

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (3): 506.doi: 10.7503/cjcu20170577
• Physical Chemistry • Previous Articles Next Articles
CHENG Weiliang1,*( ), ZHU Mengqian1, QIN Wu2,*(
), ZHU Mengqian1, QIN Wu2,*( ), HOU Cuicui2
), HOU Cuicui2
Received:2017-08-24
Online:2018-03-10
Published:2018-01-23
Contact:
CHENG Weiliang,QIN Wu
E-mail:cwl@ncepu.edu.cn;qinwu@ncepu.edu.cn
Supported by:CLC Number:
TrendMD:
CHENG Weiliang, ZHU Mengqian, QIN Wu, HOU Cuicui. Chemical Looping Combustion Characteristics of Fe2O3(104) and CO Under Synergistic Action of ZrO2/TiO2 Carrier†[J]. Chem. J. Chinese Universities, 2018, 39(3): 506.
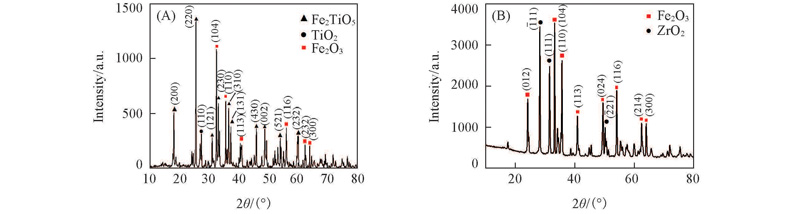
Fig.3 XRD patterns of the prepared oxygen carrieres Fe2O3(104)/TiO2(A) and Fe2O3(104)/ZrO2(B)The data are denoted as Fe2O3 different crystal face indices.
| Oxygen carrier | Surface area/(m2·g-1) | Pore volume/(mL·g-1) | Pore diameter/nm |
|---|---|---|---|
| Fe2O3(104)/TiO2 | 2.918 | 0.003 | 12.967 |
| Fe2O3(104)/ZrO2 | 3.870 | 0.006 | 3.400 |
Table 1 Specific surface and pore size of different oxygen carriers
| Oxygen carrier | Surface area/(m2·g-1) | Pore volume/(mL·g-1) | Pore diameter/nm |
|---|---|---|---|
| Fe2O3(104)/TiO2 | 2.918 | 0.003 | 12.967 |
| Fe2O3(104)/ZrO2 | 3.870 | 0.006 | 3.400 |
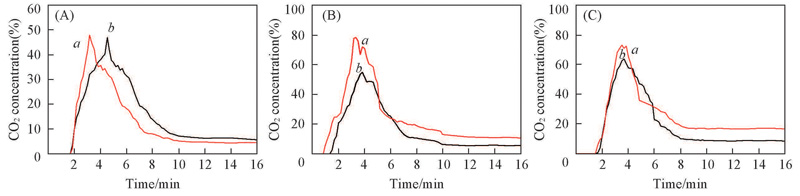
Fig.5 CO2 generation for the chemical looping combustion between Fe2O3(104)/ZrO2(a) and Fe2O3(104)/TiO2(b) and CO at 700 ℃(A), 800 ℃(B) and 900 ℃(C), respectively
| Temperature/℃ | Time/min | Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 |
|---|---|---|---|
| 700 | 6 | 0.322 | 0.308 |
| 7 | 0.309 | 0.276 | |
| 8 | 0.286 | 0.245 | |
| 16 | 0.165 | 0.135 | |
| 800 | 6 | 0.388 | 0.510 |
| 7 | 0.348 | 0.452 | |
| 8 | 0.309 | 0.410 | |
| 16 | 0.174 | 0.262 | |
| 900 | 6 | 0.462 | 0.475 |
| 7 | 0.409 | 0.438 | |
| 8 | 0.363 | 0.401 | |
| 16 | 0.207 | 0.272 |
Table 2 CO2 conversion of different oxygen carriers at different time
| Temperature/℃ | Time/min | Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 |
|---|---|---|---|
| 700 | 6 | 0.322 | 0.308 |
| 7 | 0.309 | 0.276 | |
| 8 | 0.286 | 0.245 | |
| 16 | 0.165 | 0.135 | |
| 800 | 6 | 0.388 | 0.510 |
| 7 | 0.348 | 0.452 | |
| 8 | 0.309 | 0.410 | |
| 16 | 0.174 | 0.262 | |
| 900 | 6 | 0.462 | 0.475 |
| 7 | 0.409 | 0.438 | |
| 8 | 0.363 | 0.401 | |
| 16 | 0.207 | 0.272 |
| Reaction | Correlation coefficient | Reaction | Correlation coefficient | ||
|---|---|---|---|---|---|
| Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 | Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 | ||
