

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (7): 20240063.doi: 10.7503/cjcu20240063
收稿日期:2024-02-02
出版日期:2024-07-10
发布日期:2024-04-19
通讯作者:
赵海燕
E-mail:hbhaiyanzh@163.com
基金资助:
BAI Yize, LIU Ruiduan, LU Qiuran, ZHAO Haiyan( )
)
Received:2024-02-02
Online:2024-07-10
Published:2024-04-19
Contact:
ZHAO Haiyan
E-mail:hbhaiyanzh@163.com
Supported by:摘要:
选用N,N,N-三齿席夫碱化合物2-氨基乙基苯并咪唑缩吡啶-2-甲醛(L1)和2-氨基丙基苯并咪唑缩吡啶-2-甲醛(L2)为配体, 与过渡金属Cu(II)盐反应合成了4个单核铜配合物: [Cu(L1)Cl2] (1), [Cu(L1)(SCN)2] (2), [Cu(L1)bpy](ClO4)2·CH3OH (3)和[Cu(L2)bpy](ClO4)2 (4) (bpy=2,2′-联吡啶). 通过元素分析、 红外光谱、 粉末X射线衍射、 单晶X射线衍射、 热重分析和循环伏安分析等手段对配合物进行了表征. 结果表明, 在固体状态下, 配合物1~4均为单核Cu(II)配合物, 中心Cu(II)均为畸变四方锥构型, 结构参数τ=0.088~0.340. 以3,5-二叔丁基邻苯二酚为底物, 研究了配合物1~4模拟儿茶酚氧化酶的催化活性, 采用Michaelis-Menten模型和Lineweaver-Burk图法计算了动力学参数. 结果表明, 配合物均具有儿茶酚氧化酶活性, 催化活性顺序为2>3≈4>1, 氧化速率取决于Cu(II)配位环境的畸变程度、 离去基团与中心Cu(II)的键长和配合物的空间位阻.
中图分类号:
TrendMD:
白一泽, 刘睿端, 鲁秋然, 赵海燕. N,N,N-三齿席夫碱铜(II)配合物的合成、 晶体结构及儿茶酚酶活性. 高等学校化学学报, 2024, 45(7): 20240063.
BAI Yize, LIU Ruiduan, LU Qiuran, ZHAO Haiyan. Synthesis, Crystal Structure and Catecholase Activity of Copper(II) Complexes with N,N,N⁃Tridentate Schiff Base Ligand. Chem. J. Chinese Universities, 2024, 45(7): 20240063.
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C15H14Cl2CuN4 | C17H14CuN6S2 | C26H26Cl2CuN6O9 | C26H24Cl2CuN6O8 |
| M | 384.74 | 430.00 | 700.97 | 682.95 |
| Temperature/K | 298(2) | 298(2) | 298(2) | 298(2) |
| λ/nm | 0.071073 | 0.071073 | 0.071073 | 0.071073 |
| Crystal System | Orthorhombic | Triclinic | Triclinic | Monoclinic |
| Space group | P212121 | P | P | P21/c |
| a/nm | 0.8662(2) | 0.87780(8) | 0.89274(9) | 1.70142(16) |
| b/nm | 1.2611(3) | 0.96341(9) | 1.17971(12) | 1.09415(9) |
| c/nm | 1.4058(4) | 1.23569(11) | 1.54248(17) | 1.61380(15) |
| α/(°) | 90 | 97.4300(10) | 108.060(2) | 90 |
| β/(°) | 90 | 94.2400(10) | 96.0830(10) | 105.806(2) |
