

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (1): 142.doi: 10.7503/cjcu20150369
• Physical Chemistry • Previous Articles Next Articles
LÜ Jiangwei1,*( ), QU Youpeng2, FENG Yujie3, LIU Junfeng3
), QU Youpeng2, FENG Yujie3, LIU Junfeng3
Received:2015-05-08
Online:2016-01-10
Published:2015-12-20
Contact:
LÜ Jiangwei
E-mail:pp198259@163.com
Supported by:CLC Number:
TrendMD:
LÜ Jiangwei, QU Youpeng, FENG Yujie, LIU Junfeng. Electrochemical Impedance Spectroscopy of Dichlorophenols at Boron-doped Diamond Electrodes†[J]. Chem. J. Chinese Universities, 2016, 37(1): 142.
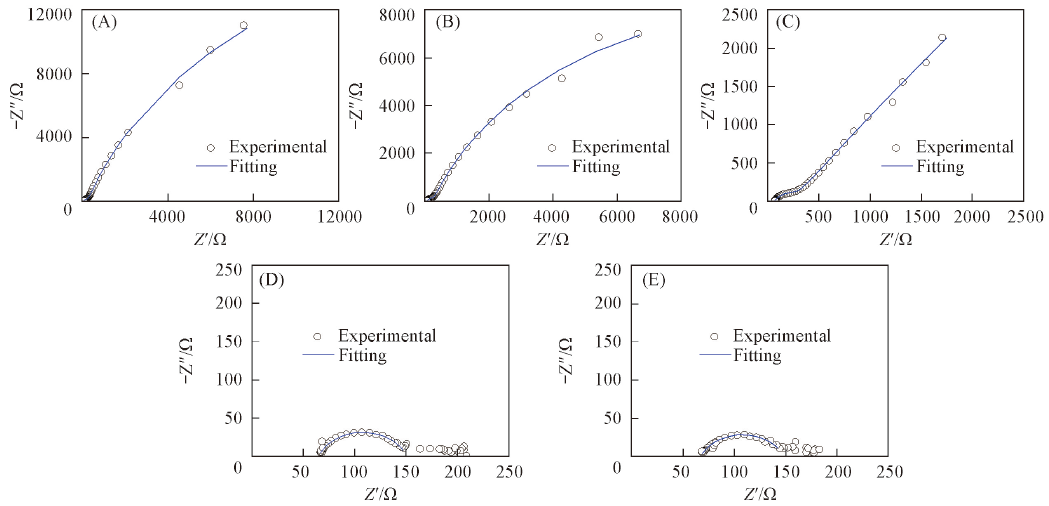
Fig.3 Nyquist plots of BDD electrodes in 1 mmol/L 2,4-DCP+0.1 mol/L Na2SO4 solution at different potentials^ (A) Open circuit potential; (B) 1.0 V; (C) 1.5 V; (D) 2.0 V; (E) 2.5 V.
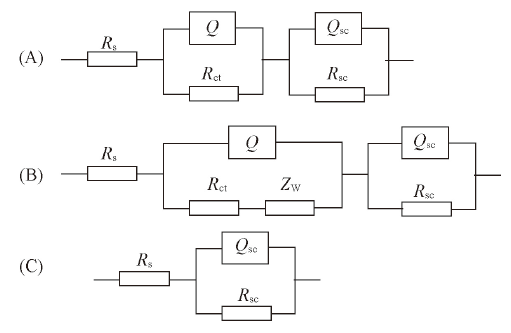
Fig.5 Equivalent circuit for electrochemical impedance spectroscopy^(A) Open circuit and 1.0 V: Rs(QRct)(QscRsc); (B) 1.5 V: Rs(Q(RctZW))(QscRsc); (C) 2.0 and 2.5 V: Rs(QscRsc).
