

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (3): 551.doi: 10.7503/cjcu20140694
• Physical Chemistry • Previous Articles Next Articles
JI Lei*( ), YU Ruimin, WANG Haoren, CHEN Liduo, WANG Huaiyuan
), YU Ruimin, WANG Haoren, CHEN Liduo, WANG Huaiyuan
Received:2014-07-24
Online:2015-03-10
Published:2015-02-04
Contact:
JI Lei
E-mail:jileiwipm@163.com
CLC Number:
TrendMD:
JI Lei, YU Ruimin, WANG Haoren, CHEN Liduo, WANG Huaiyuan. In-situ Synthesis of BiOCl/NaBiO3 Composites and Their Photocatalytic Activities†[J]. Chem. J. Chinese Universities, 2015, 36(3): 551.
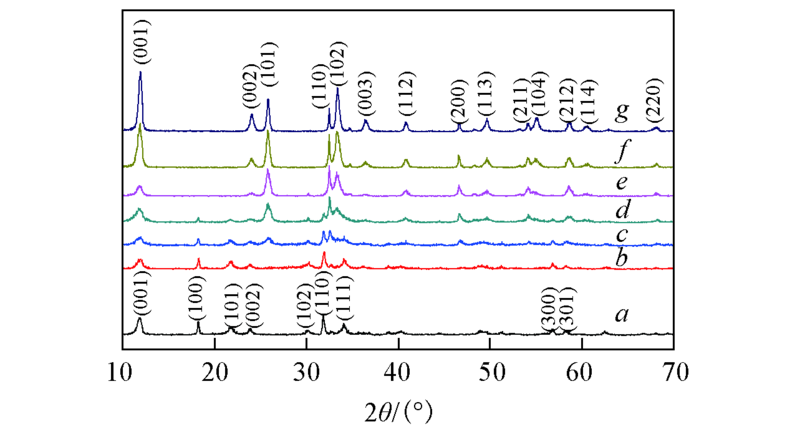
Fig.1 XRD patterns of the samples a. NaBiO3; b. 12.1%BiOCl/NaBiO3; c. 27.4%BiOCl/NaBiO3; d. 47.6%BiOCl/NaBiO3; e. 60.4%BiOCl/NaBiO3; f. 75.5%BiOCl/NaBiO3; g. BiOCl.
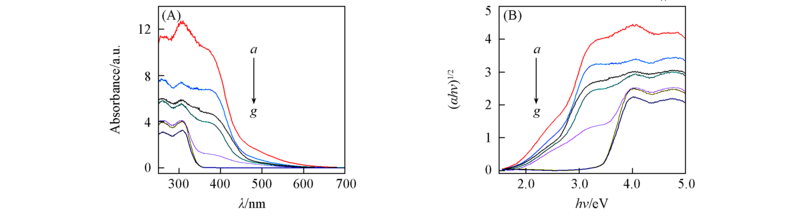
Fig.3 UV-Vis diffuse reflectance spectra(A) and (αhν)1/2-hν curves(B) of different samples a. NaBiO3; b. 12.1%BiOCl/NaBiO3; c. 27.4%BiOCl/NaBiO3; d. 47.6%BiOCl/NaBiO3; e. 60.4%BiOCl/NaBiO3;f. 75.5%BiOCl/NaBiO3; g. BiOCl.
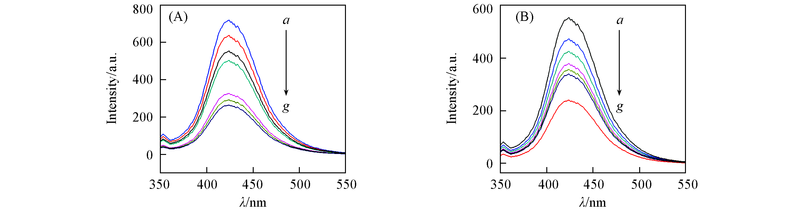
Fig.7 ·OH trapping PL spectra changes of different samples observed during visible light irradiation(A) and UV light irradiation(B) (A) a. 27.4%BiOCl/NaBiO3; b. 12.1%BiOCl/NaBiO3; c. NaBiO3; d. 47.6%BiOCl/NaBiO3; e. 60.4%BiOCl/NaBiO3; f. 75.5%BiOCl/NaBiO3; g. BiOCl. (B) a. 47.6%%BiOCl/NaBiO3; b. 27.4%BiOCl/NaBiO3; c. 12.1%BiOCl/NaBiO3; d. 60.4%BiOCl/NaBiO3; e. 75.5%BiOCl/NaBiO3; f. BiOCl; g. NaBiO3.
