

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (9): 1589.doi: 10.7503/cjcu20160289
• Articles: Inorganic Chemistry • Previous Articles Next Articles
GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo*( )
)
Received:2016-04-26
Online:2016-09-10
Published:2016-08-26
Contact:
JING Shubo
E-mail:jingsb@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
GAO Lijuan, WANG Li, WANG Shengyan, JING Shubo. Influence of Solvent on Structure of Ni(Ⅱ) Metal-organic Frameworks†[J]. Chem. J. Chinese Universities, 2016, 37(9): 1589.
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1472035 | 1472036 | 1472037 |
| Empirical formula | C23H21N5O5Ni | C20H18N4O6Ni | C22H22N4O6Ni |
| Formula weight | 505.69 | 468.69 | 497.13 |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic |
| Space group | Pnna | P21/c | P21/c |
| a/nm | 1.20404(9) | 0.59980(5) | 0.78049(16) |
| b/nm | 1.08946(7) | 0.74515(6) | 1.3323(3) |
| c/nm | 1.74538(12) | 2.2385(2) | 1.0684(2) |
| α/(°) | 90 | 90 | 90 |
| β/(°) | 90 | 100.782(5) | 94.03(3) |
| γ/(°) | 90 | 90 | 90 |
| Volume/nm3 | 2.2895(3) | 0.98281(14) | 1.1082(4) |
| Z, Dc/(Mg·m-3) | 4, 1.355 | 2, 1.571 | 1, 1.490 |
| F(000) | 928 | 484 | 516 |
| θ range/(°) | 2.05—25.10 | 1.85—25.03 | 2.45—25.05 |
| Goodness-of-fit on F2 | 1.051 | 1.065 | 1.080 |
| R1, wR2[I>2σ(I)]* | R1=0.0736, wR2=0.1523 | R1=0.0431, wR2=0.1248 | R1=0.0319, wR2=0.0727 |
| R1, wR2(all data)* | R1=0.0915, wR2=0.1598 | R1=0.0497, wR2=0.1308 | R1=0.0494, wR2=0.0809 |
| Largest difference in peak and | 639 and -617 | 745 and -603 | 226 and -351 |
| hole/(e·nm-3) |
Table 1 Crystal data and structure refinement for compounds 1—3
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1472035 | 1472036 | 1472037 |
| Empirical formula | C23H21N5O5Ni | C20H18N4O6Ni | C22H22N4O6Ni |
| Formula weight | 505.69 | 468.69 | 497.13 |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic |
| Space group | Pnna | P21/c | P21/c |
| a/nm | 1.20404(9) | 0.59980(5) | 0.78049(16) |
| b/nm | 1.08946(7) | 0.74515(6) | 1.3323(3) |
| c/nm | 1.74538(12) | 2.2385(2) | 1.0684(2) |
| α/(°) | 90 | 90 | 90 |
| β/(°) | 90 | 100.782(5) | 94.03(3) |
| γ/(°) | 90 | 90 | 90 |
| Volume/nm3 | 2.2895(3) | 0.98281(14) | 1.1082(4) |
| Z, Dc/(Mg·m-3) | 4, 1.355 | 2, 1.571 | 1, 1.490 |
| F(000) | 928 | 484 | 516 |
| θ range/(°) | 2.05—25.10 | 1.85—25.03 | 2.45—25.05 |
| Goodness-of-fit on F2 | 1.051 | 1.065 | 1.080 |
| R1, wR2[I>2σ(I)]* | R1=0.0736, wR2=0.1523 | R1=0.0431, wR2=0.1248 | R1=0.0319, wR2=0.0727 |
| R1, wR2(all data)* | R1=0.0915, wR2=0.1598 | R1=0.0497, wR2=0.1308 | R1=0.0494, wR2=0.0809 |
| Largest difference in peak and | 639 and -617 | 745 and -603 | 226 and -351 |
| hole/(e·nm-3) |
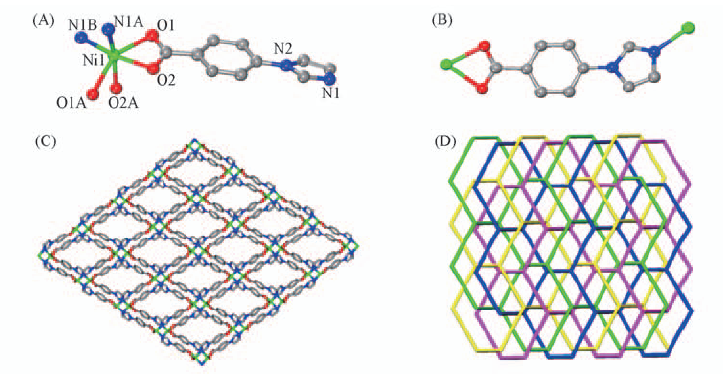
Fig.1 Coordination environment of Ni(Ⅱ) in compound 1(A), coordination mode of ligand(B), stick view of 3D structure of compound 1 along the y-axis, showing the diamond windows in the xz plane(C) and topology of the four-fold interpenetration in compound 1(D) The hydrogen atoms are omitted for clarity.
