

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 76.doi: 10.7503/cjcu20180448
• Organic Chemistry • Previous Articles Next Articles
JIANG Jing1, HUANG Yali2, ZHANG Qilong2,*( ), XU Hong2,*(
), XU Hong2,*( ), SUN Xiaohong3
), SUN Xiaohong3
Received:2018-06-20
Online:2019-01-10
Published:2018-12-18
Contact:
ZHANG Qilong,XU Hong
E-mail:gzuqlzhang@126.com;1738943269@qq.com
Supported by:CLC Number:
TrendMD:
JIANG Jing,HUANG Yali,ZHANG Qilong,XU Hong,SUN Xiaohong. Effects of Cucurbit[8]uril on the Solubility, Stability and Antioxidation of Cyanidin†[J]. Chem. J. Chinese Universities, 2019, 40(1): 76.
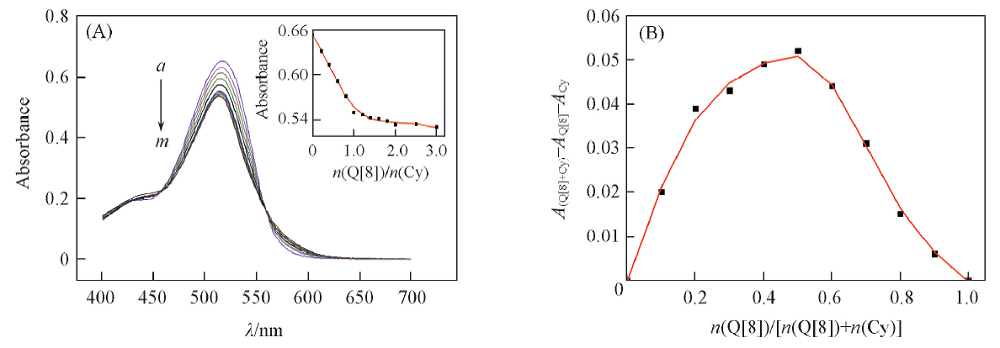
Fig.3 Changes in UV-Vis absorption spectra(A), the plot(inset) and Job’s plot(B) of Cy with Q[8](A) c(Cy)=20 μmol/L; n(Q[8])/n(Cy), a—m: 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.5, 3.0.
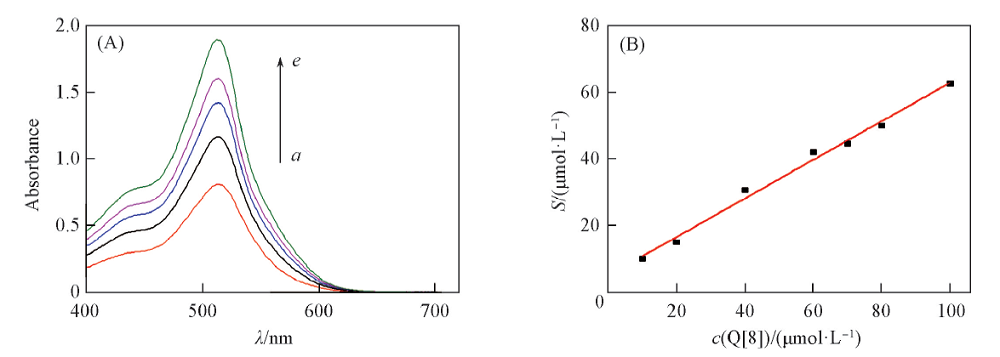
Fig.6 Changes in UV spectra of saturated Cy in the presence of different concentrations of Q8(A) and phase solubility graph of Cy in Q8 at λ=513 nm(B)c(Q[8])/(μmol·L-1): a. 20; b. 40; c. 60; d. 80; e. 100.
