Tian J. L., Bu X. H., Batten S. R., Cryst. Growth Des., 2005, 5, 1775—1780 |
| [31] | Liu Q., Ren Z.G., Deng L., Zhang W. H., Zhao X., Sun Z. R., Lang J. P., Dalton Trans., 2015, 44, 130—137 |
| [32] | Batten S.R., Robson R., Angew. Chem. Int. Ed., 1998, 37, 1460—1494 |
| [33] | Batten S.R., CrystEngComm, 2001, 3, 67—72 |
| [34] | Carlucci L., Ciani G., Proserpio D.M., Coord. Chem. Rev., 2003, 246, 247—289 |
| [35] | Yang J., Ma J.F., Batten S. R., Chem. Commun., 2012, 48, 7899—7912 |
| [36] | Zhou J.L., Wang Y. Y., Zhou M. J., Qin L., Zhang M. D., Yang Q. X., Zheng H. G., Inorg. Chem. Commun., 2014, 40, 148—150 |
| [37] | Liu B., Zhang Q., Ding H., Hu G., Du Y., Wang C., Wu J., Li S., Zhou H., Yang J., Tian Y., Dyes Pigm., 2012, 95(1), 149—160 |
| [38] | Sheldrick G.M., Acta Crystallogr. Sect. C: Cryst. Struc. Chem., 2015, 71, 3—8 |
| [39] | Sheldrick G.M., Acta Crystallogr. Sect. A, 2008, 64, 112—122 |
| [40] | Zhang Z.Y., Deng Z. P., Huo L. H., Zhao H., Gao S., Inorg. Chem., 2013, 52(10), 5914—5923 |
| [41] | Huang R.Y., Wang J. W., Xue C., Zhao S. P., Xu H., Inorg. Chim. Acta, 2014, 423, 133—138 |
| [42] | Fatma K., Bulent D., Sabriye P.O., Eser K., Dyes Pigm., 2010, 84(1), 14—18 |
| [43] | Liu L.L., Huang J. J., Wang X. L., Liu G. C., Yang S., Lin H. Y., Inorg. Chim. Acta, 2013, 394, 715—722 |
| [44] | Hao S.Y., Hou S. X., Van H. K., Cui G. H., Dalton Trans., 2017, 46(6), 1951—1962 |
| [1] | GAO Xia,PAN Huibin,QIAO Chengfang,CHEN Fengying,ZHOU Yuan,YANG Wenhua. Construction of HRP Immobilized Enzyme Reactor Based on Hierarchically Porous Metal-organic Framework and Its Dye Degradation Application† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1591. |
| [2] | XIA Yupei, WANG Chenxue, ZHENG Jinyu, LI Na, CHANG Ze, BU Xianhe. Construction of a Fe-MOF Based on Carbazole-carboxylate Ligand for CO2/CH4 Separation [J]. Chem. J. Chinese Universities, 2020, 41(11): 2415. |
| [3] | CAI Yanchao,NIU Pengfei,SHEN Zhenlu,LI Meichao. Electrocatalytic Oxidative Coupling of Primary Amines with the Medium ABNO † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2308. |
| [4] | ZHANG Jiayi,JIA Mengyang,JIANG Xiaohui,ZHANG Zhiming,YU Liangmin,WANG Xuan. Antifouling Properties of Dodecyl Benzene Sulfonic Acid Doped Polypyrrole Under Alternating Anodic-cathodic Polarization † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2396. |
| [5] | SUN Jie, MING Tingyun, QIAN Huixuan, ZHANG Manke, TAN Yong. Electrochemical Behavior of Copper Electrodeposition in BMIMPF6 Ionic Liquid [J]. Chem. J. Chinese Universities, 2018, 39(7): 1497. |
| [6] | ZHAO Bangtun, TAO Jingjing, CHEN Xiaoji, FU Huimin, ZHU Weimin. Synthesis, Structure and Electrochemistry of Tetrathiafulvalene Vinylogues Bearing Thienyl and Pyridyl Groups† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1449. |
| [7] | ZHANG Wei, ZHANG Yiwei, LI Hui, LEI Ming. Theoretical Study on the Formation Mechanism of Catalytic Active Components in Suzuki-Miyaura Cross-Coupling Reaction Catalyzed by Transition Metal Cobalt Complex† [J]. Chem. J. Chinese Universities, 2018, 39(4): 721. |
| [8] | ZHAO Bangtun, MA Shuxiu, TAO Jingjing, ZHU Weimin. Synthesis, Structures and Electrochemical Properties of Pyridine-based Tetrathiafulvalene Derivatives† [J]. Chem. J. Chinese Universities, 2017, 38(2): 193. |
| [9] | GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo. Influence of Solvent on Structure of Ni(Ⅱ) Metal-organic Frameworks† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1589. |
| [10] | ZHANG Xuena, ZHONG Xinwen, ZHONG Yan, LU Haiyan. Optimization of Conditions of the Electrochemical Detection of Methamphetamine† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1799. |
| [11] | LÜ Jiangwei, QU Youpeng, FENG Yujie, LIU Junfeng. Electrochemical Impedance Spectroscopy of Dichlorophenols at Boron-doped Diamond Electrodes† [J]. Chem. J. Chinese Universities, 2016, 37(1): 142. |
| [12] | WU Ranran, TIAN Xiaochun, WU Shenjian, LIU Yuangang, JIANG Yanxia, ZHAO Feng. Research on the Electrochemical Activity of Magnetospirillum Magneticum AMB-1† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1730. |
| [13] |
SHI Shuai, HUANG Ximing, AI Hongqi.
Mechanism Studies on Adsorption of DNA Bases and Base Pair-Zn2+ mplexes for CO2, N2 and |
| [14] | JING Weixuan, ZHOU Fan, CHEN Lujia, QI Han, JIANG Zhuangde, WANG Bing, NIU Lingling. Glucose Sensor of Spirally Hierarchical Structure with ZnO Nanowires Synthesized on a Spiralled Au Fiber† [J]. Chem. J. Chinese Universities, 2014, 35(3): 493. |
| [15] | LIANG Fangyuan, WU Ranran, CAO Changli, ZHENG Yue, YANG Zhaohui, ZHAO Feng. Research on Extracellular Electron Transfer of Acidithiobacillus Ferrooxidans† [J]. Chem. J. Chinese Universities, 2014, 35(2): 372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||