

高等学校化学学报 ›› 2026, Vol. 47 ›› Issue (2): 20250225.doi: 10.7503/cjcu20250225
徐一铭, 史燚威, 王鑫, 朱志慧, 宋志国( ), 王敏(
), 王敏( )
)
收稿日期:2025-08-16
出版日期:2026-02-10
发布日期:2025-10-16
通讯作者:
宋志国,王敏
E-mail:songzhiguo@qymail.bhu.edu.cn;wangmin@qymail.bhu.edu.cn
基金资助:
XU Yiming, SHI Yiwei, WANG Xin, ZHU Zhihui, SONG Zhiguo( ), WANG Min(
), WANG Min( )
)
Received:2025-08-16
Online:2026-02-10
Published:2025-10-16
Contact:
SONG Zhiguo, WANG Min
E-mail:songzhiguo@qymail.bhu.edu.cn;wangmin@qymail.bhu.edu.cn
Supported by:摘要:
分别以对氨基苯磺酸根(4-ABS-)和对甲基苯磺酸根(4-MBS-)为主配体、 4,4′-联吡啶(4,4′-bipy)为辅助配体, 与Zn(II)盐通过溶剂热反应合成了两种锌基配合物: [Zn(4,4′-bipy)(H2O)4]·(4-ABS)2(1, CCDC: 2171834)和 [Zn(4,4′-bipy)(H2O)4]·(4-MBS)2(2, CCDC: 2225758). 采用单晶X射线衍射(SXRD)、 傅里叶变换红外光谱(FTIR)、 热重分析(TGA)、 粉末X射线衍射(PXRD)、 氮气吸附/脱附测试和场发射扫描电子显微镜(SEM)对配合物进行了表征. 考察了配合物1和2催化合成2,3-二苯基-2,3-二氢喹唑啉-4(1H)-酮反应的性能, 并以催化效果较好的配合物1进行底物普适性实验. 结果表明, 在无溶剂条件下, 使用少量催化剂, 在短时间内可得到较高产率的产物, 并且该绿色工艺适用于多种不同的原料胺/铵和含不同取代基的芳香醛. 此外, 利用密度泛函理论(DFT)对配合物1和3种反应物进行结构优化, 通过计算分析前线分子轨道(FMO), 推测反应顺序及配合物1对3种反应物的核心活性区; 通过分析分子表面静电势(ESP)、 平均局部离子化能(ALIE)和Mulliken电荷进一步预测了配合物1的活性位点和反应底物的反应位点. 最后, 结合DFT阐述了配合物多位点协同催化的反应机理.
中图分类号:
TrendMD:
徐一铭, 史燚威, 王鑫, 朱志慧, 宋志国, 王敏. 新型三维单核Zn(II)配合物多位点协同催化单和双取代喹唑啉酮的绿色合成. 高等学校化学学报, 2026, 47(2): 20250225.
XU Yiming, SHI Yiwei, WANG Xin, ZHU Zhihui, SONG Zhiguo, WANG Min. Green Synthesis of Mono- and Disubstituted Quinazolinones by Multi-site Synergistic Catalysis of Novel Three-dimensional Single-nuclear Zn(II) Complexes. Chem. J. Chinese Universities, 2026, 47(2): 20250225.
