

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 531.doi: 10.7503/cjcu20130659
• Organic Chemistry • Previous Articles Next Articles
LI Yingjun1,*( ), LI Chunyan1, JIN Kun2, SHAO Xin1, ZHOU Xiaoxia1, LI Lina1, ZHAO Nan1, YU Yang1, LUO Tongchuan1
), LI Chunyan1, JIN Kun2, SHAO Xin1, ZHOU Xiaoxia1, LI Lina1, ZHAO Nan1, YU Yang1, LUO Tongchuan1
Received:2013-07-15
Online:2014-03-10
Published:2013-09-13
Contact:
LI Yingjun
E-mail:chemlab.lnnu@163.com
Supported by:CLC Number:
TrendMD:
LI Yingjun, LI Chunyan, JIN Kun, SHAO Xin, ZHOU Xiaoxia, LI Lina, ZHAO Nan, YU Yang, LUO Tongchuan. Synthesis and Biological Activity of Novel Imidazo[2,1-b][1,3,4]thiadiazole Derivatives†[J]. Chem. J. Chinese Universities, 2014, 35(3): 531.
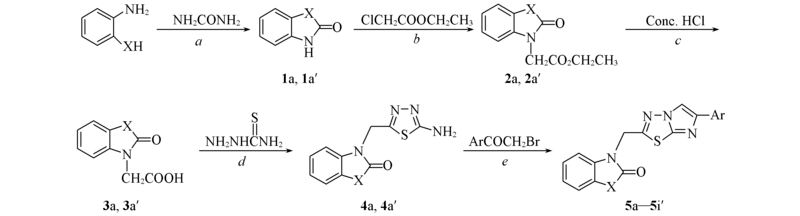
Scheme 1 Synthetic routes of the target compounds X=O; Ar=C6H5(5a), 4-CH3OC6H4(5b), 4-BrC6H4(5c), 4-ClC6H4(5d), 4-FC6H4(5e), 3-O2NC6H4(5f), 4-O2NC6H4(5g), 2-C10H7(5h), Ph-C6H4(5i); X=S, Ar=C6H5(5a'), 4-CH3OC6H4(5b'), 4-BrC6H4(5c'), 4-ClC6H4(5d'), 4-FC6H4(5e'), 3-O2NC6H4(5f'), 4-O2NC6H4(5g'), 2-C10H7(5h'), Ph-C6H4(5i').Reagents and conditions: a. Heating, 1 h, N2; b. Et3N, DMF, reflux, 7 h; c. dioxane, reflux, 2 h; d. POCl3, reflux, 4 h, NaOH; e. dry EtOH, reflux, 17 h, Na2CO3.
| Compd. | X | Ar | Responseb(%) | Compd. | X | Ar | Responseb(%) |
|---|---|---|---|---|---|---|---|
| 5a | O | C6H5 | 49.64±38.06 | 5a' | S | C6H5 | 69.37±33.14 |
| 5b | O | 4-CH3OC6H4 | 42.68±36.56 | 5b' | S | 4-CH3OC6H4 | 49.77±31.05 |
| 5c | O | 4-BrC6H4 | 94.94±15.95 | 5c' | S | 4-BrC6H4 | 29.23±9.46 |
| 5d | O | 4-ClC6H4 | 87.54±8.47 | 5d' | S | 4-ClC6H4 | 73.34±23.58 |
| 5e | O | 4-FC6H4 | 75.73±24.03 | 5e' | S | 4-FC6H4 | 85.03±18.02 |
| 5f | O | 3-O2NC6H4 | 79.76±13.04 | 5f' | S | 3-O2NC6H4 | 94.26±8.60 |
| 5g | O | 4-O2NC6H4 | 90.60±16.56 | 5g' | S | 4-O2NC6H4 | 105.16±4.92 |
| 5h | O | 2-C10H7 | 96.93±5.00 | 5h' | S | 2-C10H7 | 92.77±13.59 |
| 5i | O | 4-Ph-C6H4 | 107.87±8.98 | 5i' | S | 4-Ph-C6H4 | 89.59±13.50 |
Table 1 Responses of target compounds 5a—5i' to β2-ARa
| Compd. | X | Ar | Responseb(%) | Compd. | X | Ar | Responseb(%) |
|---|---|---|---|---|---|---|---|
| 5a | O | C6H5 | 49.64±38.06 | 5a' | S | C6H5 | 69.37±33.14 |
| 5b | O | 4-CH3OC6H4 | 42.68±36.56 | 5b' | S | 4-CH3OC6H4 | 49.77±31.05 |
| 5c | O | 4-BrC6H4 | 94.94±15.95 | 5c' | S | 4-BrC6H4 | 29.23±9.46 |
| 5d | O | 4-ClC6H4 | 87.54±8.47 | 5d' | S | 4-ClC6H4 | 73.34±23.58 |
| 5e | O | 4-FC6H4 | 75.73±24.03 | 5e' | S | 4-FC6H4 | 85.03±18.02 |
| 5f | O | 3-O2NC6H4 | 79.76±13.04 | 5f' | S | 3-O2NC6H4 | 94.26±8.60 |
| 5g | O | 4-O2NC6H4 | 90.60±16.56 | 5g' | S | 4-O2NC6H4 | 105.16±4.92 |
| 5h | O | 2-C10H7 | 96.93±5.00 | 5h' | S | 2-C10H7 | 92.77±13.59 |
| 5i | O | 4-Ph-C6H4 | 107.87±8.98 | 5i' | S | 4-Ph-C6H4 | 89.59±13.50 |
| [1] | Namath A., Chen C., Agrawal R., Patterson A. J., Seminars in Anesthesia, Perioperative Medicine and Pain,2007, 26(1), 2—9 |
| [2] | Yu J. T., Wang N. D., Ma T., Jiang H., Guan J., Tan L., Brain Res. Bull., 2011, 84(2), 111—117 |
| [3] | Schuller H. M., Cekanova M., Lung Cancer,2005, 49(1), 35—45 |