| Potential/V | Rs/ (Ω·cm2) | 105Q-Ysc/ (S·sn·cm-2) | Q-nsc | Rsc/ (Ω·cm2) | 104Q-Y/ (S·sn·cm-2) | Q-n | Rct/ (Ω·cm2) | 104ZW/ (S·s0.5·cm2) | 104Chi squared |
|---|---|---|---|---|---|---|---|---|---|
| Open circuit | 64.75 | 2.581 | 0.8292 | 174.6 | 0.953 | 0.8066 | 4.275×104 | 2.504 | |
| 1.0 | 64.66 | 2.786 | 0.8222 | 180.7 | 1.176 | 0.7912 | 2.199×104 | 4.127 | |
| 1.5 | 64.05 | 3.552 | 0.7830 | 194.8 | 2.259 | 0.7286 | 2.885×102 | 2.235 | 3.929 |
| 2.0 | 65.08 | 2.456 | 0.8047 | 85.46 | 0.108 | ||||
| 2.5 | 67.59 | 2.384 | 0.7781 | 80.56 | 1.840 |
Table 1 Simulation results of BDD electrodes in 1 mmol/L 2,4-DCP+0.1 mol/L Na2SO4 solution at different potentials*
| Potential/V | Rs/ (Ω·cm2) | 105Q-Ysc/ (S·sn·cm-2) | Q-nsc | Rsc/ (Ω·cm2) | 104Q-Y/ (S·sn·cm-2) | Q-n | Rct/ (Ω·cm2) | 104ZW/ (S·s0.5·cm2) | 104Chi squared |
|---|---|---|---|---|---|---|---|---|---|
| Open circuit | 64.75 | 2.581 | 0.8292 | 174.6 | 0.953 | 0.8066 | 4.275×104 | 2.504 | |
| 1.0 | 64.66 | 2.786 | 0.8222 | 180.7 | 1.176 | 0.7912 | 2.199×104 | 4.127 | |
| 1.5 | 64.05 | 3.552 | 0.7830 | 194.8 | 2.259 | 0.7286 | 2.885×102 | 2.235 | 3.929 |
| 2.0 | 65.08 | 2.456 | 0.8047 | 85.46 | 0.108 | ||||
| 2.5 | 67.59 | 2.384 | 0.7781 | 80.56 | 1.840 |
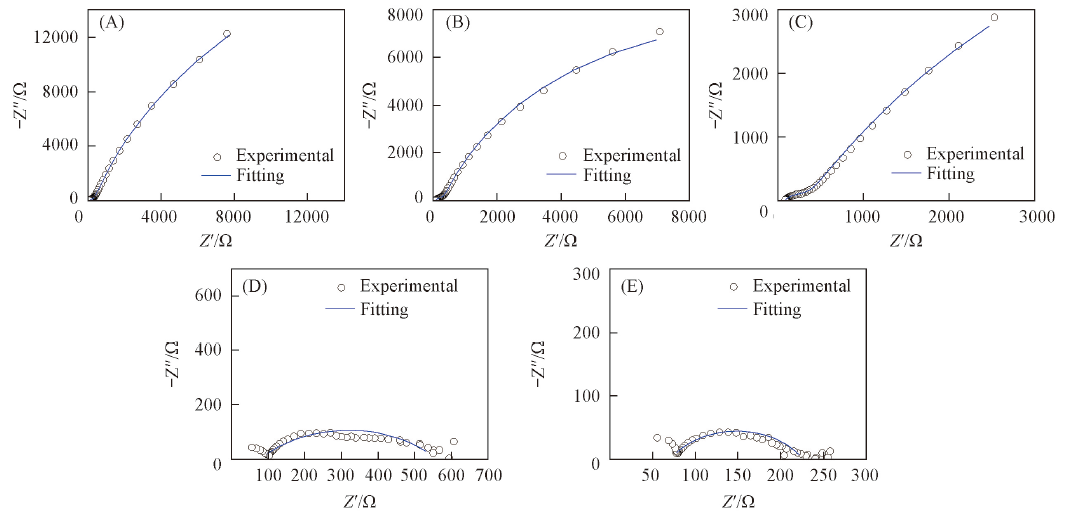
Fig.7 Nyquist plots of BDD electrodes in 1 mmol/L 2,6-DCP+0.1 mol/L Na2SO4 solution at different potentials^ (A) Open circuit potential; (B) 1.0 V; (C) 1.5 V; (D) 2.0 V; (E) 2.5 V.