| Complex | 1 | 2 | Complex | 1 | 2 |
|---|---|---|---|---|---|
| Formula | C22H8N4O10S2Zn | C24H30N2O10S2Zn | γ/(°) | 90 | 90 |
| Formula weight | 617.81 | 635.99 | V/nm3 | 1.33902(12) | 1.36890(2) |
| Crystal system | Monoclinic | Monoclinic | Z | 2 | 2 |
| Space group | P21/c | P21/c | Dc/(g·cm-3) | 1.532 | 1.543 |
| a/nm | 1.12530(5) | 1.12602(9) | F(000) | 620 | 660 |
| b/nm | 0.82811(4) | 0.81423(7) | Reflections collected | 19628 | 43157 |
| c/nm | 1.43698(8) | 1.49445(13) | Goodness⁃of⁃fit on F2 | 1.051 | 1.127 |
| α/(°) | 90 | 90 | R1, ωR2* | 0.0454, 0.1247 | 0.0502, 0.1141 |
| β/(°) | 90.543(2) | 92.426(4) | R1, ωR2(all data)* | 0.0546, 0.1328 | 0.0694, 0.1322 |
Table 1 Crystallographic parameters of complexes 1 and 2
| Complex | 1 | 2 | Complex | 1 | 2 |
|---|---|---|---|---|---|
| Formula | C22H8N4O10S2Zn | C24H30N2O10S2Zn | γ/(°) | 90 | 90 |
| Formula weight | 617.81 | 635.99 | V/nm3 | 1.33902(12) | 1.36890(2) |
| Crystal system | Monoclinic | Monoclinic | Z | 2 | 2 |
| Space group | P21/c | P21/c | Dc/(g·cm-3) | 1.532 | 1.543 |
| a/nm | 1.12530(5) | 1.12602(9) | F(000) | 620 | 660 |
| b/nm | 0.82811(4) | 0.81423(7) | Reflections collected | 19628 | 43157 |
| c/nm | 1.43698(8) | 1.49445(13) | Goodness⁃of⁃fit on F2 | 1.051 | 1.127 |
| α/(°) | 90 | 90 | R1, ωR2* | 0.0454, 0.1247 | 0.0502, 0.1141 |
| β/(°) | 90.543(2) | 92.426(4) | R1, ωR2(all data)* | 0.0546, 0.1328 | 0.0694, 0.1322 |
| Bond | 1 | 2 | Bond | 1 | 2 |
|---|---|---|---|---|---|
| Zn1—O1W | 0.2147(3) | 0.2149(3) | Zn1—O1W#1 | 0.2147(3) | 0.2149(3) |
| Zn1—O2W | 0.2152(3) | 0.2170(3) | Zn1—O2W#1 | 0.2152(3) | 0.2170(3) |
| Zn1—N1 | 0.2083(3) | 0.2083(3) | Zn1—N1#1 | 0.2083(3) | 0.2083(3) |
| O1W—Zn1—O1W#1 | 180 | 180 | O1W—Zn1—O2W | 89.46(12) | 89.48(13) |
| O1W—Zn1—O2W#1 | 90.53(12) | 90.52(13) | O2W#1—Zn1—O2W | 180 | 180 |
| N1—Zn1—O1W | 87.83(10) | 93.79(11) | N1—Zn1—O2W | 92.84(10) | 92.39(11) |