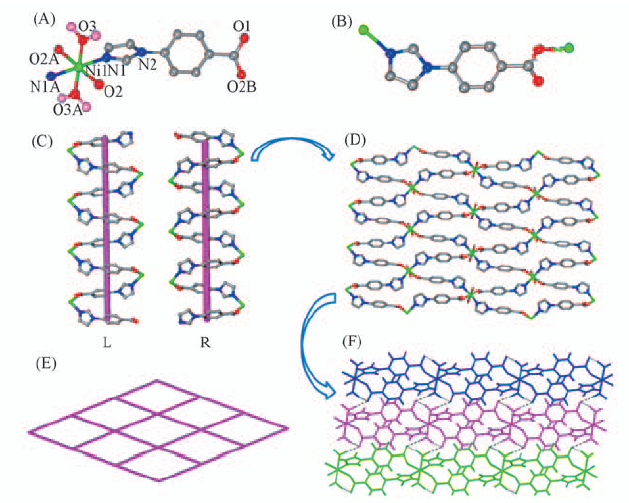
Fig.2 Coordination environment of Ni(Ⅱ) in compound 2(A), coordination mode of ligand(B), view of the left- and right- handed helical chains(C), view of the 2D layer structure constructed by the 1D helical chain(D), view of the topological structure of the 2D layer(E) and view of the 3D supramolecular structure constructed by the 2D layer via hydrogen-bond interactions(F) The hydrogen atoms are omitted for clarity.
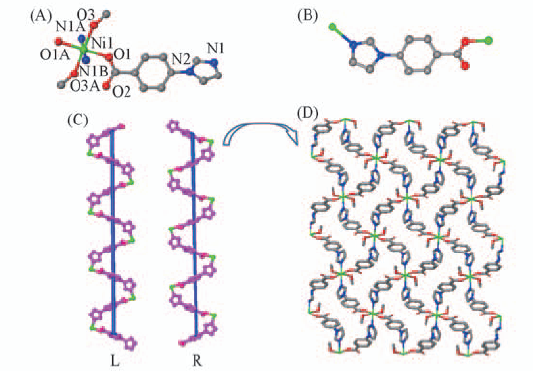
Fig.3 Coordination environment of Ni(Ⅱ) in compound 3(A), coordination mode of ligand(B), view of the left- and right-handed helical chains(C) and view of the 2D layer structure constructed by the 1D helical chain(D)
| [1] | Paz F. A. A., Klinowski J., Vilela S. M. F., Tomé J. P. C., Cavaleiro J. A. S., Rocha J., Chem. Soc. Rev., 2012, 41(3), 1088—1110 |
| [2] | Qiu S. L., Xue M., Zhu G. S., Chem. Soc. Rev., 2014, 43(16), 6116—6140 |
| [3] | Schneemann A., Bon V., Schwedler I., Senkovska I., Kaskel S., Fischer R. A., Chem. Soc. Rev., 2014, 43(16), 6062—6096 |
| [4] | Yamada T., Otsubo K., Makiura R., Kitagawa H., Chem. Soc. Rev., 2013, 42(16), 6655—6669 |
| [5] | Song Z., Li G. H., Yu Y., Shi Z., Feng S. H., Chem. Res.Chinese Universities,2009, 25(1), 1—4 |
| [6] | Singh D., Nagaraja C. M., Cryst. Growth Des., 2015, 15(7), 3356—3365 |
| [7] | Fan L. M., Fan W. L., Song W. K., Liu G. Z., Zhang X. T., Zhao X., Cryst. Eng. Comm., 2014, 16(39), 9191—9197 |
| [8] | Yan Z. H., Zhang X. W., Pang H. D., Zhang Y. H., Sun D. F., Wang L., RSC Adv., 2014, 4(96), 53608—53616 |
| [9] | Ezugwu C. I., Kabir N. A., Yusubov M., Verpoort F., Coord. Chem. Rev., 2016, 307, 188—210 |
| [10] | Chang Z., Yang D. H., Xu J., Hu T. L., Bu X. H., Adv. Mater., 2015, 27(36), 5432—5441 |
| [11] | Li C. P., Du M., Chem. Commun., 2011, 47(21), 5958—5972 |
| [12] | Manna B., Desai A. V., Kumar N., Karmakar A., Ghosh S. K., Cryst. Eng. Comm., 2015, 17(46), 8796—8800 |
| [13] | Weaver M. J., McNis G. E., Acc. Chem. Res., 1990, 23(9), 294—300 |
| [14] | Barbara P. F., Jarzeba W., Acc. Chem. Res., 1988, 21(5), 195—199 |
| [15] | Wang X. Y., Wang L., Wang Z. M., Gao S., J. Am. Chem. Soc., 2006, 128(3), 674—675 |
| [16] | Ma L. Q., Lin W. B., J. Am. Chem. Soc., 2008, 130(42), 13834—13835 |
| [17] | Pachfule P., Das R., Poddar P., Banerjee R., Cryst. Growth Des., 2011, 11(4), 1215—1222 |
| [18] | Tynan E., Jensen P., Kruger P.E., Lees A. C., Chem. Commun., 2004, (7), 776—777 |
| [19] | Wu Y., Yang G. P., Zhao Y., Wu W. P., Liu B., Wang Y. Y., Dalton Trans., 2015, 44(7), 3271—3277 |
| [20] | Liu C., Zhuo X., Cheng H., Liu C., Liu X. H., Chem. J. Chinese Universities,2015, 36(5), 831—837 |
| (刘超, 卓馨, 成慧, 刘闯, 刘新华. 高等学校化学学报, 2015, 36(5), 831—837) | |
| [21] | Huang C. T., Wu X. F., Li G. H., Gao L., Feng S. H., Chem. J. Chinese Universities,2015, 36(9), 1661—1666 |
| (黄楚婷, 吴小峰, 李光华, 高路, 冯守华. 高等学校化学学报, 2015, 36(9), 1661—1666) | |
| [22] | Li Y., Zhang S. S., Song D. T., Angew. Chem. Int. Ed., 2013, 52(2), 710—713 |
| [23] | Zhang Y., Niu Y. B., Liu T., Li Y. T., Wang M. Q., Hou J., Xu M., Mater. Lett., 2015, 161, 712—715 |
| [24] | Li J. R., Yu Q., Tao Y., Bu X. H., Ribas J., Batten S. R., Chem. Commun., 2007, (22), 2290—2292 |
| [25] | Wang K., Zou H. H., Chen Z. L., Zhang Z., Sun W. Y., Liang F. P., Dalton Trans., 2014, 43(34), 12989—12995 |
