

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (2): 193.doi: 10.7503/cjcu20160625
• Organic Chemistry • Previous Articles Next Articles
ZHAO Bangtun1,*( ), MA Shuxiu2, TAO Jingjing2, ZHU Weimin2,*(
), MA Shuxiu2, TAO Jingjing2, ZHU Weimin2,*( )
)
Received:2016-09-05
Online:2017-02-10
Published:2016-12-28
Contact:
ZHAO Bangtun,ZHU Weimin
E-mail:zbt@lynu.edu.cn;zhuwm@zzu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHAO Bangtun, MA Shuxiu, TAO Jingjing, ZHU Weimin. Synthesis, Structures and Electrochemical Properties of Pyridine-based Tetrathiafulvalene Derivatives†[J]. Chem. J. Chinese Universities, 2017, 38(2): 193.
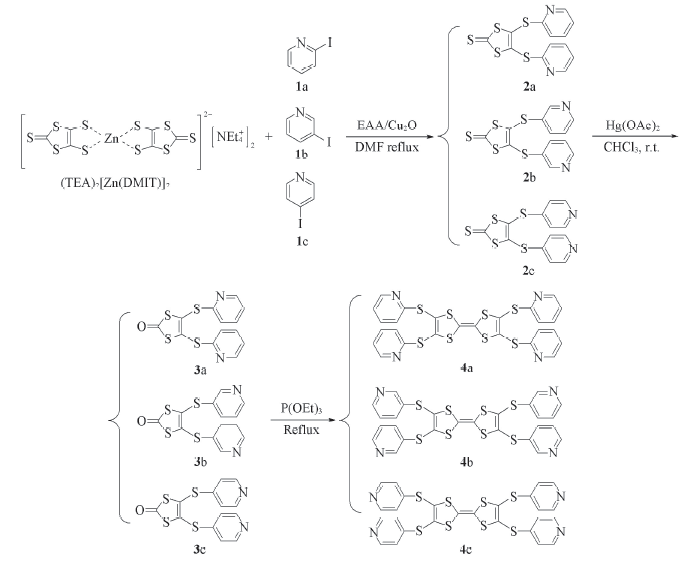
Scheme 1 General synthetic routes for 2,3,6,7-tetrakis(pyridine-2-ylthio)tetrathiafulvalene(4a), 2,3,6,7-tetrakis(pyridine-3-ylthio)tetrathiafulvalene(4b) and 2,3,6,7-tetrakis(pyridine-4-ylthio)tetrathiafulvalene(4c)
| Compound | 4b | 4c |
|---|---|---|
| Empirical formula | C26H16N4S8 | C26H16N4S8 |
| Formula mass | 640.91 | 640.91 |
| Crystal size/mm | 0.25×0.20×0.15 | 0.35×0.35×0.20 |
| Crystal system | Triclinic | Triclinic |
| Space group | P1 | P1 |
| a/nm | 0.52575(11) | 0.56065(11) |
| b/nm | 1.0461(2) | 1.0114(2) |
| c/nm | 1.2788(3) | 1.2722(3) |
| α/(°) | 92.58(3) | 102.39(3) |
| β/(°) | 92.22(3) | 91.25(3) |
| γ/(°) | 98.05(3) | 94.25(3) |
| Volume/nm3 | 0.6950(2) | 0.7021(2) |
| Z | 1 | 1 |
| Calculated density/(g·cm-3) | 1.531 | 1.516 |
| Absorption coefficient/mm-1 | 0.668 | 0.661 |
| F(000) | 328 | 328 |
| θ range for data collection/(°) | 2.60—25.50 | 2.34—24.99 |
| Limiting indices | -6≤h≤6,-12≤k≤12,-15≤l≤15 | -6≤h≤6,-11≤k≤11,-14≤l≤15 |
| Reflections collected/unique | 5819/2442(Rint=0.0471) | 5977/2470(Rint=0.0199) |
| Completeness to θ=25.00°(%) | 99.6 | 99.8 |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9064 and 0.8508 | 0.8791 and 0.8015 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2442/0/172 | 2470/0/172 |
| Goodness of fit on F2 | 1.087 | 1.084 |
| Final R indices[I>2σ(I)] | R1=0.0524, wR2=0.1497 | R1=0.0296, wR2=0.0790 |
| R index(all data) | R1=0.0656, wR2=0.1741 | R1=0.0353, wR2=0.0841 |
| Largest diff. peak and hole/(e·nm-3) | 0.521 and -0.392 | 0.206 and -0.234 |
| CCDC number | 1472060 | 1472061 |
Table 1 X-ray diffraction data for pyridine-based tetrathiafulvalenes 4b and 4c
| Compound | 4b | 4c |
|---|---|---|
| Empirical formula | C26H16N4S8 | C26H16N4S8 |
| Formula mass | 640.91 | 640.91 |
| Crystal size/mm | 0.25×0.20×0.15 | 0.35×0.35×0.20 |
| Crystal system | Triclinic | Triclinic |
| Space group | P1 | P1 |
| a/nm | 0.52575(11) | 0.56065(11) |