| γ/(°) | 90 | 112.621(2) | 95.6240(10) | 90 |
| Volume/nm3 | 1.5357(7) | 0.94774(15) | 1.5210(3) | 2.8907(5) |
| Z | 4 | 2 | 2 | 4 |
| μ/mm-1 | 1.770 | 1.386 | 0.955 | 1.000 |
| F(000) | 780 | 438 | 718 | 1396 |
| Crystal size/mm | 0.48×0.45×0.43 | 0.23×0.16×0.11 | 0.45×0.44×0.41 | 0.23×0.15×0.09 |
| Theta range/(°) | 2.85—25.02 | 2.32—25.02 | 2.53—25.02 | 2.28—25.02 |
| Index ranges | -10≤h≤8 | -6≤h≤10 | -10≤h≤10 | -20≤h≤20 |
| -14≤k≤15 | -11≤k≤10 | -14≤k≤10 | -3≤k≤10 | |
| -14≤l≤16 | -14≤l≤14 | -18≤l≤17 | -19≤l≤18 | |
| Dcalc/(g·cm-3) | 1.664 | 1.507 | 1.531 | 1.569 |
| Total reflections | 7676 | 4818 | 7606 | 14014 |
| Unique reflections | 2711 | 3287 | 5232 | 5076 |
| R1, wR2[I>2σ(I)] | 0.0264, 0.0565 | 0.0737, 0.1706 | 0.0602, 0.1471 | 0.0843, 0.1849 |
| R1, wR2(all data) | 0.0331, 0.0594 | 0.1083, 0.1840 | 0.1079, 0.1726 | 0.1648, 0.2115 |
| Goodness⁃of⁃fit | 1.056 | 1.075 | 1.052 | 1.064 |
| CCDC No. | 2325617 | 2325377 | 2325376 | 2325375 |
Table1 Crystallographic data of complexes 1—4
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C15H14Cl2CuN4 | C17H14CuN6S2 | C26H26Cl2CuN6O9 | C26H24Cl2CuN6O8 |
| M | 384.74 | 430.00 | 700.97 | 682.95 |
| Temperature/K | 298(2) | 298(2) | 298(2) | 298(2) |
| λ/nm | 0.071073 | 0.071073 | 0.071073 | 0.071073 |
| Crystal System | Orthorhombic | Triclinic | Triclinic | Monoclinic |
| Space group | P212121 | P | P | P21/c |
| a/nm | 0.8662(2) | 0.87780(8) | 0.89274(9) | 1.70142(16) |
| b/nm | 1.2611(3) | 0.96341(9) | 1.17971(12) | 1.09415(9) |
| c/nm | 1.4058(4) | 1.23569(11) | 1.54248(17) | 1.61380(15) |
| α/(°) | 90 | 97.4300(10) | 108.060(2) | 90 |
| β/(°) | 90 | 94.2400(10) | 96.0830(10) | 105.806(2) |
| γ/(°) | 90 | 112.621(2) | 95.6240(10) | 90 |
| Volume/nm3 | 1.5357(7) | 0.94774(15) | 1.5210(3) | 2.8907(5) |
| Z | 4 | 2 | 2 | 4 |
| μ/mm-1 | 1.770 | 1.386 | 0.955 | 1.000 |