| [4] | Yu N. N., Wang X. X., Yu J. T., Wang N. D., Lu R. C., Miao D., Tian Y., Tan L., Brain Res., 2010, 1317, 305—310 |
| [5] | Jindal D. P., Singh B., Coumar M. S., Bruni G., Massarelli P., Bioorg. Chem., 2005, 33(4), 310—324 |
| [6] | Liang D. Y., Shi X. Y., Li X. Q., Li J., Clark J. D., Behav. Brain Res., 2007, 181(1), 118—126 |
| [7] | Denda M., Fuziwara S., Inoue K., J. Invest. Dermatol., 2003, 121(1), 142—148 |
| [8] | Noolvi M. N., Patel H. M., Singh N., Gadad A. K., Cameotra S. S., Badiger A., Eur. J. Med. Chem., 2011, 46(9), 4411—4418 |
| [9] | Karki S. S., Panjamurthy K., Kumar S., Nambiar M., Ramareddy S. A., Chiruvella K. K., Raghavan S. C., Eur. J. Med. Chem., 2011, 46(6), 2109—2116 |
| [10] | Noolvi M. N., Patel H. M., Kamboj S., Kaur A., Mann V., Eur. J. Med. Chem., 2012, 56, 56—69 |
| [11] | Kolavi G., Hegde V., Khazia I. A., Gadad P., Bioorg. Med. Chem., 2006, 14(9), 3069—3080 |
| [12] | Lamani R. S., Shetty N. S., Kamble R. R., Khazi I. A. M., Eur. J. Med. Chem., 2009, 44(7), 2828—2833 |
| [13] | Gadad A. K., Palkar M. B., Anand K., Noolvi M. N., Boreddy T. S., Wagwade J., Bioorg. Med. Chem., 2008, 16(1), 276—283 |
| [14] | Jadhav V. B., Kulkarni M. V., Rasal V. P., Biradar S. S., Vinay M. D., Eur. J. Med. Chem., 2008, 43(8), 1721—1729 |
| [15] | Salgın-GökŞen U., Gökhan-Kelekçi N., GöktaŞ Ö., Köysal Y., Kılıç E., IŞık Ş., Aktay G., Özalp M., Bioorg. Med. Chem., 2007, 15(17), 5738—5751 |
| [16] | Önkol T., Çakir B., Şahin M. F., Yildirim E., Erol K., Turk. J. Chem., 2004, 28(4), 461—468 |
| [17] | Erol D. D., Aytemir M. D., Yulug N., Eur. J. Med. Chem., 1996, 31(9), 73l—734 |
| [18] | Petrov O., Gerova M., Petrova K., Ivanova Y., J. Heterocyclic Chem., 2009, 46(1), 44—48 |
| [19] | Ucar H., van Derpoorten K., Cacciaguerra S., Spampinato S., Stables J. P., Depovere P., Isa M., Masereel B., Delarge J., Poupaert J. H., J. Med. Chem., 1998, 41(7), 1138—1145 |
| [20] | Lamani R. S., Shetty N. S., Kamble R. R., Khazi I. A. M., Eur. J. Med. Chem., 2009, 44(7), 2828—2833 |
| [21] | Close W. J., Tiffany B. D., Spielman M. A., J. Am. Chem. Soc., 1949, 71(4), 1265—1268 |
| [22] | Li Y. J., Liu L. J., Jin K., Sun S. Q., Xu Y. T., Acta Chim. Sinica,2010, 68(16), 1577—1584 |
| (李英俊, 刘丽军, 靳焜, 孙淑琴, 许永廷. 化学学报, 2010, 68(16), 1577—1584) | |
| [23] | Potts K. T., Bhattacharjee D., Kanemasa S., J. Org. Chem., 1980, 45(24), 4985—4988 |
| [24] | Cui L., Chen J. S., Liu F. M., Xie Z. F., Sun Y. D., Wang H. Y., Chin. J. Org. Chem., 2006, 26(7), 963—966 |
| (崔蕾, 陈家胜, 刘方明, 解正峰, 孙亚栋, 王厚勇. 有机化学, 2006, 26(7), 963—966) | |
| [25] | Li Y. J., Li C. Y., Jin K., An J., Wang L. L., Zhao X., Chinese J. Magn. Reson., 2010, 27(4), 617—622 |
| (李英俊, 李春燕, 靳焜, 安静, 王雷雷, 赵星. 波谱学杂志, 2010, 27(4), 617—622) |
| [1] | ZHANG Xiaofei, LIU Jiaxin. Visible Light Induced Cyclization of O-Alkenylcarboxanilide to 2-Quinolinone [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220274. |
| [2] | HUANG Qiuhong, LI Wenjun, LI Xin. Organocatalytic Enantioselective Mannich-type Addition of 5H-Oxazol-4-ones to Isatin Derived Ketimines [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220131. |
