

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (10): 20240235.doi: 10.7503/cjcu20240235
任峻晴1, 马玉乐1, 马宇欣1, 高少琪1, 仇宇豪1, 陈国博1( ), 夏树伟1,2(
), 夏树伟1,2( ), 于良民1,2
), 于良民1,2
收稿日期:2024-05-17
出版日期:2024-10-10
发布日期:2024-08-30
通讯作者:
陈国博,夏树伟
E-mail:chenguobo@ouc.edu.cn;shuweixia@ouc.edu.cn
基金资助:
REN Junqing1, MA Yule1, MA Yuxin1, GAO Shaoqi1, QIU Yuhao1, CHEN Guobo1( ), XIA Shuwei1,2(
), XIA Shuwei1,2( ), YU Liangmin1,2
), YU Liangmin1,2
Received:2024-05-17
Online:2024-10-10
Published:2024-08-30
Contact:
CHEN Guobo, XIA Shuwei
E-mail:chenguobo@ouc.edu.cn;shuweixia@ouc.edu.cn
Supported by:摘要:
为了解决碳钢在工业应用中的腐蚀状况, 设计合成了3种含哒嗪功能结构的酰胺醚类化合物(PCAE). 采用电化学分析和表面测试等实验方法探究了其在1 mol/L盐酸中对碳钢的缓蚀性能, 结果表明, PCAE是以抑制阳极为主的混合型缓蚀剂, PCAE1和PCAE2浓度为500 mg/L时缓蚀率分别为96.8%和93.1%, 而PCAE3在100 mg/L时缓蚀率即可达到96.5%. PCAE在碳钢表面以化学吸附为主, 符合Langmuir等温式, 在缓蚀剂作用下的碳钢表面腐蚀坑明显减少, 粗糙度曲线趋于平缓. 量子化学计算结果表明, PCAE的结构中含有大量活性吸附位点, PCAE3分子最低未占据轨道(LUMO)接受金属电子形成反馈键的能力更占优势, 其支链取代基中的C2pz 和羰基O2pz 对LUMO轨道的贡献较大.
中图分类号:
TrendMD:
任峻晴, 马玉乐, 马宇欣, 高少琪, 仇宇豪, 陈国博, 夏树伟, 于良民. 含哒嗪功能结构酰胺醚类化合物的合成及其缓蚀性能的实验评价与理论模拟. 高等学校化学学报, 2024, 45(10): 20240235.
REN Junqing, MA Yule, MA Yuxin, GAO Shaoqi, QIU Yuhao, CHEN Guobo, XIA Shuwei, YU Liangmin. Synthesis of Pyridazine-containing Amide Ethers and Experimental Evaluation and Theoretical Studies of Their Corrosion Inhibition. Chem. J. Chinese Universities, 2024, 45(10): 20240235.
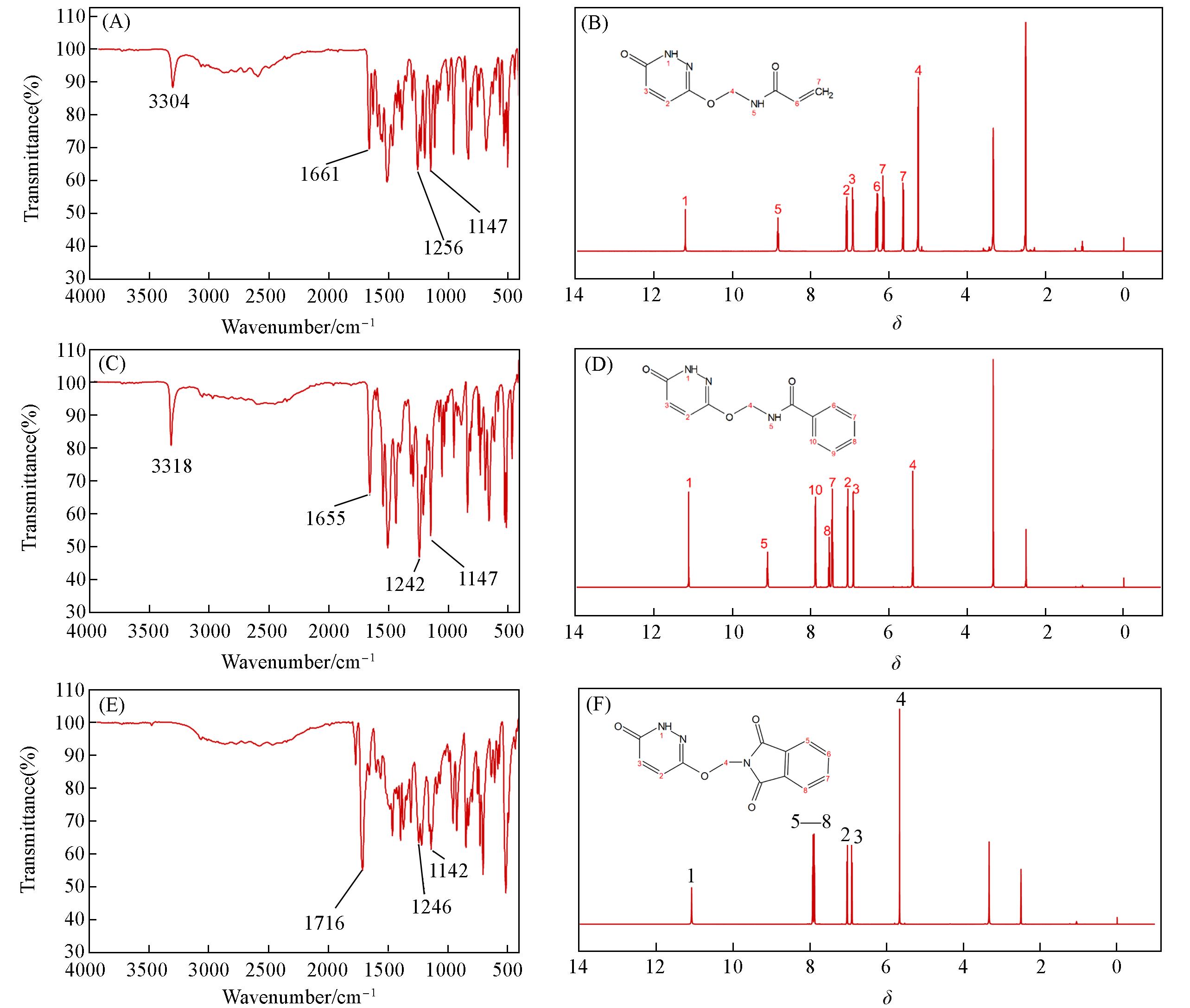
Fig.1 FTIR spectra(A, C, E) and 1H NMR spectra(B, D, F) of PCAE1(A, B), PCAE2(C, D), PCAE3(E, F)Insets: molecular structures of PCAE1—PCAE3, respectively.
| Molecule | c/(mg·L-1) | Ecorr/mV | BA/(mV·dec-1) | BC/(mV·dec-1) | icorr/(μA·cm-2) | IEP(%) |
|---|---|---|---|---|---|---|
| 0(Blank) | -450.7 | 46.31 | -117.37 | 117.90 | — | |
| PCAE1 | 100 | -438.71 | 51.14 | -106.29 | 13.98 | 88.1 |