| F(000) | 780 | 438 | 718 | 1396 |
| Crystal size/mm | 0.48×0.45×0.43 | 0.23×0.16×0.11 | 0.45×0.44×0.41 | 0.23×0.15×0.09 |
| Theta range/(°) | 2.85—25.02 | 2.32—25.02 | 2.53—25.02 | 2.28—25.02 |
| Index ranges | -10≤h≤8 | -6≤h≤10 | -10≤h≤10 | -20≤h≤20 |
| -14≤k≤15 | -11≤k≤10 | -14≤k≤10 | -3≤k≤10 | |
| -14≤l≤16 | -14≤l≤14 | -18≤l≤17 | -19≤l≤18 | |
| Dcalc/(g·cm-3) | 1.664 | 1.507 | 1.531 | 1.569 |
| Total reflections | 7676 | 4818 | 7606 | 14014 |
| Unique reflections | 2711 | 3287 | 5232 | 5076 |
| R1, wR2[I>2σ(I)] | 0.0264, 0.0565 | 0.0737, 0.1706 | 0.0602, 0.1471 | 0.0843, 0.1849 |
| R1, wR2(all data) | 0.0331, 0.0594 | 0.1083, 0.1840 | 0.1079, 0.1726 | 0.1648, 0.2115 |
| Goodness⁃of⁃fit | 1.056 | 1.075 | 1.052 | 1.064 |
| CCDC No. | 2325617 | 2325377 | 2325376 | 2325375 |
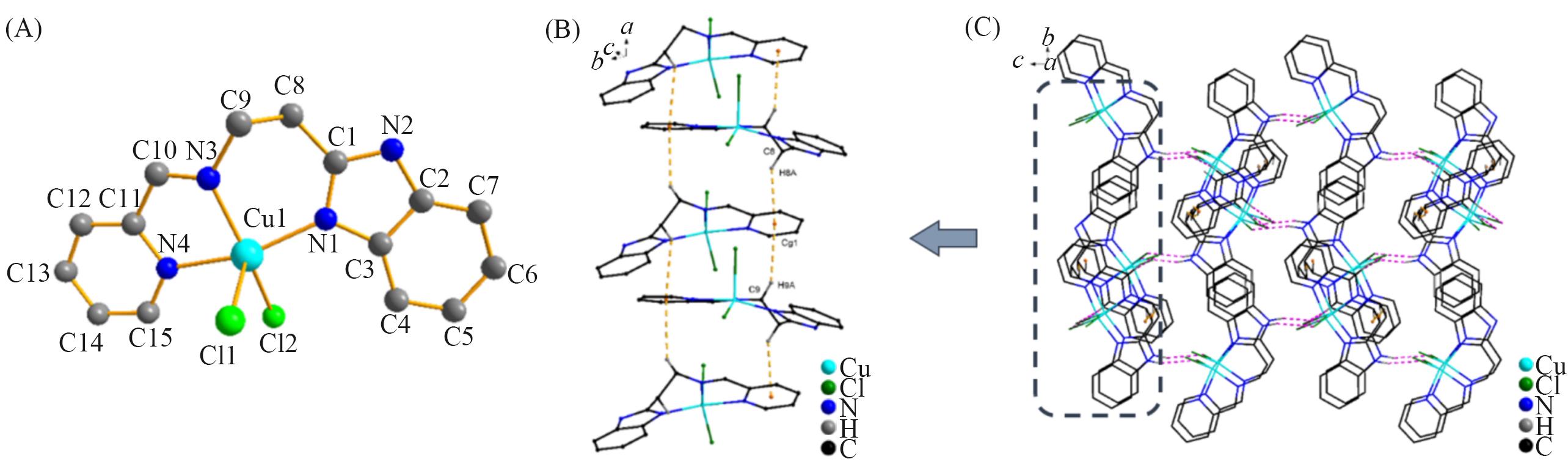
Fig.1 Molecular structure of complex 1 with atom labels(A, hydrogen atoms are omitted for clarity), a perspective view of C—H∙∙∙π interactions in the crystalline architecture of complex 1(orange dashed line)(B) and a view of the 3D molecular network fabricated by N—H∙∙∙Cl and C—H∙∙∙Cl hydrogen interactions and C—H∙∙∙π interactions in complex 1(C)
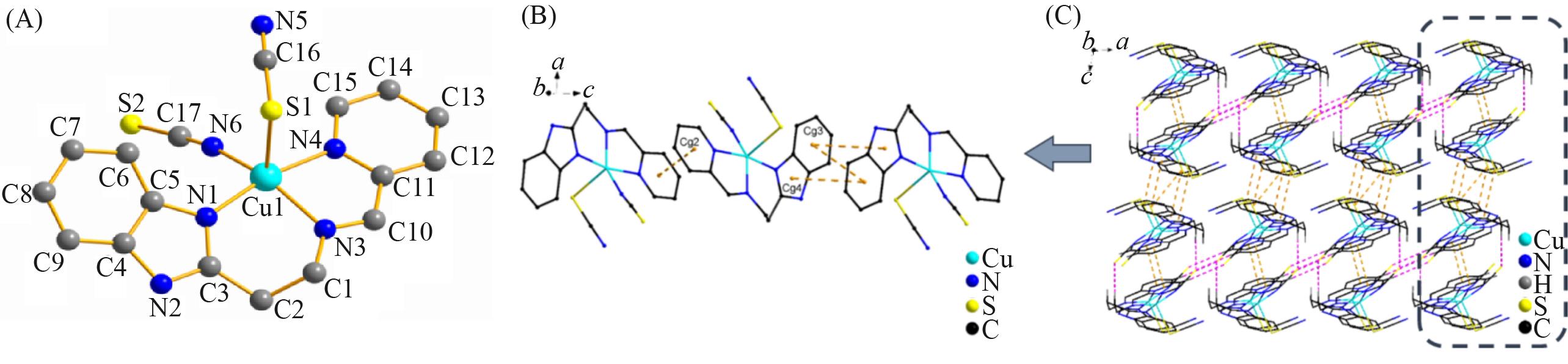
Fig.2 Molecular structure of complex 2 with atom labels(A, hydrogen atoms are omitted for clarity),a perspective view of π∙∙∙π interactions in the crystalline architecture of complex 2(orange dashed line)(B) and a view of the 3D molecular network fabricated by C—H∙∙∙S hydrogen interactions and π∙∙∙π interactions in complex 2(C)
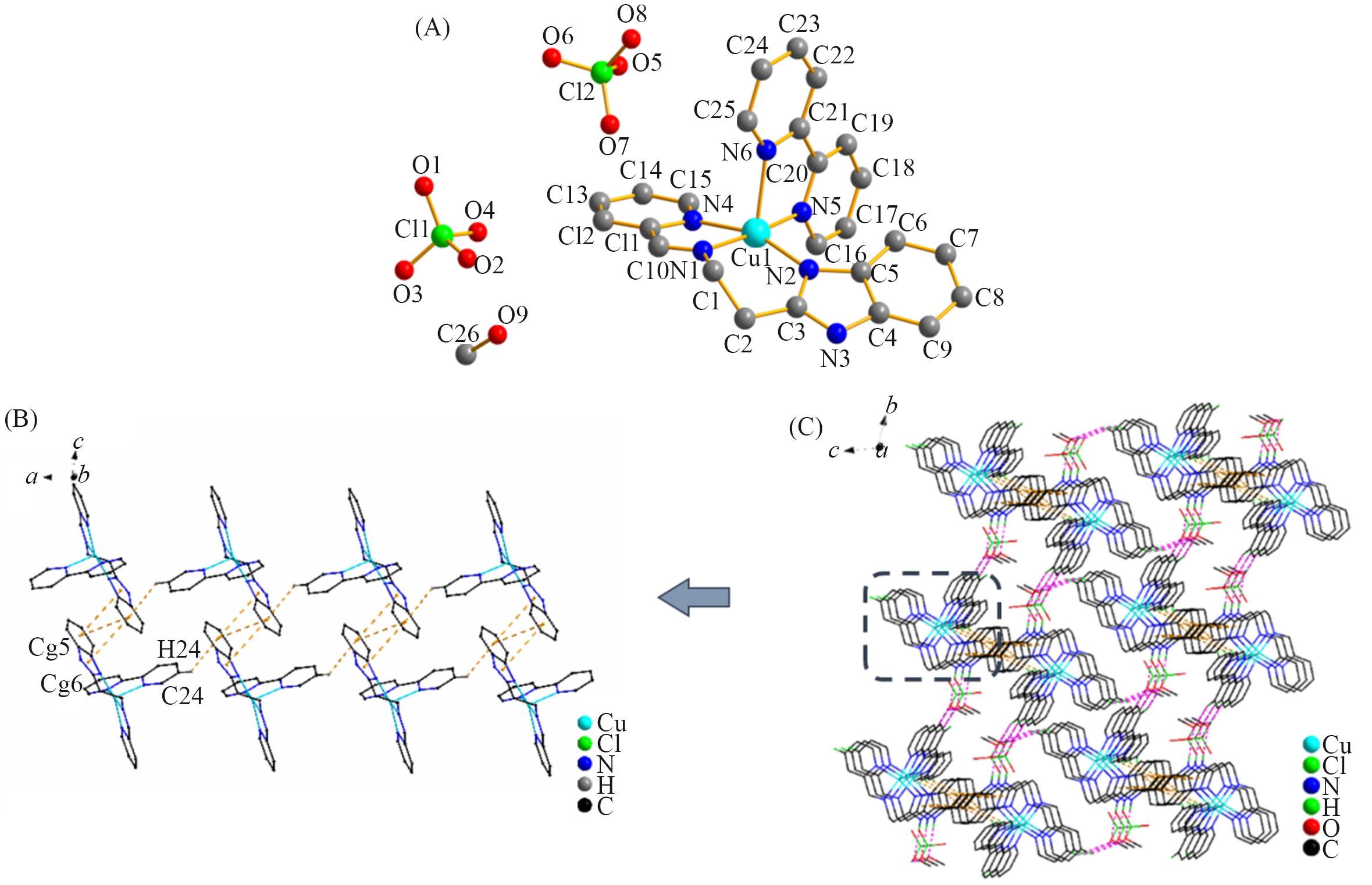
Fig.3 Molecular structure of complex 3 with atom labels(A, hydrogen atoms are omitted for clarity), a perspective view of C—H∙∙∙π and π∙∙∙π interactions in the crystalline architecture of complex 3(orange dashed line)(B) and a view of the 3D molecular network fabricated by N—H∙∙∙O, O—H∙∙∙ O and C—H∙∙∙O hydrogen interactions and π∙∙∙π interactions in complex 3(C)