| [3] | GE Yicong, NIE Wanli, SUN Guofeng, CHEN Jiaxuan, TIAN Chong. Silver-catalyzed [5+1] Cyclization of 2-Vinylanilines with Benzisoxazoles [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220142. |
| [4] | LI Jing, SU Wei, WANG Xueyuan, FU Peng, SUN Yan. Synthesis and Characterization of Antihypertensive Drug Aranidipine and Its Related Impurities [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210663. |
| [5] | ZHAO Ying, QIAO Ling, ZHAO Guofeng, CHEN Li. Synthesis and Biological Activity of Lycorine Derivatives Containing Malate Ester [J]. Chem. J. Chinese Universities, 2021, 42(9): 2789. |
| [6] | LI Pengjie, ZHOU Chunni, WANG Zetian, ZHENG Ziang, ZHANG Yumin, WANG Liang, XIAO Biao. Rhodium⁃catalyzed C—H Alkenylation of Indoles and Vinyltriethoxysilane [J]. Chem. J. Chinese Universities, 2021, 42(8): 2450. |
| [7] | DONG Xinrui, XIA Zhe, WANG Zhenxue, BIAN Qiang, LI Huabin. Design, Synthesis and Biological Activity of Pyrazole-4-carboxamides Compounds Containing 1,2,4,5-Tetrasubstituted Phenyl [J]. Chem. J. Chinese Universities, 2020, 41(12): 2759. |
| [8] | NAN Jiang, CHEN Pu, MA Yangmin. Acid-promoted [5+1] Annulation of 2-Vinylanilines with Diazo Compounds to 2-Arylquinolines [J]. Chem. J. Chinese Universities, 2020, 41(11): 2457. |
| [9] | PAN Yixiao, LI Yanwen, HAN Jiahong, ZHAO Haoqiang, FENG Yu, DING Xiangyuan, XU Lijin, FAN Qinghua, SHI Qian. Synthesis of 1,2,3,4-Tetrahydroquinoxalines Through a One-pot Tandem Reaction Involving Cyclization and Hydrogenation of Imine and Amide Moieties [J]. Chem. J. Chinese Universities, 2020, 41(10): 2239. |
| [10] | MA Jinyu, LIU Shuanglei, ZHANG Zhenguo, JIN Junyang, JIA Zhenhua. B(C6F5)3-Catalyzed Synthesis of 3,3′-Bisindolylmethane Derivatives [J]. Chem. J. Chinese Universities, 2020, 41(10): 2225. |
| [11] | LIU Chang, ZHANG Pengfei, LI Pengfei. Asymmetric Organocatalytic Enantioselective [1+4]-Annulation of Morita-Baylis-Hillman Carbonates with Thiazolyl Enones for Assembling of Dihydrofurans Featuring Thiazole Skeleton [J]. Chem. J. Chinese Universities, 2020, 41(10): 2272. |
| [12] | CHEN Danyi, ZHANG Fumei, HE Dan, ZHANG Zimei, ZHONG Fen, WEN Simiaomiao, LIU Qixing, ZHOU Haifeng. Synthesis of Chiral Phenylbenzothiazole Methanol via Transfer Hydrogenation Catalyzed by Ruthenium Complexes† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2264. |
| [13] | ZHANG Chenglu, SUN Yuedong, WANG Jing, HE Yu, ZHANG Yanpeng, ZHANG Lu, SONG Fulu. Synthesis and Application of Quinolinone Derivative Fluorescent Probe for High Selective Detection of Hg2+ [J]. Chem. J. Chinese Universities, 2020, 41(8): 1785. |
| [14] | REN Yushuang, GUO Yuanyuan, LIU Xueyi, SONG Jie, ZHANG Chuan. Platinum(Ⅳ) Prodrug-grafted Phosphorothioate DNA and Its Self-assembled Nanostructure for Targeted Drug Delivery [J]. Chem. J. Chinese Universities, 2020, 41(8): 1721. |
| [15] | ZHOU Chunni, ZHENG Ziang, PENG Wangming, WANG Hongbo, ZHANG Yumin, WANG Liang, XIAO Biao. Microwave Assisted Rhodium-catalyzed C—H Activation/cyclization of Diaryl Phosphoramides and Alkynes † [J]. Chem. J. Chinese Universities, 2020, 41(4): 726. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||