| N1—Zn1—N1#1 | 180 | 180 | N1—Zn1—O1W#1 | 92.17(10) | 86.21(11) |
| N1—Zn1—O2W#1 | 87.16(10) | 87.61(11) |
Table 2 Selected bond lengths(nm) and bond angles(°) of complexes 1 and 2*
| Bond | 1 | 2 | Bond | 1 | 2 |
|---|---|---|---|---|---|
| Zn1—O1W | 0.2147(3) | 0.2149(3) | Zn1—O1W#1 | 0.2147(3) | 0.2149(3) |
| Zn1—O2W | 0.2152(3) | 0.2170(3) | Zn1—O2W#1 | 0.2152(3) | 0.2170(3) |
| Zn1—N1 | 0.2083(3) | 0.2083(3) | Zn1—N1#1 | 0.2083(3) | 0.2083(3) |
| O1W—Zn1—O1W#1 | 180 | 180 | O1W—Zn1—O2W | 89.46(12) | 89.48(13) |
| O1W—Zn1—O2W#1 | 90.53(12) | 90.52(13) | O2W#1—Zn1—O2W | 180 | 180 |
| N1—Zn1—O1W | 87.83(10) | 93.79(11) | N1—Zn1—O2W | 92.84(10) | 92.39(11) |
| N1—Zn1—N1#1 | 180 | 180 | N1—Zn1—O1W#1 | 92.17(10) | 86.21(11) |
| N1—Zn1—O2W#1 | 87.16(10) | 87.61(11) |
| Complex | D—H···A | D—H/nm | H···A/nm | D···A/nm | ∠DHA/(°) |
|---|---|---|---|---|---|
| 1 | O1W—H1WA···O(1) | 0.085 | 0.193 | 0.2765(4) | 165.1 |
| O1W—H1WB···O(2)#3 | 0.085 | 0.204 | 0.2861(4) | 160.9 | |
| O2W—H2WA···O(3)#1 | 0.085 | 0.195 | 0.2792(4) | 170.9 | |
| 2 | O2W—H2WA···O(2)#3 | 0.085 | 0.200 | 0.2817(5) | 159.6 |
| O2W—H2WB···O(3)#4 | 0.085 | 0.213 | 0.2859(5) | 142.9 | |
| O1W—H1WA···O(1) | 0.085 | 0.214 | 0.2827(4) | 137.8 | |
| O1W—H1WB···O(1)#3 | 0.085 | 0.220 | 0.2878(2) | 136.6 |
Table 3 Hydrogen bond lengths and bond angles of complexes 1 and 2*
| Complex | D—H···A | D—H/nm | H···A/nm | D···A/nm | ∠DHA/(°) |
|---|---|---|---|---|---|
| 1 | O1W—H1WA···O(1) | 0.085 | 0.193 | 0.2765(4) | 165.1 |
| O1W—H1WB···O(2)#3 | 0.085 | 0.204 | 0.2861(4) | 160.9 | |
| O2W—H2WA···O(3)#1 | 0.085 | 0.195 | 0.2792(4) | 170.9 | |
| 2 | O2W—H2WA···O(2)#3 | 0.085 | 0.200 | 0.2817(5) | 159.6 |
| O2W—H2WB···O(3)#4 | 0.085 | 0.213 | 0.2859(5) | 142.9 | |
| O1W—H1WA···O(1) | 0.085 | 0.214 | 0.2827(4) | 137.8 | |
| O1W—H1WB···O(1)#3 | 0.085 | 0.220 | 0.2878(2) | 136.6 |
| Entry | R1 | R2 | Catalyst | Time/min | Yield(%) | m.p./℃ | |