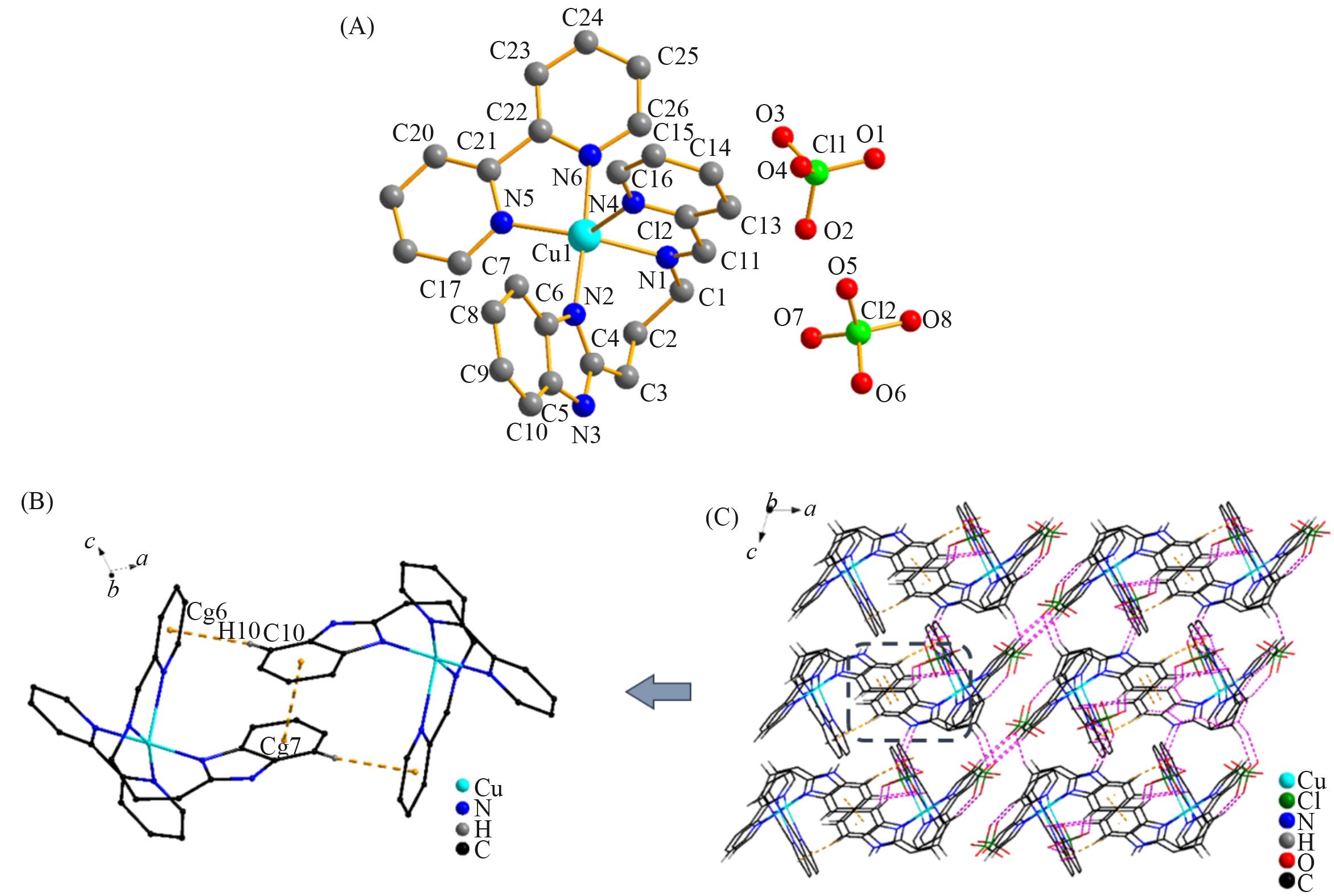
Fig.4 Molecular structure of complex 4 with atom labels(A, hydrogen atoms are omitted for clarity), a perspective view of C—H∙∙∙π and π∙∙∙π interactions in the crystalline architecture of complex 4 (orange dashed line)(B) and a view of the 3D molecular network fabricated by N—H∙∙∙O and C—H∙∙∙O hydrogen interactions and π∙∙∙π interactions in complex 4(C)
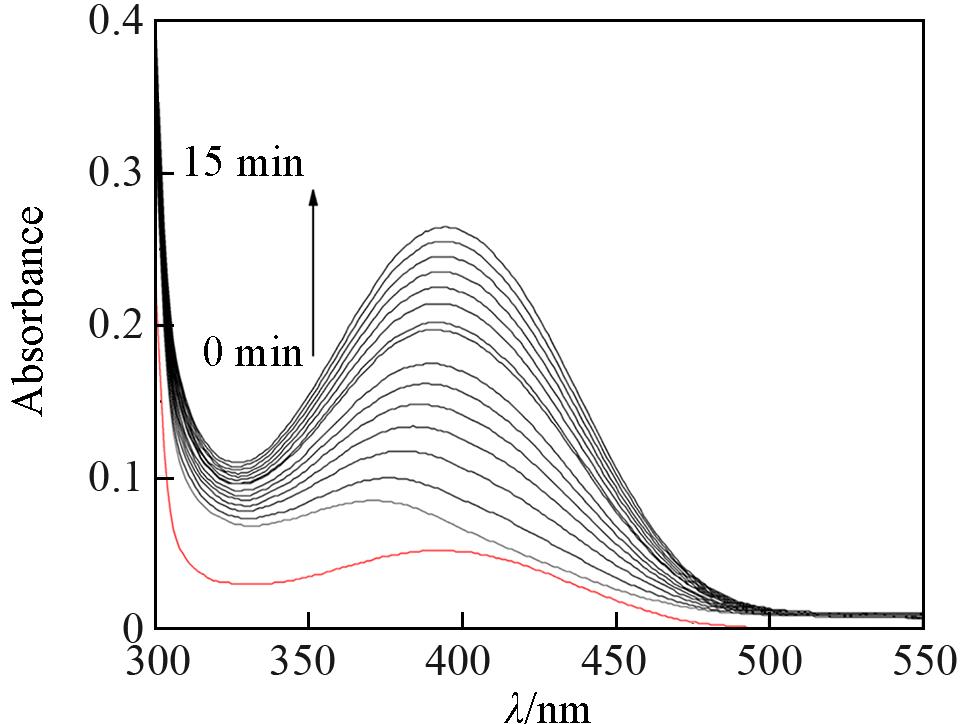
Fig.5 UV⁃Vis spectra showing the increase of 3,5⁃DTBQ band after addition of 1×10-4 mol/L DMF solution of complex 1 to 100 fold methanolic solution of 3,5⁃DTBC
| Complex* | τ | dCu—X/nm | vmax/(mol·L-1·s-1) | Km/(mol·L-1) | Kcat/(h-1) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.088 | 0.24967(10) | 1.14×10-4 | 1.95×10-3 | 12.56 | This work |
| 2 | 0.270 | 0.2605(2) | 3.40×10-4 | 1.49×10-2 | 37.41 | This work |
| 3 | 0.180 | 0.2182(4) | 2.35×10-4 | 9.13×10-3 | 25.90 | This work |
| 4 | 0.340 | 0.2317(9) | 2.31×10-4 | 3.54×10-3 | 25.45 | This work |
| [Cu(HL1)Cl2] | 0.043 | 0.25719(8) | 4.54×10-4 | 0.5221 | 163.30 | [ |
| [Cu(sal⁃ppzH)Cl2] | 0.015 | 0.2694 | 1.64×10-4 | 0.53 | 11.82 | [ |
[Cu(L2)(phen)] [Cu(L2)(phen)]∙5H2O | 0.44 | 0.2251(4) | 5.67×10-7 | 2.4×10-3 | 62 | [ |
| 0.68 | 0.2118(3) | |||||
| [Cu(L3)(phen)](ClO4) | 0.56 | 0.2195(15) | 4.67×10-7 | 2.3×10-3 | 52 | [ |
| [Cu(L4)(bipy)]ClO4 | 0.67 | 0.21423(19) | 1.16×10-8 | 4.64×10-3 | 83.59 | [ |
| [Cu(L4)(phen)]ClO4 | 0.62 | 0.21911(19) | 1.02×10-8 | 2.95×10-3 | 73.57 | [ |
Table 2 Kinetic parameters and structure parameters for the oxidation of 3,5-DTBC catalyzed by Cu(II) complexes
| Complex* | τ | dCu—X/nm | vmax/(mol·L-1·s-1) | Km/(mol·L-1) | Kcat/(h-1) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.088 | 0.24967(10) | 1.14×10-4 | 1.95×10-3 | 12.56 | This work |