| Phase interface | 0.8474 | 0.9308 | Shrink the sphere | 0.9553 | 0.9953 |
| First order | 0.9921 | 0.9494 | Power function law | 0.8995 | 0.9625 |
| Secondary | 0.9099 | 0.1601 | Valensi | 0.9398 | 0.9787 |
| Three-stage | 0.7816 | 0.0528 | Jander | 0.9873 | 0.9650 |
| Shrink the cylinder | 0.9288 | 0.9875 | G-B | 0.9607 | 0.9828 |
Table 3 Correlation coefficients calculated using different kinetic models
| Reaction | Correlation coefficient | Reaction | Correlation coefficient | ||
|---|---|---|---|---|---|
| Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 | Fe2O3(104)/TiO2 | Fe2O3(104)/ZrO2 | ||
| Phase interface | 0.8474 | 0.9308 | Shrink the sphere | 0.9553 | 0.9953 |
| First order | 0.9921 | 0.9494 | Power function law | 0.8995 | 0.9625 |
| Secondary | 0.9099 | 0.1601 | Valensi | 0.9398 | 0.9787 |
| Three-stage | 0.7816 | 0.0528 | Jander | 0.9873 | 0.9650 |
| Shrink the cylinder | 0.9288 | 0.9875 | G-B | 0.9607 | 0.9828 |
| [1] | Richter Horst J., Knoche Karl F.,Am. Chem. Soc., 1983, 71—85 |
| [2] | Ishida M., Jin H., Ind. Eng. Chem. Res., 1996, 35(7), 2469—2472 |
| [3] | Fan L. S., Zeng L., Wang W., Luo S. W., Energy Environ. Sci., 2012, 5(6), 7254—7280 |
| [4] | Adanez J., Abad A., Garcia-Labiano F., Gayan P., de Diego L. F., Prog. Energy Combust. Sci., 2012, 38(2), 215—282 |
| [5] | Zhang Y., Doroodchi E., Moghtaderi B., Energy Fuels, 2012, 26(1), 287—295 |
| [6] | He F., Li H.B., Zhao Z. L.,Int. J. Chem. Eng., 2009, 1—16 |
| [7] | Lyngfelt A., Leckner B., Mattisson T., Chem. Eng. Sci., 2001, 56(10), 3101—3113 |
| [8] | Johansson M., Mattisson T., Lyngfelt A., J. Therm. Sci., 2006, 10(3), 93—107 |
| [9] | Saha C., Bhattacharya S., Int. J. Chem. Eng., 2011, 36(18), 12048—12057 |
| [10] | Cho P., Mattisson T., Lyngfelt A., Fuels, 2004, 83(9), 1215—1225 |
| [11] | Wei G. Q., He F., Huang Z., Zhao Q., Li X. A., Li H. B., Chem. Ind. Eng. Prog., 2012, 31(4), 713—725 |
| (魏国强, 何方, 黄振, 赵坤, 李新爱, 李海滨.化工进展,2012, 31(4), 713—725) | |
| [12] | Zhao H. B., Liu L. M., Wang B. W., Xu D., Jiang L. L., Zheng C. G., Energy Fuels, 2008, 22(2), 898—905 |
| [13] | Mattisson T., Johansson M., Lyngfelt M., Energy Fuels, 2003, 17(3), 643—651 |
| [14] | Ad nez-Rubio I., Gayn P., Abad A., García-Labiano F., de Diego L. F., Adnez J., Chem. Eng. J., 2014, 256, 69—84 |
| [15] | Dennis J. S., Scott S. A., Fuels, 2010, 89(7), 1623—1640 |
| [16] | Lee J. B., Park C. S., Choi S. I., Song Y. W., Kim Y. H., Yang H. S., J. Ind. Engin. Chem., 2005, 11(1), 96—102 |
| [17] | Yang J. B., Cai N. S., Li Z. S., Energy. Fuels, 2007, 21(6), 3360—3368 |
| [18] | Guo L., Zhao H. B., Ma J. C., Mei D. F., Zheng C. G., Chem. Engin. Techn., 2014, 37(7), 1211—1219 |