| b/nm | 1.0461(2) | 1.0114(2) |
| c/nm | 1.2788(3) | 1.2722(3) |
| α/(°) | 92.58(3) | 102.39(3) |
| β/(°) | 92.22(3) | 91.25(3) |
| γ/(°) | 98.05(3) | 94.25(3) |
| Volume/nm3 | 0.6950(2) | 0.7021(2) |
| Z | 1 | 1 |
| Calculated density/(g·cm-3) | 1.531 | 1.516 |
| Absorption coefficient/mm-1 | 0.668 | 0.661 |
| F(000) | 328 | 328 |
| θ range for data collection/(°) | 2.60—25.50 | 2.34—24.99 |
| Limiting indices | -6≤h≤6,-12≤k≤12,-15≤l≤15 | -6≤h≤6,-11≤k≤11,-14≤l≤15 |
| Reflections collected/unique | 5819/2442(Rint=0.0471) | 5977/2470(Rint=0.0199) |
| Completeness to θ=25.00°(%) | 99.6 | 99.8 |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9064 and 0.8508 | 0.8791 and 0.8015 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2442/0/172 | 2470/0/172 |
| Goodness of fit on F2 | 1.087 | 1.084 |
| Final R indices[I>2σ(I)] | R1=0.0524, wR2=0.1497 | R1=0.0296, wR2=0.0790 |
| R index(all data) | R1=0.0656, wR2=0.1741 | R1=0.0353, wR2=0.0841 |
| Largest diff. peak and hole/(e·nm-3) | 0.521 and -0.392 | 0.206 and -0.234 |
| CCDC number | 1472060 | 1472061 |
| Compound | ||||||||
|---|---|---|---|---|---|---|---|---|
| 4a | 0.613 | 0.552 | 0.061 | 0.792 | 0.732 | 0.060 | 0.5825 | 0.762 |
| 4b | 0.762 | 0.691 | 0.071 | 1.046 | 0.981 | 0.065 | 0.726 | 1.014 |
| 4c | 0.855 | 0.787 | 0.068 | 1.127 | 1.029 | 0.098 | 0.821 | 1.078 |
Table 2 Electrochemical data(V) of compounds 4a, 4b and 4c measured by cyclic voltammetry
| Compound | ||||||||
|---|---|---|---|---|---|---|---|---|
| 4a | 0.613 | 0.552 | 0.061 | 0.792 | 0.732 | 0.060 | 0.5825 | 0.762 |
| 4b | 0.762 | 0.691 | 0.071 | 1.046 | 0.981 | 0.065 | 0.726 | 1.014 |
| 4c | 0.855 | 0.787 | 0.068 | 1.127 | 1.029 | 0.098 | 0.821 | 1.078 |
| Compound | HOMO/eV | LUMO/eV | Eg/eV | IE/eV |
|---|---|---|---|---|
| 4a | -4.64 | -1.10 | 3.54 | 5.96 |
| 4b | -4.71 | -1.36 | 3.35 | 6.06 |
| 4c | -5.12 | -1.69 | 3.42 | 6.46 |
Table 3 Molecular orbital energy of compounds 4a, 4b and 4c
| Compound | HOMO/eV | LUMO/eV | Eg/eV | IE/eV |
|---|---|---|---|---|
| 4a | -4.64 | -1.10 | 3.54 | 5.96 |
| 4b | -4.71 | -1.36 | 3.35 | 6.06 |
| 4c | -5.12 | -1.69 | 3.42 | 6.46 |
| [1] | Yamada J., Sugimoto T., TTF Chemistry Fundamentals and Applications of Tetrathiafulvalene, Springer, Verlag, Berlin,2004, 36, 98 |
| [2] | Nielsen M. B., Lomholt C., Becher J., Chem. Soc. Rev., 2000, 29, 153—164 |
| [3] | Segura J. L., Martín N., Angew. Chem. Int. Ed., 2001, 40, 1372—1409 |
| [4] | Nazario M., Luis S., María Ángeles H., Beatriz I., Dirk M. G., Acc. Chem. Res., 2007, 40, 1015—1024 |