| 2 | 0.270 | 0.2605(2) | 3.40×10-4 | 1.49×10-2 | 37.41 | This work |
| 3 | 0.180 | 0.2182(4) | 2.35×10-4 | 9.13×10-3 | 25.90 | This work |
| 4 | 0.340 | 0.2317(9) | 2.31×10-4 | 3.54×10-3 | 25.45 | This work |
| [Cu(HL1)Cl2] | 0.043 | 0.25719(8) | 4.54×10-4 | 0.5221 | 163.30 | [ |
| [Cu(sal⁃ppzH)Cl2] | 0.015 | 0.2694 | 1.64×10-4 | 0.53 | 11.82 | [ |
[Cu(L2)(phen)] [Cu(L2)(phen)]∙5H2O | 0.44 | 0.2251(4) | 5.67×10-7 | 2.4×10-3 | 62 | [ |
| 0.68 | 0.2118(3) | |||||
| [Cu(L3)(phen)](ClO4) | 0.56 | 0.2195(15) | 4.67×10-7 | 2.3×10-3 | 52 | [ |
| [Cu(L4)(bipy)]ClO4 | 0.67 | 0.21423(19) | 1.16×10-8 | 4.64×10-3 | 83.59 | [ |
| [Cu(L4)(phen)]ClO4 | 0.62 | 0.21911(19) | 1.02×10-8 | 2.95×10-3 | 73.57 | [ |
| 1 | Mondal S., Chakraborty M., Mondal A., Pakhira B., Blake A. J., Sinn E., Chattopadhyay S. K., New J. Chem., 2018, 42(12), 9588—9597 |
| 2 | Chhabra V., Kundu B. K., Ranjan R., Pragti, Mobin S. M., Mukhopadhyay S., Inorg. Chim. Acta, 2020, 502, 119389 |
| 3 | Balakrishnan N., Haribabu J., Dhanabalan A. K., Swaminathan S., Sun S., Dibwe D. F., Bhuvanesh N., Awale S., Karvembu R., Dalton Trans., 2020, 49(27), 9411—9424 |
| 4 | Thio Y., Yang X., Vittal J. J., Dalton Trans., 2014, 43(9), 3545—3556 |
| 5 | Dey S. K., Mukherjee A., Coord. Chem. Rev., 2016, 310, 80—115 |
| 6 | Ramadan A. E. M. M., Shaban S.Y., Ibrahim M. M., Abdel⁃Rahman A. A. H., Sallam S. A., Al-Harbi S. A., Omar W., New J. Chem., 2020, 44(16), 6331—6345 |
| 7 | Dasgupta S., Chakraborty P., Kundu P., Kara H., Aullón G., Zangrando E., Das D., CrystEngComm, 2019, 21(46), 7094—7107 |
| 8 | Marcon M., Crespi S., Pielmeier A., König B., Chem. Commun., 2023, 59(7), 948—951 |
| 9 | Das K., Dolai S., Vojtíšek P., Manna S. C., Polyhedron, 2018, 149, 7—16 |
| 10 | Kahrović E., Zahirović A., Višnjevac A., Osmanković I., Turkušić E., Kurtagić H., Croat. Chem. Acta, 2018, 91(2), 195—207 |