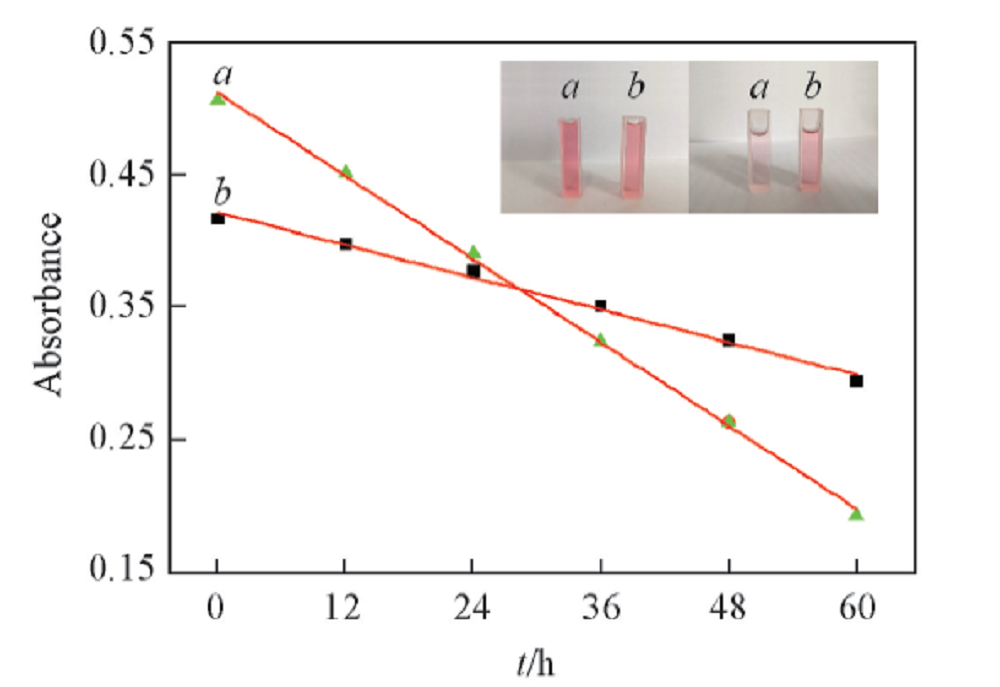
Fig.7 Changes in UV absorption intensity of Cy(a) and Q8/Cy(b) inclusion complex over timeInsets are changes in color over time. Left: 0 h; right: 60 h. a. c(Cy)=20 μmol/L; b. n([Q8])/n(Cy)=1:1.
| [1] | Zuo Y., Tian F., Cereals & Oils,2014, 27(7), 1—5 |
| (左玉, 田芳. 粮食与油脂, 2014, 27(7), 1—5) | |
| [2] | Yang X.J., Zhao X. Y., Ma Y., Wu Q. B., Ma R. S.,China Food Addit., 2005, (4), 40—42 |
| (杨秀娟, 赵晓燕, 马越, 吴秋波, 马荣山. 中国食品添加剂, 2005, (4), 40—42) | |
| [3] | Pina F., Melo M. J., Laia C. A., Parola A. J., Lima J. C., Chem. Soc. Rev.,2012, 41(2), 869—908 |
| [4] | Kong J. M., Chia L. S., Goh N. K., Chia T. F., Brouillard R., Phytochem.,2003, 64(5), 923—933 |
| [5] | Tong X. W., Han H., Wang L., Dong C., Chem. Res. Appl.,2017, 29(3), 401—406 |
| (童馨苇, 韩辉, 王丽, 董川. 化学研究与应用, 2017, 29(3), 401—406) | |
| [6] | Brouillard R., Lang J., Cheminform.,1990, 21(44), 755—761 |
| [7] | Bornsek S. M., Ziberna L., Polak T., Vanzo A., Ulrih N. P., Abram V., Tramer F., Passamonti S., Food Chem.,2012, 134(4), 1878—1884 |
| [8] | Colak N., Torun H., Gruz J., Strnad M., Hermosín-Gutiérrez I., Hayirlioglu-Ayaz S., Ayaz F. A., Food Chem.,2016, 201, 339—349 |
| [9] | Duarte L. J., Chaves V. C., Marcus V. P. D. S. N., Calvete E., Li M. C., Ciraolo E., Ghigo A., Hirsch E., Claudia M. O. S., Reginatto F. H., Dalmarco E. M., Food Chem.,2018, 247, 56—65 |
| [10] | Lamy S., Lafleur R., Bédard V., Moghrabi A., Barrette S., Gingras D., Béliveau R., J. Cell. Biochem.,2007, 100(1), 100—111 |
| [11] | Liang S., Jiang Z. C., Feng J., China J. Biotech,,2017, 37(11), 101—108 |
| (梁姗, 蒋子川, 冯均. 中国生物工程杂志, 2017, 37(11), 101—108) | |
| [12] | Liu Y., Song X., Han Y., Zhou F., Zhang D., Ji B., Hu J., Lv Y., Cai S., Wei Y., Gao F., Jia X., J. Agric. Food Chem.,2011, 59(1), 356—363 |
| [13] | Cassidy A., Bertoia M., Chiuve S., Flint A., Forman J., Rimm E. B., Am. J. Clin. Nutr.,2016, 104(3), 587—594 |
| [14] | Marko D., Puppel N., Tjaden Z., Jakobs S., Pahlke G., Mol. Nutr. Food Res.,2004, 48(4), 318—325 |
| [15] | Yi L., Chen C. Y., Jin X., Mi M. T., Yu B., Chang H., Ling W. H., Zhang T., FEBS Lett.,2010, 584(3), 583—590 |
| [16] | Yang L. Y., Liu Y. X., Fan Z. L., J. Food Nutr. Sci.,2017, 6(3), 137—142 |