| [5] | Canevet D., Sallé M., Zhang G.X., Zhu D. B.,Chem. Commun., 2009, (17), 2245—2269 |
| [6] | Bergkamp J. J., Decurtins S., Liu S. X., Chem. Soc. Rev., 2015, 44, 863—874 |
| [7] | Zhao B. T., Chen X. H., Ma S. X., Zhu W. M., Chem. Res. Chinese Universities,2015, 31(6), 930—935 |
| [8] | Wen Z., Kan Y. H., Yan W. Y., Ding Y. Y., Wang. X. L., Chem. J. Chinese Universities,2013, 34(6), 1483—1489 |
| (温智, 阚玉和, 闫文艳, 丁艳艳, 王新龙. 高等学校化学学报, 2013, 34(6), 1483—1489) | |
| [9] | Li Q., Geng Y., Duan Y. A., Wang G. Y., Su Z. M., Chem. J. Chinese Universities,2014, 35(7), 1471—1479 |
| (李倩, 耿允, 段雨爱, 王光宇, 苏忠民. 高等学校化学学报, 2014, 35(7), 1471—1479) | |
| [10] | Fabrice P., Boris L. G., Olivier M., Olivier C., Lahcéne O., Acc. Chem. Res., 2015, 48, 2834—2842 |
| [11] | Chahma M., Wang X. S., Van des Est A., Pilkington M., J. Org. Chem., 2006, 71, 2750—2755 |
| [12] | Goeb S., Bivaud S., Croué V., Vajpayee V., Allain M., Sallé M., Materials,2014, 7, 611—622 |
| [13] | Zhao Y. P., Wu L. Z., Si G., Liu Y., Xue H., Zhang L. P., Tung C. H., J. Org. Chem., 2007, 72, 3632—3639 |
| [14] | Balandier J.Y., Belyasmine A., Sallé M.,Eur. J. Org. Chem., 2008, (2), 269—276 |
| [15] | Ota A., Ouahab L., Golhen S., Cador O., Yoshida Y., Saito G., New J. Chem., 2005, 29, 1135—1140 |
| [16] | Wang Q., Day P., Griffiths J. P., Nie H., Wallis J. D., New J. Chem., 2006, 30, 1790—1800 |
| [17] | Benhaoua C., Mazari M., Mercier N., Le D. F., Sallé M., New J. Chem., 2008, 32, 913—916 |
| [18] | Jia C., Zhang D., Guo X., Wan S., Xu W., Zhu D.,Synthesis, 2002, (15), 2177—2182 |
| [19] | Sun J. B., Lu X. F., Shao J. F., Cui Z. L., Shao Y., Jiang G. Y., Yu W., Shao X. F., RSC Adv., 2013, 3, 10193—10196 |
| [20] | Sun J. B., Lu X. F., Shao J. F., Li X. X., Zhang S. X., Wang B. L., Zhao J. L., Shao Y. L., Fang R., Wang Z. H., Yu W., Shao X. F., Chem. Eur. J., 2013, 19, 12517—12525 |
| [21] | Sun J. B., Lu X. F., Ishikawa M., Nakano Y., Zhang S. X., Zhao J. L., Shao Y. L., Wang Z. H., Yamochi H., Shao X. F., J. Mater. Chem. C,2014, 2, 8071—8076 |
| [22] | Lu X., Sun J., Zhang S., Ma L., Liu L., Qi H., Shao Y., Shao X., Beilstein J. Org. Chem., 2015, 11, 1043—1051 |
| [23] | Yu Z. X., Cheong P. H., Liu P., Legault C. Y., Wender P. A., Houk K. N., J. Am. Chem. Soc., 2008, 130, 2378—2379 |
| [1] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [2] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [3] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [4] | CHANG Hui, YAO Shuangquan, HAN Wenjia, KANG Xiena, ZHANG Li, LI Xinping, ZHANG Zhao. Highly Solvatochromic Terpyridine Compounds for Identification of Butanol Isomers [J]. Chem. J. Chinese Universities, 2021, 42(3): 902. |