| 200 | -448.79 | 52.92 | -116.74 | 13.51 | 88.5 | |
| 300 | -451.57 | 67.60 | -100.78 | 9.67 | 91.8 | |
| 400 | -441.75 | 55.56 | -108.19 | 7.20 | 93.9 | |
| 500 | -444.23 | 58.80 | -101.15 | 5.86 | 95.0 | |
| PCAE2 | 100 | -429.22 | 40.22 | -120.47 | 18.29 | 84.5 |
| 200 | -442.90 | 40.43 | -114.64 | 17.84 | 84.9 | |
| 300 | -446.42 | 44.88 | -102.89 | 16.54 | 86.0 | |
| 400 | -406.25 | 32.82 | -119.56 | 11.72 | 90.0 | |
| 500 | -419.06 | 34.91 | -109.76 | 10.16 | 91.4 | |
| PCAE3 | 20 | -448.20 | 52.04 | -118.78 | 37.51 | 68.2 |
| 40 | -442.56 | 52.50 | -110.70 | 32.85 | 72.1 | |
| 60 | -450.88 | 52.33 | -107.54 | 14.72 | 87.5 | |
| 80 | -447.76 | 48.81 | -95.24 | 11.73 | 90.1 | |
| 100 | -435.33 | 48.31 | -111.39 | 4.99 | 95.8 |
Table 1 PDP parameters for carbon steel in 1 mol/L HCl with different concentrations of PCAE
| Molecule | c/(mg·L-1) | Ecorr/mV | BA/(mV·dec-1) | BC/(mV·dec-1) | icorr/(μA·cm-2) | IEP(%) |
|---|---|---|---|---|---|---|
| 0(Blank) | -450.7 | 46.31 | -117.37 | 117.90 | — | |
| PCAE1 | 100 | -438.71 | 51.14 | -106.29 | 13.98 | 88.1 |
| 200 | -448.79 | 52.92 | -116.74 | 13.51 | 88.5 | |
| 300 | -451.57 | 67.60 | -100.78 | 9.67 | 91.8 | |
| 400 | -441.75 | 55.56 | -108.19 | 7.20 | 93.9 | |
| 500 | -444.23 | 58.80 | -101.15 | 5.86 | 95.0 | |
| PCAE2 | 100 | -429.22 | 40.22 | -120.47 | 18.29 | 84.5 |
| 200 | -442.90 | 40.43 | -114.64 | 17.84 | 84.9 | |
| 300 | -446.42 | 44.88 | -102.89 | 16.54 | 86.0 | |
| 400 | -406.25 | 32.82 | -119.56 | 11.72 | 90.0 | |
| 500 | -419.06 | 34.91 | -109.76 | 10.16 | 91.4 | |
| PCAE3 | 20 | -448.20 | 52.04 | -118.78 | 37.51 | 68.2 |
| 40 | -442.56 | 52.50 | -110.70 | 32.85 | 72.1 | |
| 60 | -450.88 | 52.33 | -107.54 | 14.72 | 87.5 | |