| Potential/V | Rs/ (Ω·cm2) | 105Q-Ysc/ (S·sn·cm2) | Q-nsc | Rsc/ (Ω·cm2) | 104Q-Y/ (S·sn·cm2) | Q-n | Rct/ (Ω·cm2) | 104ZW/ (S·s0.5·cm2) | 104 Chi squared |
|---|---|---|---|---|---|---|---|---|---|
| Open circuit | 80.09 | 4.190 | 0.7480 | 235.7 | 0.915 | 0.8126 | 5.463×104 | 3.524 | |
| 1.0 | 80.01 | 4.168 | 0.7464 | 228.8 | 1.143 | 0.8049 | 1.981×104 | 4.312 | |
| 1.5 | 79.07 | 4.428 | 0.6757 | 270.7 | 6.330 | 0.8709 | 4.972×103 | 7.753 | 7.975 |
| 2.0 | 77.39 | 15.720 | 0.5278 | 483.5 | 46.89 | ||||
| 2.5 | 71.23 | 6.029 | 0.6632 | 154.6 | 13.48 |
Table 2 Simulation results of BDD electrodes in 1 mmol/L 2,6-DCP+0.1 mol/L Na2SO4 solution at different potentials
| Potential/V | Rs/ (Ω·cm2) | 105Q-Ysc/ (S·sn·cm2) | Q-nsc | Rsc/ (Ω·cm2) | 104Q-Y/ (S·sn·cm2) | Q-n | Rct/ (Ω·cm2) | 104ZW/ (S·s0.5·cm2) | 104 Chi squared |
|---|---|---|---|---|---|---|---|---|---|
| Open circuit | 80.09 | 4.190 | 0.7480 | 235.7 | 0.915 | 0.8126 | 5.463×104 | 3.524 | |
| 1.0 | 80.01 | 4.168 | 0.7464 | 228.8 | 1.143 | 0.8049 | 1.981×104 | 4.312 | |
| 1.5 | 79.07 | 4.428 | 0.6757 | 270.7 | 6.330 | 0.8709 | 4.972×103 | 7.753 | 7.975 |
| 2.0 | 77.39 | 15.720 | 0.5278 | 483.5 | 46.89 | ||||
| 2.5 | 71.23 | 6.029 | 0.6632 | 154.6 | 13.48 |
| [1] | Carey J. J., Christ J. C. S., Lowery S. N., Method of Electrolysis Employing A Doped Diamond Anode to Oxidize Solutes in Wastewater, US 5399247,1995-03-21 |
| [2] | Troster I., Fryda M., Herrmann D., Schafer L., Hanni W., Perret A., Blaschke M., Kraft A., Stadelmann M., Diamond Relat. Mater., 2002, 11(3—6SI), 640—645 |
| [3] | Moreira F.C., Garcia-Segura S., Boaventura R., Brillas E., Vilar V., Appl.Catal. B., Environ., 2014, 160, 492—505 |
| [4] | Groenen-Serrano K., Weiss-Hortala E., Savall A., Spiteri P., Electrocatalysis,2013, 4(4), 346—352 |
| [5] | Iniesta J., Michaud P. A., Panizza M., Cerisola G., Aldaz A., Comninellis C., Electrochim. Acta, 2001, 46(23), 3573—3578 |
| [6] | Zhi J. F., Tian R. H., Process Chem., 2005, 17(1), 55—63 |
| (只金芳, 田如海. 化学进展, 2005, 17(1), 55—63) | |
| [7] | Wang S. S., Chen G. H., Yang F. L., Thin Solid Films, 2009, 517(12), 3559—3561 |
| [8] | Panizza M., Cerisola G., Electrochim.Acta,2005, 51(2), 191—199 |
| [9] | Habka N., Pinault M. A., Mer C., Jomard F., Barjon J., Nesladek M., Bergonzo P., Phys. Status Solidi A, 2008, 205(9), 2169—2172 |
| [10] | Liang L. Q., Huang W. M., Lin H. B., Chem. J. Chinese Universities, 2015, 36(8), 1606—1611 |
| (梁龙琪, 黄卫民, 林海波. 高等学校化学学报,2015, 36(8), 1606—1611) | |
| [11] | Tian Y., Chen X. M., Shang C., Chen G. H., J. Electrochem. Soc., 2006, 153(7), J80—J85 |
| [12] | Huang W. M., Lin H. B., Chem. J. Chinese Universities, 2015, 36(9), 1765—1770 |
| (黄卫民, 林海波. 高等学校化学学报, 2015, 36(9), 1765—1770) | |
| [13] | Zhi J. F., Wang H. B., Nakashima T., Rao T. N., Fujishima A., J. Phys.Chem. B, 2003, 107(48), 13389—13395 |
| [14] | Lv J. W., Feng Y. J., Liu J. F., Qu Y. P., Cui F. Y., Appl. Surf. Sci., 2013, 283, 900—905 |
| [15] | Xu J. S., Granger M. C., Wang J., Chen Q. Y., Witek M. A., Hupert M. L., Hanks A., Swain G. M., Sakaguchi I., Nishitani-Gamo M., Ando T., Diamond Materials VI, 2000, 99(32), 403—415 |
| [16] | Zhao G. H., Li M. L., Qi Y., Hu H. K., China Environ. Sci., 2005, 25(3), 370—374 |