|---|---|---|---|---|---|---|---|
| Found | Repored | ||||||
| 1* | 262 | 46.2 | |||||
| 2 | C6H5 | C6H5 | Complex 1 | 20 | 92.3 | 214—215 | 214—215[ |
| 3 | C6H5 | C6H5 | Complex 2 | 35 | 88.2 | 214—215 | 214—215[ |
| 4 | C6H5 | 2⁃Cl―C6H4 | Complex 1 | 35 | 87.1 | 217—218 | 214—217[ |
| 5 | C6H5 | 4⁃Cl―C6H4 | Complex 1 | 34 | 85.8 | 219—220 | 219—220[ |
| 6 | C6H5 | 4⁃CH3O―C6H4 | Complex 1 | 40 | 89.1 | 204—205 | 204—205[ |
| 7 | 4⁃Cl―C6H4 | C6H5 | Complex 1 | 32 | 84.1 | 211—212 | 210—212[ |
| 8 | 4⁃CH3―C6H4 | C6H5 | Complex 1 | 37 | 82.6 | 197—198 | 196—199[ |
| 9 | 4⁃CH3―C6H4 | 4⁃NO2―C6H4 | Complex 1 | 31 | 90.3 | 213—214 | 210—212[ |
| 10 | Et | C6H5 | Complex 1 | 67 | 82.3 | 135—136 | 134—137[ |
| 11 | Et | 4⁃Cl―C6H4 | Complex 1 | 31 | 80.1 | 134—135 | 132—135[ |
| 12 | Et | 3⁃NO2―C6H4 | Complex 1 | 19 | 91.5 | 177—178 | 176—178[ |
| 13 | NH4OAc | C6H5 | Complex 1 | 28 | 92.1 | 219—220 | 218—219[ |
| 14 | NH4OAc | 4⁃Cl―C6H4 | Complex 1 | 24 | 86.4 | 206—207 | 205—206[ |
| 15 | NH4OAc | 2⁃NO2―C6H4 | Complex 1 | 15 | 91.4 | 193—194 | 193—194[ |
| 16 | NH4OAc | 3⁃NO2―C6H4 | Complex 1 | 18 | 87.2 | 215—217 | 216—217[ |
| 17 | NH4OAc | 4⁃CH3―C6H4 | Complex 1 | 23 | 88.7 | 232—233 | 233—234[ |
| 18 | NH4OAc | 4⁃CH3O―C6H4 | Complex 1 | 26 | 90.4 | 192—193 | 192—193[ |
Table 4 Investigation of the universality of the catalysts
| Entry | R1 | R2 | Catalyst | Time/min | Yield(%) | m.p./℃ | |
|---|---|---|---|---|---|---|---|
| Found | Repored | ||||||
| 1* | 262 | 46.2 | |||||
| 2 | C6H5 | C6H5 | Complex 1 | 20 | 92.3 | 214—215 | 214—215[ |
| 3 | C6H5 | C6H5 | Complex 2 | 35 | 88.2 | 214—215 | 214—215[ |
| 4 | C6H5 | 2⁃Cl―C6H4 | Complex 1 | 35 | 87.1 | 217—218 | 214—217[ |
| 5 | C6H5 | 4⁃Cl―C6H4 | Complex 1 | 34 | 85.8 | 219—220 | 219—220[ |
| 6 | C6H5 | 4⁃CH3O―C6H4 | Complex 1 | 40 | 89.1 | 204—205 | 204—205[ |
| 7 | 4⁃Cl―C6H4 | C6H5 | Complex 1 | 32 | 84.1 | 211—212 | 210—212[ |