| 80 | -447.76 | 48.81 | -95.24 | 11.73 | 90.1 | |
| 100 | -435.33 | 48.31 | -111.39 | 4.99 | 95.8 |
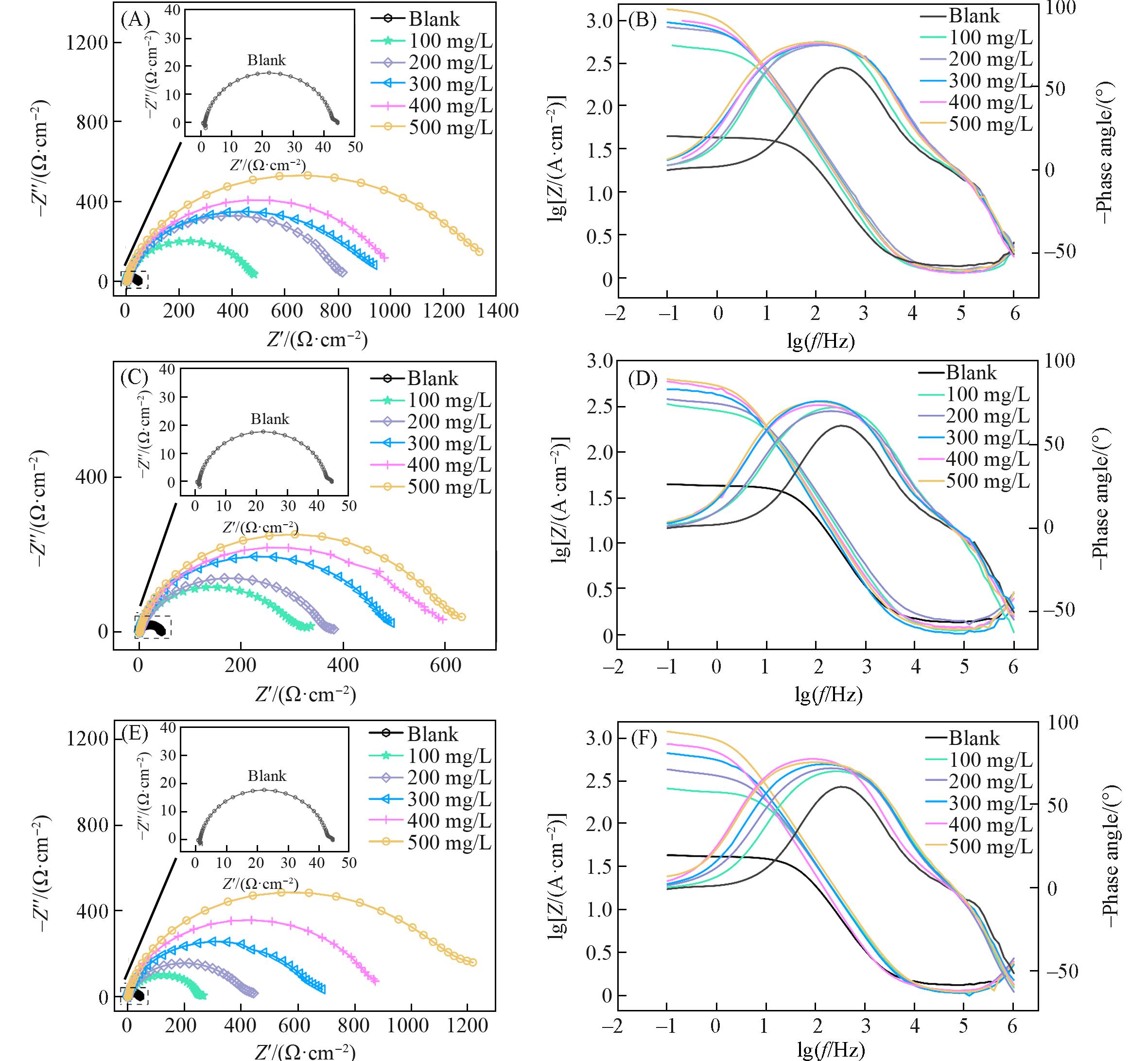
Fig.3 Nyquist curves(A, C, E) and bode curves(B, D, F) of carbon steel in 1 mol/L HCl containing different concentrations of PCAE1(A, B), PCAE2(C, D) and PCAE3(E, F)
| Molecule | c/(mg·L-1) | Rs/(Ω·cm2) | Cdl/(μF·cm-2) | n | Rct/(Ω·cm2) | IEEIS(%) |
|---|---|---|---|---|---|---|
| 0(Blank) | 1.384 | 141.3 | 0.89 | 41.6 | — | |
| PCAE1 | 100 | 1.168 | 109.7 | 0.91 | 470.2 | 91.2 |
| 200 | 1.219 | 84.1 | 0.88 | 799.0 | 94.8 | |
| 300 | 1.124 | 93.9 | 0.88 | 879.5 | 95.3 | |
| 400 | 1.127 | 96.2 | 0.88 | 990.3 | 95.8 | |
| 500 | 1.205 | 91.6 | 0.89 | 12980 | 96.8 | |
| PCAE2 | 100 | 1.102 | 101.1 | 0.88 | 286.2 | 85.5 |
| 200 | 1.393 | 105.5 | 0.86 | 355.4 | 88.3 | |
| 300 | 1.065 | 123.4 | 0.90 | 467.9 | 91.1 | |
| 400 | 1.198 | 121.4 | 0.87 | 545.7 | 92.4 | |
| 500 | 1.148 | 113.4 | 0.89 | 600.2 | 93.1 | |
| PCAE3 | 20 | 1.096 | 108.1 | 0.86 | 249.2 | 83.3 |
| 40 | 1.117 | 110.4 | 0.87 | 396.9 | 89.5 | |
| 60 | 1.095 | 98.0 | 0.88 | 628.4 | 93.4 | |
| 80 | 1.228 | 113.8 | 0.91 | 849.2 | 95.1 | |
| 100 | 1.137 | 83.8 | 0.88 | 1181.0 | 96.5 |
Table 2 EIS parameters of carbon steel in 1 mol/L HCl with different concentrations of PCAE
| Molecule | c/(mg·L-1) | Rs/(Ω·cm2) | Cdl/(μF·cm-2) | n | Rct/(Ω·cm2) | IEEIS(%) |
|---|---|---|---|---|---|---|
| 0(Blank) | 1.384 | 141.3 | 0.89 | 41.6 | — | |
| PCAE1 | 100 | 1.168 | 109.7 | 0.91 | 470.2 | 91.2 |
| 200 | 1.219 | 84.1 | 0.88 | 799.0 | 94.8 | |
| 300 | 1.124 | 93.9 | 0.88 | 879.5 | 95.3 | |
| 400 | 1.127 | 96.2 | 0.88 | 990.3 | 95.8 | |
| 500 | 1.205 | 91.6 | 0.89 | 12980 | 96.8 | |
| PCAE2 | 100 | 1.102 | 101.1 | 0.88 | 286.2 | 85.5 |
| 200 | 1.393 | 105.5 | 0.86 | 355.4 | 88.3 | |