| [19] | Zhu X., Li K. Z., Wei Y. G., Wang H., Sun L. Y., Energy Fuels, 2014, 28(2), 754—760 |
| [20] | Wang C. P., Cui H. R., Di H. S., Guo Q. J., Huang F., Energy Fuels, 2014, 28(6), 4162—4166 |
| [21] | Azimi G., Leion H., Mattisson T., Rydén M., Snijkers F., Lyngfelt A., Ind. Eng. Chem. Res., 2014, 53(25), 10358—10365 |
| [22] | Ksepko E., Siriwardane R. V., Tian H. J., Simonyi T., Sciazko M., Energy Fuels, 2012, 26(4), 2461—2472 |
| [23] | Wang B. W., Yan R., Zhao H. B., Zheng Y., Liu Z. H., Zheng C. G., Energy Fuels, 2011, 25(7), 3344—3354 |
| [24] | Moghtaderi B., Song H., Energy Fuels, 2010, 24(10), 5359—5368 |
| [25] | Wang S. Z., Wang G. X., Jiang F., Luo M., Li H. Y., Energy Environ. Sci., 2010, 3(9), 1353—1360 |
| [26] | Liu L., Zachariah M. R., Energy Fuels, 2013, 27(8), 4977—4983 |
| [27] | Bao J., Li Z., Cai N., Research, 2013, 52(18), 6119—6128 |
| [28] | Chen D. Q., Shen L. H., Xiao J., Song T., Gu H. M., Zhang S. W., J. Fuel. Chem. Techno., 2012, 40(3), 267—272 |
| (陈定千, 沈来宏, 肖军, 宋涛, 顾海明, 张思文.燃料化学学报,2012, 40(3), 267—272) | |
| [29] | Xie X. W., Li Y., Liu Z. Q., Haruta M., Shen W. J., Nature, 2009, 458(7239), 746—749 |
| [30] | Zhou X., Xu Q., Lei W., Zhang T., Qi X., Liu G., Deng K., Yu J., Small, 2014, 10, 674—679 |
| [31] | Li Y. L., Zhao J. Z., Zhao Y., Hao X. L., Hou Z. Y., Chem. Res. Chinese Universities, 2013, 29(6), 1040—1044 |
| [32] | Guan Y., Wang C., Wang B., Ma J., Xu X. M., Sun Y. F., Liu F. M., Liang X. S., Gao Y., Lu G. Y., Chem. Res. Chinese Universities, 2013, 29(5), 837—840 |
| [33] | Zhu J., Simon N. K. Y., Deng D., Cryst. Growth Des., 2014, 14(6), 2811—2817 |
| [34] | Liu X. H., Zhang J., Wu S. H., Yang D. J., Liu P., Zhang H. M., Wang S. R., Yao X. D., Zhu G. S., Zhao H. J., RSC Adv., 2012, 2, 6178—6184 |
| [35] | Qin W., Wang Y., Lin C. F., Hu X. Q., Dong C. Q., Energy Fuels, 2015, 29(2), 1210—1218 |
| [36] | Qin W., Lin C. F., Cheng W. L., Xiao X. B., Chem. J. Chinese Universities, 2015, 36(1), 116—123 |
| (覃吴, 林常枫, 程伟良, 肖显斌.高等学校化学学报,2015, 36(1), 116—123) | |
| [37] | Qin W., Lin C. F., Long D. T., Xiao X. B., Dong C. Q., Acta Phys. Chim. Sin., 2015, 31(4), 667—675 |
| [38] | Qin W., Wang Y., Dong C. Q., Zhang J. J., Chen Q. L., Yang Y. P., Applied Surface Science, 2013, 282, 718—723 |
| [39] | Chen D. Q., Shen L. H., Xiao J., Song T., Gu H. M., Zhang S. W., Proc. Chin. Soc. Electrical Eng., 2013, 33(20), 40—45 |
| (陈定千, 沈来宏, 肖军, 宋涛, 顾海明, 张恩文.中国电机工程学报,2013, 33(20), 40—45) | |
| [40] | Qin W., Lin C. F., Wang J. Y., Xiao X. B., Dong C. Q., Wei L., Energies, 2016, 9(8), 656 |