| 11 | Magherusan A. M, Nelis D. N., Twamley B., McDonald A. R., Dalton Trans., 2018, 47(43), 15555—15564 |
| 12 | Dede B, Özen N., Görgülü G., J. Mol. Struct., 2018, 1163, 357—367 |
| 13 | Ghosh A. K., Ali A., Singh Y., Purohit C. S., Ghosh R., Inorg. Chim. Acta, 2018, 474, 156—163 |
| 14 | Das M., Afsan Z., Basak D., Arjmand F., Ray D., Dalton Trans., 2019, 48(4), 1292—1313 |
| 15 | Mandal A., Sarkar A., Adhikary A., Samanta D., Das D., Dalton Trans., 2020, 49(43), 15461—15472 |
| 16 | Chakraborty M., Mondal A., Chattopadhyay S. K., New J. Chem., 2020, 44(30), 12916—12925 |
| 17 | Jana N. C., Ghorai P., Brandão P., Jagličić Z., Panja A., Dalton Trans., 2021, 50(42), 15233—15247 |
| 18 | Chatterjee A., Seikh M. M., Chowdhury S., Ghosh R., Inorg. Chim. Acta, 2021, 521, 120345 |
| 19 | Shyamal M., Mandal T. K., Panja A., Saha A., RSC Adv., 2014, 4(96), 53520—53530 |
| 20 | Bhunia A., Manna S., Mistri S., Paul A., Manne R. K., Santra M. K., Bertolasi V., Manna S. C., RSC Adv., 2015, 5(83), 67727—67737 |
| 21 | Beyazit N., Çatıkkaş B., Bayraktar Ş., Demetgül C., J. Mol. Struct., 2016, 1119, 124—132 |
| 22 | Kumari S., Mahato A. K., Maurya A., Singh V. K., Kesharwani N., Kachhap P., Koshevoy I. O., Haldar C., New J. Chem., 2017, 41(22), 13625—13646 |
| 23 | Selvakumaran B., Murali M., Sathya V., Inorg. Chim. Acta, 2023, 553, 121514 |
| 24 | Das M., Kundu B. K., Tiwari R., Mandal P., Nayak D., Ganguly R., Mukhopadhyay S., Inorg. Chim. Acta, 2018, 469, 111—122 |
| 25 | Bhunia A., Vojtíšek P., Bertolasi V., Manna S. C., J. Mol. Struct., 2019, 1189, 94—101 |
| 26 | Bhunia A, Vojtíšek P., Manna S. C., J. Mol. Struct., 2019, 1179, 558—567 |
| 27 | Castro K. A. D. F., Figueira F., Paz F. A. A., Tomé J. P. C., da Silva R. S., Nakagaki S., Neves M. G. P. M. S., Cavaleiro J. A. S., Simões M. M. Q., Dalton Trans., 2019, 48(23), 8144—8152 |