| 300 | 1.065 | 123.4 | 0.90 | 467.9 | 91.1 | |
| 400 | 1.198 | 121.4 | 0.87 | 545.7 | 92.4 | |
| 500 | 1.148 | 113.4 | 0.89 | 600.2 | 93.1 | |
| PCAE3 | 20 | 1.096 | 108.1 | 0.86 | 249.2 | 83.3 |
| 40 | 1.117 | 110.4 | 0.87 | 396.9 | 89.5 | |
| 60 | 1.095 | 98.0 | 0.88 | 628.4 | 93.4 | |
| 80 | 1.228 | 113.8 | 0.91 | 849.2 | 95.1 | |
| 100 | 1.137 | 83.8 | 0.88 | 1181.0 | 96.5 |
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 2.084 | 2pz | 1.860 | C1 | 2p | 3.584 | 2pz | 3.201 |
| N2 | 2p | 19.085 | 2px | 0.736 | N2 | 2p | 12.936 | 2pz | 11.553 |
| 2pz | 17.040 | N3 | 2p | 20.365 | 2px | 0.766 | |||
| N3 | 2p | 18.039 | 2px | 0.692 | 2pz | 18.190 | |||
| 2pz | 16.128 | C4 | 2p | 3.988 | 2pz | 3.557 | |||
| C4 | 2p | 10.814 | 2pz | 9.666 | C5 | 2p | 24.627 | 2px | 0.941 |
| C6 | 2p | 7.462 | 2pz | 6.677 | 2pz | 22.024 | |||
| O7 | 2p | 24.647 | 2px | 0.927 | C6 | 2p | 27.699 | 2px | 1.047 |
| 2pz | 22.024 | 2pz | 24.751 | ||||||
| O8 | 2p | 15.362 | 2pz | 13.564 | O7 | 2p | 2.704 | 2pz | 2.415 |
| H19 | 1s | 0.767 | 1s | 0.767 | O8 | 0.585 | 2pz | 0.521 | |
Table 3 NAO analysis of the contribution of the major atoms to the frontier orbitals in PCAE1 molecules
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 2.084 | 2pz | 1.860 | C1 | 2p | 3.584 | 2pz | 3.201 |
| N2 | 2p | 19.085 | 2px | 0.736 | N2 | 2p | 12.936 | 2pz | 11.553 |
| 2pz | 17.040 | N3 | 2p | 20.365 | 2px | 0.766 | |||
| N3 | 2p | 18.039 | 2px | 0.692 | 2pz | 18.190 | |||
| 2pz | 16.128 | C4 | 2p | 3.988 | 2pz | 3.557 | |||
| C4 | 2p | 10.814 | 2pz | 9.666 | C5 | 2p | 24.627 | 2px | 0.941 |
| C6 | 2p | 7.462 | 2pz | 6.677 | 2pz | 22.024 | |||
| O7 | 2p | 24.647 | 2px | 0.927 | C6 | 2p | 27.699 | 2px | 1.047 |
| 2pz | 22.024 | 2pz | 24.751 | ||||||