| [26] | Tian C. B., Chen R. P., He C., Li W. J., Wei Q., Zhang X. D., Du S. W., Chem. Commun., 2014, 50(15), 1915—1917 |
| [27] | Zhao J. P., Han S. D., Zhao R., Yang Q., Chang Z., Bu X. H., Inorg. Chem., 2013, 52(6), 2862—2869 |
| [28] | Liu Q., Yu L. L., Wang Y., Ji Y. Z., Horvat J., Cheng M. L., Jia X. Y., Wang G. X., Inorg. Chem., 2013, 52(6), 2817—2822 |
| [29] | Mao J. J., Yang L. F., Yu P., Wei X. W., Mao L. Q., Electrochem. Commun., 2012, 19, 29—31 |
| [30] | Wang H. L., Zhang D. P., Jiang J. Z., Cryst. Eng. Comm., 2010, 12(4), 1096—1102 |
| [31] | Blatov V.A., Peaskov M.V., Acta Crystallogr. Sect. B: Struct. Sci., 2006, 62, 457—466 |
| [32] | Fatma K., Bulent D., Sabriye P. O., Eser K., Dyes Pigments,2010, 84(1), 14—18 |
| [1] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [2] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [3] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [4] | GUO Biao, ZHAO Chencan, LIU Xinxin, YU Zhou, ZHOU Lijing, YUAN Hongming, ZHAO Zhen. Effects of Surface Hydrothermal Carbon Layer on the Photocatalytic Activity of Magnetic NiFe2O4 Octahedron [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220472. |
| [5] | XING Peiqi, LU Tong, LI Guanghua, WANG Liyan. Controllable Syntheses of Two Cd(II) Metal-organic Frameworks Possessing Related Structures [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220218. |
| [6] | SHI Xiaofan, ZHU Jian, BAI Tianyu, FU Zixuan, ZHANG Jijie, BU Xianhe. Research Status and Progress of MOFs with Application in Photoelectrochemical Water-splitting [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210613. |
| [7] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [8] | LI Wen, QIAO Junyi, LIU Xinyao, LIU Yunling. Zirconium-based Metal-Organic Framework with Naphthalene for Fluorescent Detection of Nitroaromatic Explosives in Water [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210654. |
| [9] | WANG Jie, HUO Haiyan, WANG Yang, ZHANG Zhong, LIU Shuxia. General Strategy for In situ Synthesis of NENU-n Series Polyoxometalate-based MOFs on Copper Foil [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210557. |
| [10] | LIU Xueguang, YANG Xiaoshan, MA Jingjing, LIU Weisheng. Separating Methyl Blue Selectively from the Mixture of Dyes by Europium Metal-organic Frameworks [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210715. |
| [11] | XU Fei, LI Gangmei, HAN Songde, WANG Guoming. Photochromism and Photomagnetism in Two Dinuclear Lanthanide Complexes [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210337. |
| [12] | MO Zongwen, ZHANG Xuewen, ZHOU Haolong, ZHOU Dongdong, ZHANG Jiepeng. Guest-responses of A Porous Coordination Polymer Based on Synergistic Hydrogen Bonds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210576. |
| [13] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [14] | LI Shurong, WANG Lin, CHEN Yuzhen, JIANG Hailong. Research Progress of Metal⁃organic Frameworks on Liquid Phase Catalytic Chemical Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210575. |
| [15] | ZHANG Chi, SUN Fuxing, ZHU Guangshan. Synthesis, N2 Adsorption and Mixed-matrix Membrane Performance of Bimetal Isostructural CAU-21 [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210578. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||