| 8 | 4⁃CH3―C6H4 | C6H5 | Complex 1 | 37 | 82.6 | 197—198 | 196—199[ |
| 9 | 4⁃CH3―C6H4 | 4⁃NO2―C6H4 | Complex 1 | 31 | 90.3 | 213—214 | 210—212[ |
| 10 | Et | C6H5 | Complex 1 | 67 | 82.3 | 135—136 | 134—137[ |
| 11 | Et | 4⁃Cl―C6H4 | Complex 1 | 31 | 80.1 | 134—135 | 132—135[ |
| 12 | Et | 3⁃NO2―C6H4 | Complex 1 | 19 | 91.5 | 177—178 | 176—178[ |
| 13 | NH4OAc | C6H5 | Complex 1 | 28 | 92.1 | 219—220 | 218—219[ |
| 14 | NH4OAc | 4⁃Cl―C6H4 | Complex 1 | 24 | 86.4 | 206—207 | 205—206[ |
| 15 | NH4OAc | 2⁃NO2―C6H4 | Complex 1 | 15 | 91.4 | 193—194 | 193—194[ |
| 16 | NH4OAc | 3⁃NO2―C6H4 | Complex 1 | 18 | 87.2 | 215—217 | 216—217[ |
| 17 | NH4OAc | 4⁃CH3―C6H4 | Complex 1 | 23 | 88.7 | 232—233 | 233—234[ |
| 18 | NH4OAc | 4⁃CH3O―C6H4 | Complex 1 | 26 | 90.4 | 192—193 | 192—193[ |
| Atom | Charge/eV | Atom | Charge/eV | Atom | Charge/eV |
|---|---|---|---|---|---|
| Zn1 | 14.56 | O4 | -22.64 | O10 | -17.09 |
| S1 | 33.42 | O5 | -19.35 | N1 | -9.09 |
| S2 | 33.36 | O6 | -16.74 | N2 | -12.03 |
| O1 | -22.18 | O7 | -15.81 | N3 | -9.28 |
| O2 | -23.37 | O8 | -17.36 | N4 | -11.73 |
| O3 | -20.79 | O9 | -17.52 | N5 | -10.62 |
Table 5 Mulliken charge of part atoms of complex 1
| Atom | Charge/eV | Atom | Charge/eV | Atom | Charge/eV |
|---|---|---|---|---|---|
| Zn1 | 14.56 | O4 | -22.64 | O10 | -17.09 |
| S1 | 33.42 | O5 | -19.35 | N1 | -9.09 |
| S2 | 33.36 | O6 | -16.74 | N2 | -12.03 |
| O1 | -22.18 | O7 | -15.81 | N3 | -9.28 |
| O2 | -23.37 | O8 | -17.36 | N4 | -11.73 |
| O3 | -20.79 | O9 | -17.52 | N5 | -10.62 |
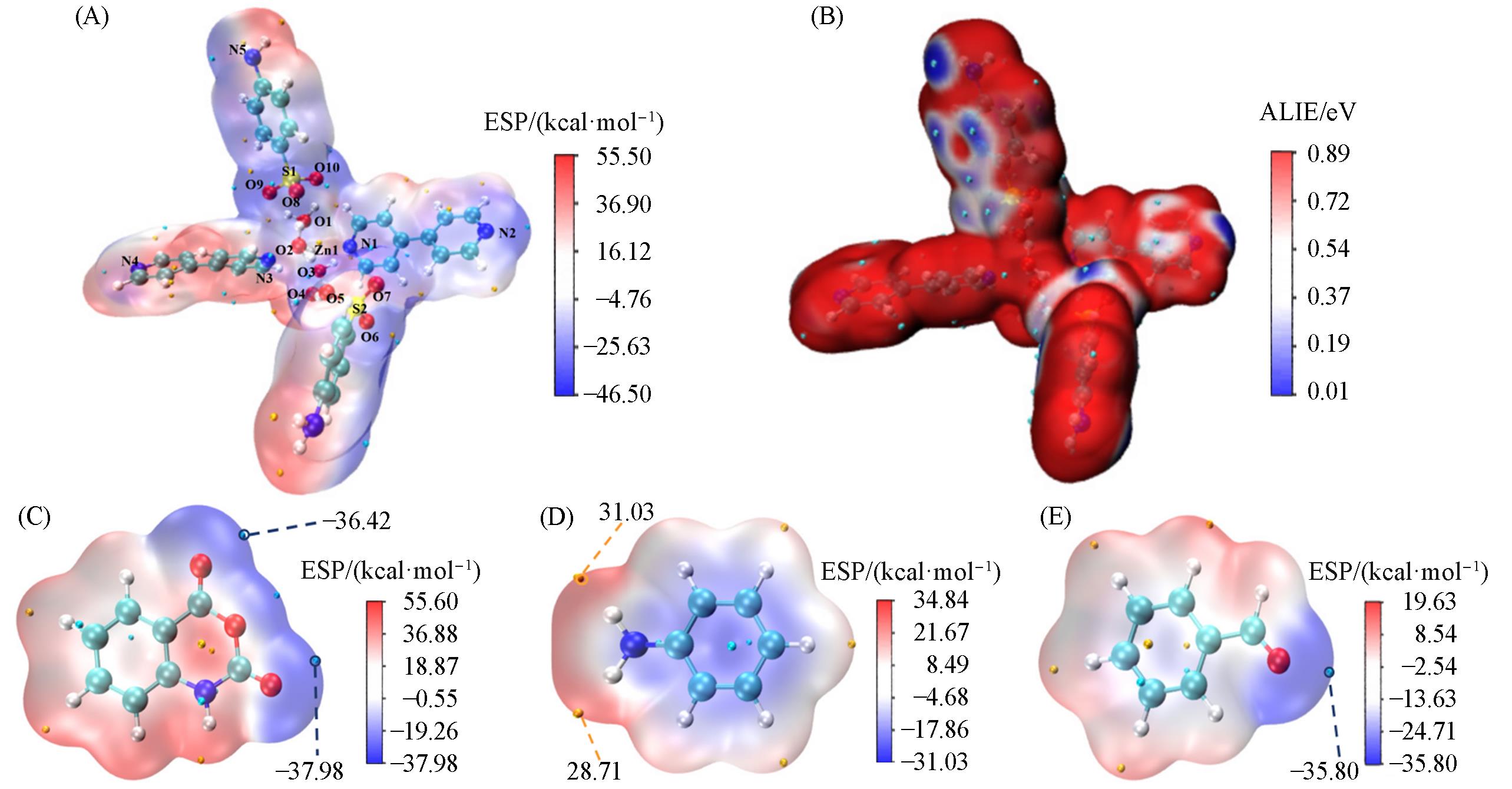
Fig.9 Surface electrostatic potential diagram(A), surface⁃averaged localised ionisation energy colour⁃filled plot(B) for complex 1 and surface electrostatic potential of indirubic anhydride(C), aniline(D) and benzaldehyde(E)1 kcal·mol-1=4.184 kJ·mol-1.
| [1] | Li Z. H., Zhao L., Bian Y. Q., Li Y., Qu J., Feng S., Curr. Top. Med. Chem., 2022, 22(12), 1035—1044 |