| O8 | 2p | 15.362 | 2pz | 13.564 | O7 | 2p | 2.704 | 2pz | 2.415 |
| H19 | 1s | 0.767 | 1s | 0.767 | O8 | 0.585 | 2pz | 0.521 | |
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 2.055 | 2py | 1.481 | C1 | 2p | 3.531 | 2px | 0.666 |
| N2 | 2p | 19.058 | 2px | 3.557 | 2py | 2.544 | |||
| 2py | 13.734 | N2 | 2p | 12.911 | 2px | 2.424 | |||
| 2pz | 1.766 | 2py | 9.296 | ||||||
| N3 | 2p | 17.871 | 2px | 3.320 | 2pz | 1.190 | |||
| 2py | 12.838 | N3 | 2p | 20.376 | 2px | 3.806 | |||
| 2pz | 1.713 | 2py | 14.684 | ||||||
| C4 | 2p | 10.832 | 2px | 1.954 | 2pz | 1.885 | |||
| 2py | 7.874 | C4 | 2p | 4.011 | 2px | 0.736 | |||
| 2pz | 1.004 | 2py | 2.904 | ||||||
| C6 | 2p | 7.511 | 2px | 1.428 | C5 | 2p | 24.459 | 2px | 4.533 |
| 2py | 5.382 | 2py | 17.632 | ||||||
| 2pz | 0.701 | 2pz | 2.295 | ||||||
| O7 | 2p | 24.643 | 2px | 4.644 | C6 | 2p | 27.676 | 2px | 5.171 |
| 2py | 17.716 | 2py | 19.954 | ||||||
| 2pz | 2.283 | 2pz | 2.550 | ||||||
| O8 | 2p | 15.118 | 2px | 2.826 | O7 | 2p | 2.678 | 2px | 0.507 |
| 2py | 11.089 | 2py | 1.929 | ||||||
| 2pz | 1.203 | ||||||||
Table 4 NAO analysis of the contribution of the major atoms to the frontier orbitals in PCAE2 molecules
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 2.055 | 2py | 1.481 | C1 | 2p | 3.531 | 2px | 0.666 |
| N2 | 2p | 19.058 | 2px | 3.557 | 2py | 2.544 | |||
| 2py | 13.734 | N2 | 2p | 12.911 | 2px | 2.424 | |||
| 2pz | 1.766 | 2py | 9.296 | ||||||
| N3 | 2p | 17.871 | 2px | 3.320 | 2pz | 1.190 | |||
| 2py | 12.838 | N3 | 2p | 20.376 | 2px | 3.806 | |||
| 2pz | 1.713 | 2py | 14.684 | ||||||
| C4 | 2p | 10.832 | 2px | 1.954 | 2pz | 1.885 | |||
| 2py | 7.874 | C4 | 2p | 4.011 | 2px | 0.736 | |||
| 2pz | 1.004 | 2py | 2.904 | ||||||
| C6 | 2p | 7.511 | 2px | 1.428 | C5 | 2p | 24.459 | 2px | 4.533 |