| [1] | Fujishima A., Honda K., Nature,1972, 238(5358), 37—38 |
| [2] | Carey J. H., Lawrence J., Tosine H. M., Bull. Environ. Contam. Toxicol., 1976, 16(6), 697—701 |
| [3] | Linsebigler A. L., Lu G., Yates J. T., Chem. Rev., 1995, 95, 735—758 |
| [4] | Zhu S. Y., Xu D. F., Fang S., Geng Z., Yang X., Chem. J. Chinese Universities,2014, 35(6), 1286—1292 |
| (朱遂一, 徐东方, 方帅, 耿直, 杨霞. 高等学校化学学报, 2014, 35(6), 1286—1292) | |
| [5] | Zhang Z., Chen A. P., Ma L., He H. B., Li C. Z., Chem. J. Chinese Universities,2013, 34(3), 656—661 |
| (张哲, 陈爱平, 马磊, 何洪波, 李春忠. 高等学校化学学报, 2013, 34(3), 656—661) | |
| [6] | Zhang L. S., Wang H. L., Chen Z. G., Wong P. K., Liu J. S., Appl. Catal. B: Environ., 2011, 106(1/2), 1—13 |
| [7] | Liu Y. F., Zhu Y. Y., Xu J., Bai X. J., Zong R. L., Zhu Y. F., Appl. Catal. B: Environ., 2013, 142/143, 561—567 |
| [8] | Yu J. Q., Zhang Y., Kudo A., J. Solid State Chem., 2009, 182(2), 223—228 |
| [9] | Lin X. P., Huang T., Huang F. Q., Wang W. D., Shi J. L., J. Phys. Chem. B,2006, 110(48), 24629—24634 |
| [10] | Wang W. D., Huang F. Q., Lin X. P., Scripta Mater., 2007, 56(8), 669—672 |
| [11] | Wang W. D., Huang F. Q., Lin X. P., Yang J. H., Catal. Commun., 2008, 9(1), 8—12 |
| [12] | Wang C. H., Shao C. L., Liu Y. C., Zhang L., Scripta Mater., 2008, 59(3), 332—335 |
| [13] | Wang C. Y., Zhang H., Li F., Zhu L. Y., Environ. Sci. Technol., 2010, 44(17), 6843—6848 |
| [14] | Chen F., Liu H. Q., Bagwasi S., Shen X. X., Zhang J. L., J. Photochem. Photobiol. A: Chem., 2010, 215(1), 76—80 |
| [15] | Gondal M. A., Chang X. F., Yamani Z. H., Chem. Eng. J., 2010, 165(1), 250—257 |
| [16] | Huang W. L., Zhu Q. S., Comput. Mater. Sci., 2008, 43(4), 1101—1108 |
| [17] | Mao X. M., Li X. L., Wang Y. W., Fan C. M., Zhang H., Chem. Eng. J., 2014, 247, 241—249 |
| [18] | Zhang K. L., Liu C. M., Huang F. Q., Zheng C., Wang W. D., Appl. Catal. B: Environ., 2006, 68(3/4), 125—129 |
| [19] | Cao B. C., Dong P. Y., Cao S., Wang Y. H., J. Am. Ceram. Soc., 2013, 96(2), 544—548 |
| [20] | Cheng H., Huang B., Qin X., Chem. Commun., 2012, 48(1), 97—99 |
| [21] | Liu B. T., Xu W. J., Sun T., Chen M., Tian L. L., Wang J. B., New J. Chem., 2014, 38(6), 2273—2277 |
| [22] | Shamaila S., Sajjad A. K. L., Chen F., Zhang J. L., J. Colloid Interface Sci., 2011, 356(2), 465—472 |
| [23] | Chai S. Y., Kim Y. J., Jung M. H., Chakraborty A. K., Jung D., Lee W. I., J. Catal., 2009, 262(1), 144—149 |
| [24] | Nethercot A .H., Phys. Rev. Lett., 1974, 33(18), 1088—1091 |
| [25] | Nasr C., Vinodgopal K., Fisher L., Hotchandani S., Chattopadhyay A. K., Kamat P. V., J. Phys. Chem., 1996, 100(20), 8436—8442 |
| [26] | Soni S. S., Henderson M. J., Bardeau J. F., Gibaud A., Adv. Mater., 2008, 20(8), 1493—1498 |
| [27] | Yin M. C., Li Z. S., Kou J. H., Zou Z. G., Environ. Sci. Technol., 2009, 43(21), 8361—8366 |
| [28] | Li G. T., Wong K. H., Zhang X. W., Hu C., Yu J. C., Chan R. C. Y., Wong P. K., Chemosphere,2009, 76(9), 1185—1191 |
| [29] | Zhang L. S., Wong K. H., Yip H. Y., Hu C., Yu J. C., Chan C. Y., Wong P. K., Erviron. Sci. Technol., 2010, 44(4), 1392—1398 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [3] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [4] | LIU Shuwei, JIN Hao, YIN Wanzhong, ZHANG Hao. Gemcitabine/polypyrrole Composite Nanoparticles for Chemo-photothermal Combination Ovarian Cancer Therapy [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220345. |
| [5] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [6] | ZHOU Leilei, CHENG Haiyang, ZHAO Fengyu. Research Progress of CO2 Hydrogenation over Pd-based Heterogeneous Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220279. |
| [7] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [8] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [9] | QIU Liqi, YAO Xiangyang, HE Liangnian. Visible-light-driven Selective Reduction of Carbon Dioxide Catalyzed by Earth-abundant Metalloporphyrin Complexes [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220064. |
| [10] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [11] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [12] | REN Nana, XUE Jie, WANG Zhifan, YAO Xiaoxia, WANG Fan. Effects of Thermodynamic Data on Combustion Characters of 1,3-Butadiene [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220151. |
| [13] | GAO Zhiwei, LI Junwei, SHI Sai, FU Qiang, JIA Junru, AN Hailong. Analysis of Gating Characteristics of TRPM8 Channel Based on Molecular Dynamics [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220080. |
| [14] | YU Pengdong, GUAN Xinghua, WANG Dongdong, XIN Zhirong, SHI Qiang, YIN Jinghua. Preparation and Properties of Novel Optical and Thermal Dual Response Shape Memory Polymers [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220085. |
| [15] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||