| [2] | Gatadi S., Pulivendala G., Gour J., Malasala S., Bujji S., Parupalli R., Shaikh M., Godugu C., Nanduri S., J. Mol. Struct., 2020, 1200, 127097 |
| [3] | Ghodge B., Kshirsagar A., Navghare V., Beni⁃Suef Univ. J. Basic Appl. Sci., 2020, 9(1), 1—12 |
| [4] | Seifu G. W., Birhan Y. S., Beshay B. Y., Hymete A., Bekhit A. A., BMC Chem., 2022, 16(1), 107 |
| [5] | Charoensutthivarakul S., Lohawittayanan D., Kanjanasirirat P., Jearawuttanakul K., Seemakhan S., Chabang N., Schlaepp P., Tantivess V., Limboonreung T., Phanchana M., Molecules, 2023, 28(7), 2999 |
| [6] | Mahala S., Singh S., Rakesh P., Bhuvanesh N., Joshi H. J., Org. Chem., 2025, 90(10), 3789—3795 |
| [7] | Ibrahim M. K., Eissa I. H., Abdallah A. E., Metwaly A. M., Radwan M. M., ElSohly M. A., Bioorg. Med. Chem., 2017, 25(4), 1496—1513 |
| [8] | Pathak S., Malhotra V., Nath R., Shanker K., Cent. Nerv. Syst. Agents Med. Chem., 2014, 14(1), 34—38 |
| [9] | Cheng D. P., Yan X. H., Pu Y. Q., Shen J., Xu X. L., Yan J. Z., Eur. J. Org. Chem., 2021,(6), 944—950 |
| [10] | Salehi P., Dabiri M., Zolfigol M. A., Baghbanzadeh M., Synlett, 2005, 7, 1155—1157 |
| [11] | Ghasemzadeh M. A., Mirhosseini⁃Eshkevari B., Nanoscale Adv., 2023, 5(24), 7031—7041 |
| [12] | Ghashang M., Orient. J. Chem., 2012, 28(3), 1213—1218 |
| [13] | Mitra B., Pariyar G. C., Ghosh P., RSC Adv., 2021, 11(3), 1271—1281 |
| [14] | Wang M., Zhang T. T., Liang Y., Gao J. J., Monatsh. Chem., 2012, 143, 835—839 |
| [15] | Hassaballah A. I., El-Ziaty A. K., Ewies E. F., Zayed E. M., Mohamed G. G., Inorg. Chem. Commun., 2023, 155, 110989 |
| [16] | Chen H. P., Gong Y. R., Chu Q. Q., Pang X., Huang X. J., Tian X. D., Yang W. T., Pan Q. H., Su Z. M., Wang X. L., Chem. Res. Chin. Univ., 2023, 39(6), 954—959 |
| [17] | Yin M. R., Yan Q. Q., Li B., Yong G. P., J. Solid State Chem., 2022, 305, 122672 |
| [18] | Wang Z. H., Su R. H., Wang G. D., Shi W. J., Hou L. J., Environ. Chem. Eng., 2023, 11(5), 110488 |