| 2py | 5.382 | 2py | 17.632 | ||||||
| 2pz | 0.701 | 2pz | 2.295 | ||||||
| O7 | 2p | 24.643 | 2px | 4.644 | C6 | 2p | 27.676 | 2px | 5.171 |
| 2py | 17.716 | 2py | 19.954 | ||||||
| 2pz | 2.283 | 2pz | 2.550 | ||||||
| O8 | 2p | 15.118 | 2px | 2.826 | O7 | 2p | 2.678 | 2px | 0.507 |
| 2py | 11.089 | 2py | 1.929 | ||||||
| 2pz | 1.203 | ||||||||
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 1.995 | 2py | 1.968 | C11 | 2p | 12.994 | 2px | 1.374 |
| N2 | 2p | 19.384 | 2py | 19.106 | 2pz | 14.033 | |||
| N3 | 2p | 17.562 | 2py | 17.289 | C12 | 2p | 12.554 | 2px | 0.901 |
| C4 | 2p | 11.160 | 2py | 10.996 | 2pz | 9.778 | |||
| C6 | 2p | 7.892 | 2py | 7.773 | C13 | 2p | 12.547 | 2px | 0.904 |
| O7 | 2p | 25.245 | 2py | 24.890 | 2pz | 9.766 | |||
| O8 | 2p | 14.371 | 2py | 14.288 | C14 | 2p | 13.020 | 2px | 1.376 |
| H23 | 1s | 0.728 | 1s | 0.728 | 2pz | 13.988 | |||
| H24 | 1s | 0.687 | 1s | 0.687 | C15 | 2p | 1.810 | 2pz | 2.265 |
| C16 | 2p | 9.132 | 2px | 0.679 | |||||
| 2pz | 7.161 | ||||||||
| C17 | 2p | 9.125 | 2px | 0.679 | |||||
| 2pz | 7.161 | ||||||||
| C18 | 2p | 1.816 | 2pz | 2.265 | |||||
| O19 | 2p | 12.571 | 2px | 1.124 | |||||
| 2pz | 11.512 | ||||||||
| O20 | 2p | 12.575 | 2px | 1.121 | |||||
| 2pz | 11.560 | ||||||||
Table 5 NAO analysis of the contribution of the major atoms to the frontier orbitals in PCAE3 molecules
| Atom | HOMO | Atom | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subshell | S(%) | Orbital | O(%) | Subshell | S(%) | Orbital | O(%) | ||
| C1 | 2p | 1.995 | 2py | 1.968 | C11 | 2p | 12.994 | 2px | 1.374 |
| N2 | 2p | 19.384 | 2py | 19.106 | 2pz | 14.033 | |||
| N3 | 2p | 17.562 | 2py | 17.289 | C12 | 2p | 12.554 | 2px | 0.901 |
| C4 | 2p | 11.160 | 2py | 10.996 | 2pz | 9.778 | |||