| (杨蕾玉, 柳雅馨, 樊梓鸾. 食品与营养科学, 2017, 6(3), 137—142) | |
| [17] | Assaf K. I., Nau W. M., Chem. Soc. Rev.,2015, 44(2), 394—418 |
| [18] | Day A. I., Blanch R. J., Arnold A. P., Lorenzo S., Lewis G. R., Dance I., Angew. Chem. Int. Ed. Eng.,2002, 41(2), 275—277 |
| [19] | Mandadapu V., Day A. I., Ghanem A., Chirality,2014, 26(11), 712—723 |
| [20] | Liu J., Lan Y., Yu Z., Tan C. S., Parker R. M., Abell C., Scherman O. A., Acc. Chem. Res.,2017, 50(2), 208—217 |
| [21] | Bai D., Wang X., Gao Z. Z., Qiu S. C., Tao Z., Zhang J. X., Xiao X., Chinese J. Org. Chem.,2017, 37(8), 2022—2027 |
| (白东, 王鑫, 高中政, 邱胜超, 陶朱, 张建新, 肖昕. 有机化学, 2017, 37(8), 2022—2027) | |
| [22] | Yi J. M., Xiao X., Zhang Y. Q., Xue S. F., Tao Z., Zhang J. X., Acta Chim. Sin.,2014, 72(8), 949—955 |
| (易君明, 肖欣, 张云黔, 薛赛凤, 陶朱, 张建新. 化学学报, 2014, 72(8), 949—955) | |
| [23] | Qiang M., Yang H., Xu W. C., Tan Y. B., Chem. Res. Chinese Universities,2012, 28(6), 1101—1106 |
| [24] | Kuok K. I., Li S., Wyman I. W., Wang R., Ann. N. Y. Acad. Sci.,2017, 1398(1), 108—119 |
| [25] | Li S., Chan J. Y., Li Y., Bardelang D., Zheng J., Yew W. W., Chan D. P., Lee S. M., Wang R.,Org. Biomol. Chem. ,2016, 14(31), 7563—7569 |
| [26] | Li B., Meng Z., Li Q., Huang X., Kang Z., Dong H., Chen J., Sun J., Dong Y., Li J., Jia X., Sessler J. L., Meng Q., Li C., Chem. Sci.,2017, 8(6), 4458—4464 |
| [27] | Xu Z. L., Lian X. W., Li M. J., Zhang X. D., Wang Y., Tao Z., Zhang Q. J., Chem. Res. Chinese Universities,2017, 33(5), 736—741 |
| [28] | Uzunova V D., Cullinane C., Brix K., Org. Biomol. Chem.,2010, 8(9), 2037—2042 |
| [29] | Oun R., Floriano R. S., Isaacs L., Rowan E. G., Wheate N. J., Toxicol. Res.(Camb), 2014, 3(6), 447—455 |
| [30] | Held B., Tang H., Natarajan P., da S. C. P., de Oliveira Silva V., Bohne C., Quina F. H., Photochem. Photobiol. Sci.,2016, 15(6), 752—757 |
| [31] | Basílio N., Cabrita L., Pina F., J. Agric. Food Chem.,2015, 63(35), 7624—7629 |
| [32] | Basílio N., Pina F., Molecules,2016, 21(11), 1502 |
| [33] | Basílio N., Petrov V., Pina F. Chempluschem.,2016, 80(12), 1779—1785 |