| [41] | Jankovic B., Adnadevic B., Menitus S., Thermochim. Acta, 2007, 456(1), 48—55 |
| [1] | DENG Guixian, LI Kongzhai, CHENG Xianming, GU Zhenhua, LU Chunqiang, ZHU Xing. Red Mud as Oxygen Carrier for Chemical Looping Combustion of Methane: Reactivity and Cyclic Performance† [J]. Chem. J. Chinese Universities, 2018, 39(2): 327. |
| [2] | ZENG Liangpeng, HUANG Fan, ZHU Xing, ZHENG Min, LI Kongzhai. Chemical Looping Conversion of Methane over CeO2-based and Co3O4-based Co3O4-CeO2 Oxygen Carriers:Controlling of Product Selectivity† [J]. Chem. J. Chinese Universities, 2017, 38(1): 115. |
| [3] | XUE Peng, WEI Yana, YUE Fan, ZHANG Yi, WANG Jide. Effects of Functional Groups in α-Amino Acids on Reversible Oxygenation Performance of Co(Ⅱ) Complexes† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1769. |
| [4] | QIN Wu, LIN Changfeng, CHENG Weiliang, XIAO Xianbin. Enhancing the Activity of Iron Based Oxygen Carrier via Surface Controlled Preparation for Lignite Chemical Looping Combustion† [J]. Chem. J. Chinese Universities, 2015, 36(1): 116. |
| [5] | WEN Hong-Mei, ZHANG Xu, LI Hui, YUE Fan, WANG Ji-De. Contrast Study of the Oxygenation of Co(Ⅱ) Complexes with Different Bi-/Poly-dentate Ligands [J]. Chem. J. Chinese Universities, 2013, 34(10): 2262. |
| [6] | ZHANG Xin-Cun, YUE Fan, HUANG Yan, FU Pei, CHENG Xiang, WANG Ji-De. Reversible Oxygenation Properties of 2,3-Diaminopropanoic Acid Cobalt Complex [J]. Chem. J. Chinese Universities, 2012, 33(07): 1370. |
| [7] | FU Ji-Hong, LI Jun-Fang, FU Pei, YUE Fan, ZHANG Xu-Long, WANG Ji-De*. Oxygenation Reaction of Histidine Cobalt Complex by Electrospray Tandem Mass Spectrometry [J]. Chem. J. Chinese Universities, 2011, 32(7): 1483. |
| [8] | YAN Kun-Ping*, HUI Wen-Li, LUO Chao, XIE Yu-Dou, PENG Ming-Li, CHEN Chao*. Preparation of Hemoglobin-based Oxygen Carrier with Low Oxygen Affinity and Arterial Blood Pressure Responses in Rat [J]. Chem. J. Chinese Universities, 2011, 32(4): 897. |
| [9] | LIN Juan-Juan, ZHUANG Zhao-Di, ZHOU Shi-Jian . Studies on Nonlinear Kinetics for KIO3-KSCN-H+ Reaction System [J]. Chem. J. Chinese Universities, 2000, 21(4): 613. |
| [10] | KONG De-Ling, JIA Yong-Hui, YU Yao-Ting, TONG Ming-Rong, Wang Zi-Hui. Oxygen Carrier Based on Immobilized Hemoglobin(Ⅱ)——Hemoglobin Covalently Bond to Periodate Oxycellulose [J]. Chem. J. Chinese Universities, 1998, 19(10): 1584. |
| [11] | DING Jun-Qi, HE Xu-Min, ZOU Wei, XIA Hai-Ping, DING Ma-Tai, CAI Qi-Rui . Studies on the Oxygen Enrichment of Chitosan Membrane(Ⅱ) [J]. Chem. J. Chinese Universities, 1992, 13(8): 1126. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||