| [19] | Yu Q. S., Qi C., Gu S. X., Yang X. D., Jiang Q., Wei R. B., Shi P. F., Acta Chim. Sinica, 2025, 83(6), 579—587 |
| 于千水, 戚聪, 顾顺心, 杨欣达, 姜琴, 魏荣斌, 施鹏飞. 化学学报, 2025, 83(6), 579—587 | |
| [20] | Sun A. H., Yang Y. H., Liu Y. F., Ding L., Duan P., Yang W. T., Pan Q. H., Cryst. Growth Des., 2021, 21(9), 4971—4978 |
| [21] | Wang X., Qi J. Y., Yang R. J., Song Z. G., Wang M., Chem. J. Chinese Univerties, 2024, 45(10), 20240297 |
| 王鑫, 祁金阳, 杨瑞杰, 宋志国, 王敏. 高等学校化学学报, 2024, 45(10), 20240297 | |
| [22] | Wang T., Astruc D., Coord. Chem. Rev., 2025, 524, 216300 |
| [23] | Erxleben A., Coord. Chem. Rev., 2003, 246(1/2), 203—228 |
| [24] | Samanta A., Shit M., Karan A. K., Manik N. B., Sinha C., Khanra S., J. Mol. Struct., 2025, 1321, 139866 |
| [25] | Atwood J. L., Barbour L. J., Hardie M. J., Raston C. L., Coord. Chem. Rev., 2001, 222(1), 3—32 |
| [26] | Côté A. P., Shimizu G. K., Coord. Chem. Rev., 2003, 245(1/2), 49—64 |
| [27] | Dayal S., Burda C., J. Am. Chem. Soc., 2008, 130(10), 2890—2891 |
| [28] | Lu W. G., Su C. Y., Lu T. B., Jiang L., Chen J. M., J. Am. Chem. Soc., 2006, 128(1), 34—35 |
| [29] | Bruker S., SMART(Version 5.628), SAINT(Version 6.45), SADABS, Bruker AXS Inc., Madison, WI, 2001 |
| [30] | Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., J. Appl. Crystallogr., 2009, 42, 339—341 |
| [31] | Sheldrick G. M., SHELXL-97, University of Göttingen, Göttingen, 1997 |
| [32] | Lu T., Chen F. W., J. Comput. Chem., 2012, 33(5), 580—592 |
| [33] | Humphrey W., Dalke A., Schulten K., J. Mol. Graphics, 1996, 14(1), 33—38 |
| [34] | Xia X, Wang S., Yang X. Q., Fan R. Wei R. Z., Liu Z., Tang Q. J., Chin. J. Inorg. Chem., 2021, 37(12), 2133—2140 |
| 夏祥, 王胜, 杨兴乾, 樊荣, 魏润芝, 刘峥, 唐群基. 无机化学学报, 2021, 37(12), 2133—2140 | |
| [35] | Shi Y. W., Yang R. J., Zhang Y. C., Wang X., Wang M., Song Z. G., J. Synth. Cryst., 2024, 53(9), 1583—1590 |