| C6 | 2p | 7.892 | 2py | 7.773 | C13 | 2p | 12.547 | 2px | 0.904 |
| O7 | 2p | 25.245 | 2py | 24.890 | 2pz | 9.766 | |||
| O8 | 2p | 14.371 | 2py | 14.288 | C14 | 2p | 13.020 | 2px | 1.376 |
| H23 | 1s | 0.728 | 1s | 0.728 | 2pz | 13.988 | |||
| H24 | 1s | 0.687 | 1s | 0.687 | C15 | 2p | 1.810 | 2pz | 2.265 |
| C16 | 2p | 9.132 | 2px | 0.679 | |||||
| 2pz | 7.161 | ||||||||
| C17 | 2p | 9.125 | 2px | 0.679 | |||||
| 2pz | 7.161 | ||||||||
| C18 | 2p | 1.816 | 2pz | 2.265 | |||||
| O19 | 2p | 12.571 | 2px | 1.124 | |||||
| 2pz | 11.512 | ||||||||
| O20 | 2p | 12.575 | 2px | 1.121 | |||||
| 2pz | 11.560 | ||||||||
| Molecule | EHOMO/eV | ELUMO/eV | (ELUMO(PCAE)-EHOMO(Fe))/eV | (ELUMO(Fe)-EHOMO(PCAE))/eV | η |
|---|---|---|---|---|---|
| PCAE1 | -6.379 | -1.910 | 5.900 | 6.129 | 2.235 |
| PCAE2 | -6.394 | -1.921 | 5.889 | 6.144 | 2.237 |
| PCAE3 | -6.438 | -2.551 | 5.259 | 6.188 | 1.944 |
| Fe[ | -7.810 | -0.250 | — | — | — |
Table 6 Global quantum chemical parameters of PCAE molecules
| Molecule | EHOMO/eV | ELUMO/eV | (ELUMO(PCAE)-EHOMO(Fe))/eV | (ELUMO(Fe)-EHOMO(PCAE))/eV | η |
|---|---|---|---|---|---|
| PCAE1 | -6.379 | -1.910 | 5.900 | 6.129 | 2.235 |
| PCAE2 | -6.394 | -1.921 | 5.889 | 6.144 | 2.237 |
| PCAE3 | -6.438 | -2.551 | 5.259 | 6.188 | 1.944 |
| Fe[ | -7.810 | -0.250 | — | — | — |
| Molecule | Atom | Atom | ||||
|---|---|---|---|---|---|---|
| PCAE1 | C1 | 0.0737 | 0.0717 | O8 | 0.0313 | 0.1013 |
| N2 | 0.0731 | 0.0990 | C9 | 0.0112 | 0.0203 | |
| N3 | 0.1269 | 0.1203 | N10 | 0.0028 | 0.0045 | |
| C4 | 0.0752 | 0.0879 | C11 | 0.0031 | 0.0054 | |
| C5 | 0.1452 | 0.0407 | C12 | 0.0008 | 0.0014 | |
| C6 | 0.1634 | 0.0718 | C13 | 0.0024 | 0.0043 | |
| O7 | 0.0839 | 0.2134 | O14 | 0.0042 | 0.0082 | |