| 28 | Santra A., Brandao P., Mondal G., Bera P., Jana A., Bhattacharyya I., Pramanik C., Bera P., Polyhedron, 2020, 176, 114277 |
| 29 | Biswas B. K., Saha S., Biswas N., Chowdhury M., Frontera A., Rizzoli C., Choudhury R. R., Choudhury C. R., J. Mol. Struct., 2020, 1217, 128398 |
| 30 | Sarkar S., Kim M., Lee H. I., Bull. Korean Chem. Soc., 2021, 42(7), 1037—1046 |
| 31 | Mukherjee S., Pal C. K., Kotakonda M., Joshi M., Shit M., Ghosh P., Choudhury A. R., Biswas B., J. Mol. Struct., 2021, 1245, 131057 |
| 32 | Selvakumaran B., Murali M., Inorg. Chim. Acta, 2022, 534, 120819 |
| 33 | Sýs M., Kocábová J., Klikarová J., Novák M., Jirásko R., Obluková M., Mikysek T., Sokolová R., Dalton Trans., 2022, 51(36), 13703—13715 |
| 34 | Mandal S., Naskar R., Mondal A. S., Bera B., Mondal T. K., Dalton Trans., 2023, 52(18), 5983—5998 |
| 35 | Maity R., Maity M., Jana K., Maity T., Sepay N., Samanta B. C., New J. Chem., 2023, 47(5), 2673—2681 |
| 36 | Roy B. C., Dutta B., Basak D., Debnath S., Ray D., Mahapatra T. S., New J. Chem., 2023, 47(25), 11928—11944 |
| 37 | Chirinos J., Ibarra D., Morillo Á., Llovera L., González T., Zárraga J., Larreal O., Guerra M., Polyhedron, 2021, 203, 115232 |
| 38 | Zhang Q., Han Y., Jiao Y. H., Chin. J. Inorg. Chem., 2016, 32(1), 131—138 |
| 张前, 韩燕, 焦元红. 无机化学学报, 2016, 32(1), 131—138 | |
| 39 | Zhao H. Y., Yang F. L., Li N., Wang X. J., J. Mol. Struct., 2017, 1148, 62—72 |
| 40 | Sheldrick G. M., Program for Crystal Structures Refinement: SHELXL⁃97, University of Göttingen, Göttingen, 1997 |
| 41 | Addison A.W., Rao T. N., Reedijk J., van Rijn J., Verschoor G. C., J. Chem. Soc., Dalton Trans., 1984, (7), 1349—1356 |
| 42 | Mukherjee T., Pessoa J. C., Kumar A., Sarkar A. R., Dalton Trans., 2013, 42(7), 2594—2607 |