| 史燚威, 杨瑞杰, 张迎春, 王鑫, 王敏, 宋志国. 人工晶体学报, 2024, 53(9), 1583—1590 | |
| [36] | Chen J. X., Wu D. Z., He F., Liu M. C., Wu H. Y., Ding J. C., Su W. K., Tetrahedron Lett., 2008, 49(23), 3814—3818 |
| [37] | Lu N., Meng L., Chen D. Z., Zhang G. Q., J. Mol. Catal., 2011, 339(1/2), 99—107 |
| [38] | Sun C., Liu M., Sun H., Hang F., Sun N., Chen D., Int. J. Quantum Chem., 2015, 115(2), 59—67 |
| [39] | Lu N., Meng L., Chen D., Zhang G. J., Phys. Chem. A, 2012, 116(1), 670—679 |
| [1] | 李浩静, 葛常威, 钟启迪, 闫红, 孙国辉. 1,4-二氢吡啶衍生物光物理性质的理论研究[J]. 高等学校化学学报, 2026, 47(2): 20250250. |
| [2] | 胡煜腾, 桑丽霞, 杜春旭. 等离激元金属及其温升作用下MoS2-H2O的界面性质[J]. 高等学校化学学报, 2025, 46(5): 20240569. |
| [3] | 顼兴宇, 解晓明, 邱萍. 2-苯基-3-氨基氮杂环丁烷开环形成噻唑或噁唑的机理研究[J]. 高等学校化学学报, 2025, 46(5): 20240547. |
| [4] | 杨思伟, 黄旭日. B, N共掺杂富勒烯C70作为氧还原和氧析出非金属电催化剂的理论研究[J]. 高等学校化学学报, 2025, 46(4): 20240490. |
| [5] | 黄智瑶, 李丽, 徐华卿, 杨一凡, 韦瑶瑶, 刘国魁, 夏其英. N掺杂石墨烯缺陷材料催化OER/ORR的第一性原理研究[J]. 高等学校化学学报, 2025, 46(2): 20240430. |
| [6] | 田阳, 郭其景, 杨华春, 刘海霞, 薛峰峰, 易浩, 宋少先. 氢氟酸体系锰杂质络合机制: 基于DFT的配位结构与反应路径解析[J]. 高等学校化学学报, 2025, 46(12): 20250189. |
| [7] | 白一泽, 刘睿端, 鲁秋然, 赵海燕. N,N,N-三齿席夫碱铜(II)配合物的合成、 晶体结构及儿茶酚酶活性[J]. 高等学校化学学报, 2024, 45(7): 20240063. |
| [8] | 何军, 朱傲阳, 魏雨晨, 朱怡全, 蒋莉, 何孝军. 三维氮掺杂分级多孔碳纳米片的制备及储锌性能[J]. 高等学校化学学报, 2024, 45(7): 20240099. |
| [9] | 陈俊杰, 张瑞丹, 陈越. 单层GeTe在锂/钠/钾离子电池中潜在应用的第一性原理研究[J]. 高等学校化学学报, 2024, 45(7): 20240148. |
| [10] | 张硕, 赵刘洋, 黄昊, 吴爱民, 李爱魁. 基于第一性原理高价元素Mo稳定层状富锂锰基材料的氧框架机制[J]. 高等学校化学学报, 2024, 45(5): 20240035. |
| [11] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [12] | 陈晴晴, 李江涛, 黄欣蓉, 顾芳, 王海军. 氢键流体中Janus粒子的过量熵[J]. 高等学校化学学报, 2024, 45(2): 20230443. |
| [13] | 王鑫, 祁金阳, 杨瑞杰, 宋志国, 王敏. 基于苯磺酸配体构筑的Cu(II)配合物的合成、 表征及催化性能[J]. 高等学校化学学报, 2024, 45(10): 20240297. |
| [14] | 富忠恒, 陈翔, 姚楠, 余乐耕, 沈馨, 张睿, 张强. 固态电解质锂离子输运机制研究进展[J]. 高等学校化学学报, 2023, 44(5): 20220703. |
| [15] | 鲍春竹, 向中华. 非热解共价有机聚合物基氧还原电催化材料[J]. 高等学校化学学报, 2023, 44(5): 20220715. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||