| (赵国华, 李明利, 祁源, 胡惠康. 中国环境科学, 2005, 25(3), 370—374) | |
| [17] | Zhao G. H., Li M. L., Wu W. W., Li R. B., He X. C., Environ. Sci., 2004, 25(5), 163—167 |
| (赵国华, 李明利, 吴薇薇, 李荣斌, 何贤昶. 环境科学, 2004, 25(5), 163—167) | |
| [18] | Liu F. B., Li X. M., Wang J. D., Liu B., Chen D. R., Chinese Sci. Bull., 2006, 51(11), 1344—1348 |
| ( 刘峰斌, 李学敏, 汪家道, 刘兵, 陈大融. 科学通报, 2006, 51(11), 1344—1348) | |
| [19] | Cui K., Wang J. D., Feng D., Chen D. R., J. Funct. Mater., 2015, 46(7), 7076—7080 |
| ( 崔凯, 汪家道, 冯东, 陈大融. 功能材料, 2015, 46(7), 7076—7080) | |
| [20] | Feng Y. J., Lv J. W., Liu J. F., Gao N., Peng H. Y., Chen Y. Q., Appl. Surf. Sci., 2011, 257(8), 3433—3439 |
| [21] | Zha Q.X.,Introduction to Kinetics of Electrode Process, Science Press, Beijing, 2002, 385 |
| (查全性. 电极过程动力学导论, 北京: 科学出版社, 2002, 385) |
| [1] | LIN Gaoxin, WANG Jiacheng. Progress and Perspective on Molybdenum Disulfide with Single-atom Doping Toward Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220321. |
| [2] | WANG Sicong, PANG Beibei, LIU Xiaokang, DING Tao, YAO Tao. Application of XAFS Technique in Single-atom Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220487. |
| [3] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [4] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [5] | HAN Fuchao, LI Fujin, CHEN Liang, HE Leiyi, JIANG Yunan, XU Shoudong, ZHANG Ding, QI Lu. Enhance of CoSe2/C Composites Modified Separator on Electrochemical Performance of Li-S Batteries at High Sulfur Loading [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220163. |
| [6] | WANG Ruhan, JIA Shunhan, WU Limin, SUN Xiaofu, HAN Buxing. CO2-involved Electrochemical C—N Coupling into Value-added Chemicals [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220395. |
| [7] | WANG Lijun, LI Xin, HONG Song, ZHAN Xinyu, WANG Di, HAO Leiduan, SUN Zhenyu. Efficient Electrocatalytic CO2 Reduction to CO by Tuning CdO-Carbon Black Interface [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220317. |
| [8] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [9] | XIA Tian, WAN Jiawei, YU Ranbo. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220162. |
| [10] | ZHANG Hongwei, CHEN Wen, ZHAO Meiqi, MA Chao, HAN Yunhu. Research Progress of Single Atom Catalysts in Electrochemistry [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220129. |
| [11] | WU Jun, HE Guanchao, FEI Huilong. Self-supported Film Electrodes Decorated with Single Atoms for Energy Electrocatalysis [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220051. |
| [12] | CHEN Changli, MI Wanliang, LI Yujing. Research Progress of Single Atom Catalysts in Electrochemical Hydrogen Cycling [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220065. |
| [13] | CHEN Zhaoyang, XUE Yurui, LI Yuliang. Synthesis and Applications of Graphdiyne Based Zerovalent Atomic Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220063. |
| [14] | DING Qin, ZHANG Zixuan, XU Peicheng, LI Xiaoyu, DUAN Limei, WANG Yin, LIU Jinghai. Effects of Cu, Ni and Co Hetroatoms on Constructions and Electrocatalytic Properties of Fe-based Carbon Nanotubes [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220421. |
| [15] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||