| [5] | LI Kangming, LI Yansai, YI Yangjie, XU Leitao, YE Jiao, OU Xiaoming, LI Jianming, HU Aixi. Design, Synthesis and Biological Activity of 5-Pyrazole Carboxamides † [J]. Chem. J. Chinese Universities, 2020, 41(4): 716. |
| [6] | YIN Shaoyun, ZHANG Luyin, WANG Zheng, PAN Mei. Glass-like Cuprous Iodide Complexes with Photoluminescence Tuning and Two-photon Emission Properties † [J]. Chem. J. Chinese Universities, 2020, 41(4): 646. |
| [7] | TIAN Xia,YANG Fuqun,YUAN Wei,ZHAO Lei,YAO Lei,ZHEN Xiaoli,HAN Jianrong,LIU Shouxin. Synthesis, Structure and Recognition Properties of Macrocyclic Crown Ethers with Oxadiazole † [J]. Chem. J. Chinese Universities, 2020, 41(3): 490. |
| [8] | LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis † [J]. Chem. J. Chinese Universities, 2020, 41(2): 253. |
| [9] | LIN Junjie, WANG Shuang, LI Weiqiang, CUI Xin, HUANG Chao. Efficient Synthesis of Pyridine [2,3-d]pyrimidine Derivatives by Catalyst-free Tandem Cyclization Under Microwave Irradiation [J]. Chem. J. Chinese Universities, 2020, 41(12): 2749. |
| [10] | QIN Liulei,LIU Yang,GUAN Xiaoqin,ZHENG Xiaoyuan,ZHANG Ziyu,LIU Zunqi. Synthesis and Switchable Dielectric Properties of an Inorganic-organic Hybrid Complex [H2(DABCO)CuCl4]·H2O † [J]. Chem. J. Chinese Universities, 2020, 41(1): 70. |
| [11] | WANG Lin, ZHANG Yanhui, Arzugul Muslim, LAN Haidie. Morphology and Size Regulation of Polyaniline Induced by PS-b-P2VP as Template and Its Electrochemical Characters [J]. Chem. J. Chinese Universities, 2019, 40(8): 1748. |
| [12] | LIU Shufeng,QIU Haiyan,JIANG Tao,ZHANG Yehua,ZHAO Yun,CHENG Hanwen,CHEN Yong. Ion Transfer Behavior of Protonated Phenazopyridine at the Liquid/Liquid Interface Modified by Functionalized Hybrid Mesoporous Silica Membrane† [J]. Chem. J. Chinese Universities, 2019, 40(5): 973. |
| [13] | LI Bing,WANG Xuemin,BAI Fengying,LIU Shuqing. Synthesises, Structures and Antibacterial Activities of a Series of Rare Earth Nitrogen Heterocyclic Complexes† [J]. Chem. J. Chinese Universities, 2019, 40(4): 632. |
| [14] | CAI Yanchao,NIU Pengfei,SHEN Zhenlu,LI Meichao. Electrocatalytic Oxidative Coupling of Primary Amines with the Medium ABNO † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2308. |
| [15] | ZHANG Jiayi,JIA Mengyang,JIANG Xiaohui,ZHANG Zhiming,YU Liangmin,WANG Xuan. Antifouling Properties of Dodecyl Benzene Sulfonic Acid Doped Polypyrrole Under Alternating Anodic-cathodic Polarization † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2396. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||