| [1] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [2] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [3] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [4] | LI Weihui, LI Haobo, ZENG Cheng, LIANG Haoyue, CHEN Jiajun, LI Junyong, LI Huiqiao. Hot-pressed PVDF-based Difunctional Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210629. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | WANG Shoubai, WU Xiuming, WU Jinming, TANG Yanfeng, SHU Chen, ZHONG Min, HUANG Wei, YAN Deyue. Synthesis and Properties of Soluble Transparent Polyimides Containing tert-Butyl and Isobutyl Groups [J]. Chem. J. Chinese Universities, 2021, 42(9): 2944. |
| [7] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [8] | WANG Hongning, HUANG Li, SONG Fujiao, ZHU Ting, HUANG Weiqiu, ZHONG Jing, CHEN Ruoyu. Synthesis and VOCs Adsorption Properties of Hollow Carbon Nanospheres [J]. Chem. J. Chinese Universities, 2021, 42(6): 1704. |
| [9] | WANG Xianwei, KE Hongjun, YUAN Hang, LU Gewu, LI Liying, MENG Xiangsheng, SONG Shulin, WANG Zhen. High Temperature Resistant and Soluble Polyimide Resins and Their Composites [J]. Chem. J. Chinese Universities, 2021, 42(6): 2041. |
| [10] | WANG Kunhua, YAO Jisong, YANG Junnan, SONG Yonghui, LIU Yuying, YAO Hongbin. Synthesis and Device Optimization of Highly Efficient Metal Halide Perovskite Light-emitting Diodes [J]. Chem. J. Chinese Universities, 2021, 42(5): 1464. |
| [11] | LIU Yao, DENG Zhengtao. Fast Synthesis of Highly Luminescent Two-dimensional Tin-halide Perovskites by Anti-solvent Method [J]. Chem. J. Chinese Universities, 2021, 42(12): 3774. |
| [12] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| [13] | WANG Tingting, LEI Yuhan, LIN Yujuan, HUANG Jialing, LIU Cuie, ZHENG Fengying, LI Shunxing. Preparation of Liposome-terminated CsPbX3(X=Cl,Br,I) Nanocrystals and Applications in Light-emitting Diode Devices [J]. Chem. J. Chinese Universities, 2020, 41(8): 1896. |
| [14] | ZHANG Danwei, WANG Hui, LI Zhanting. Water-soluble Regular Three-dimensional Supramolecular and Covalent Organic Polymers † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1139. |
| [15] | WU Chunxiao, AI Xin, CHEN Yingxin, CUI Zhiyuan, LI Feng. Effects of Introducing Halogen Atoms to Biphenylmethyl Radical on Photostability, Photophysical and Electroluminescent Properties † [J]. Chem. J. Chinese Universities, 2020, 41(5): 972. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||