| 43 | Franco E., López⁃Torres E., Mendiola M. A., Sevilla M. T., Polyhedron, 2000, 19(4), 441—451 |
| 44 | Sreedaran S., Bharathi K. S., Rahiman A. K., Jagadish L., Kaviyarasan V., Narayanan V., Polyhedron, 2008, 27(13), 2931—2938 |
| 45 | Kundu B. K., Ranjan R., Mukherjee A., Mobin S. M., Mukhopadhyay S., J. Inorg. Biochem., 2019, 195, 164—173 |
| [1] | 施耐克, 张娅, SANSON Andrea, 王蕾, 陈骏. Zn(NCN)单轴的负热膨胀性及机理研究[J]. 高等学校化学学报, 2022, 43(6): 20220124. |
| [2] | 徐丹丹, 邹修成, 罗静, 刘仁. 吩噻嗪席夫碱可见光引发剂的合成与性能[J]. 高等学校化学学报, 2022, 43(4): 20210857. |
| [3] | 岳胜利, 武光宝, 李星, 李康, 黄高胜, 唐翌, 周惠琼. 准二维钙钛矿太阳能电池的研究进展[J]. 高等学校化学学报, 2021, 42(6): 1648. |
| [4] | 田霞,杨福群,袁伟,赵雷,姚雷,甄小丽,韩建荣,刘守信. 含噁二唑大环冠醚的合成、 结构及金属离子识别性能[J]. 高等学校化学学报, 2020, 41(3): 490. |
| [5] | 刘东枚,苏雅静,李姗姗,许奇炜,李夏. 4-(4-羧基苯氧基)间苯二甲酸构筑的过渡金属配位聚合物: 合成、 晶体结构、 荧光传感与光催化[J]. 高等学校化学学报, 2020, 41(2): 253. |
| [6] | 秦刘磊,刘洋,关小琴,郑晓媛,张子钰,刘尊奇. 无机-有机杂化化合物[(H2DABCO)CuCl4]·H2O的合成及开关型介电性质[J]. 高等学校化学学报, 2020, 41(1): 70. |
| [7] | 李冰, 王学敏, 白凤英, 刘淑清. 稀土氮杂环配合物的合成、 结构及抑菌活性[J]. 高等学校化学学报, 2019, 40(4): 632. |
| [8] | 王冬梅, 刘子华, 李光华, 刘云凌, 李春霞. 铟基双金属配位聚合物的合成、 结构及荧光性质[J]. 高等学校化学学报, 2018, 39(9): 1886. |
| [9] | 田欢, 张梦龙, 王莉莎, 童碧海, 赵卓. 4,13-二硫杂苯并-18-冠-6的合成及对Ag+的选择性萃取[J]. 高等学校化学学报, 2018, 39(6): 1191. |
| [10] | 赵国政, 范荣荣, 颜熹琳, 唐维, 唐明峰, 贾建峰, 武海顺. 高压下晶体5-硝胺基-3,4-二硝基吡唑肼结构转变的周期性密度泛函理论研究[J]. 高等学校化学学报, 2018, 39(2): 292. |
| [11] | 李铮, 李睿, 李夏. 2-(3'-羧基苯氧基)苯甲酸和含氮配体构筑的过渡金属配合物的合成、 晶体结构及荧光性质[J]. 高等学校化学学报, 2018, 39(11): 2363. |
| [12] | 陈奇丹, 唐俊杰, 方千荣. 高稳定性的含氟多级孔共价有机框架材料[J]. 高等学校化学学报, 2018, 39(11): 2357. |
| [13] | 贠吉星, 胡志莉, 李禹蒙, 金晶, 陈冲, 鄢欣, 刘永华, 丁榆, 迟玉贤, 牛淑云. 由芳香羧酸构筑的系列Ni(Ⅱ)配合物的合成、 结构及光电性能[J]. 高等学校化学学报, 2018, 39(10): 2161. |
| [14] | 王秋爽, 郑晓丽, 屈相龙, 李睿, 李夏. 1,3-二(4-吡啶基)-丙烷与邻苯二甲酸构筑的过渡金属配合物的合成、结构和荧光性质[J]. 高等学校化学学报, 2017, 38(7): 1125. |
| [15] | 辛丙靖, 李鹏, 罗力莎, 夏添, 李光华. 一维链状卤化亚铜化合物的溶剂热原位合成、 结构及性质[J]. 高等学校化学学报, 2017, 38(4): 530. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||