| PCAE2 | C1 | 0.0735 | 0.0714 | N10 | 0.0034 | 0.0055 |
| N2 | 0.0730 | 0.0987 | C11 | 0.0032 | 0.0051 | |
| N3 | 0.1269 | 0.1194 | C12 | 0.0003 | 0.0003 | |
| C4 | 0.0750 | 0.0873 | O13 | 0.0054 | 0.0099 | |
| C5 | 0.1445 | 0.0405 | C14 | 0.0010 | 0.0019 | |
| C6 | 0.1632 | 0.0718 | C15 | 0.0008 | 0.0020 | |
| O7 | 0.0835 | 0.2124 | C16 | 0.0011 | 0.0019 | |
| O8 | 0.0307 | 0.0996 | C17 | 0.0009 | 0.0019 | |
| C9 | 0.0110 | 0.0198 | C18 | 0.0008 | 0.0013 | |
| PCAE3 | C1 | 0.0012 | 0.0721 | C11 | 0.1019 | 0.0033 |
| N2 | 0.0011 | 0.1005 | C12 | 0.0609 | 0.0011 | |
| N3 | 0.0024 | 0.1186 | C13 | 0.0609 | 0.0011 | |
| C4 | 0.0020 | 0.0885 | C14 | 0.1019 | 0.0033 | |
| C5 | 0.0008 | 0.0414 | C15 | 0.0489 | 0.0017 | |
| C6 | 0.0015 | 0.0736 | C16 | 0.0635 | 0.0019 | |
| O7 | 0.0020 | 0.2170 | C17 | 0.0635 | 0.0019 | |
| O8 | 0.0049 | 0.0964 | C18 | 0.0489 | 0.0017 | |
| C9 | 0.0123 | 0.0195 | C19 | 0.1315 | 0.0054 | |
| N10 | 0.0217 | 0.0023 | C20 | 0.1314 | 0.0053 |
Table 7 Fukui function of atoms in PCAE
| Molecule | Atom | Atom | ||||
|---|---|---|---|---|---|---|
| PCAE1 | C1 | 0.0737 | 0.0717 | O8 | 0.0313 | 0.1013 |
| N2 | 0.0731 | 0.0990 | C9 | 0.0112 | 0.0203 | |
| N3 | 0.1269 | 0.1203 | N10 | 0.0028 | 0.0045 | |
| C4 | 0.0752 | 0.0879 | C11 | 0.0031 | 0.0054 | |
| C5 | 0.1452 | 0.0407 | C12 | 0.0008 | 0.0014 | |
| C6 | 0.1634 | 0.0718 | C13 | 0.0024 | 0.0043 | |
| O7 | 0.0839 | 0.2134 | O14 | 0.0042 | 0.0082 | |
| PCAE2 | C1 | 0.0735 | 0.0714 | N10 | 0.0034 | 0.0055 |
| N2 | 0.0730 | 0.0987 | C11 | 0.0032 | 0.0051 | |
| N3 | 0.1269 | 0.1194 | C12 | 0.0003 | 0.0003 | |
| C4 | 0.0750 | 0.0873 | O13 | 0.0054 | 0.0099 | |
| C5 | 0.1445 | 0.0405 | C14 | 0.0010 | 0.0019 | |
| C6 | 0.1632 | 0.0718 | C15 | 0.0008 | 0.0020 | |
| O7 | 0.0835 | 0.2124 | C16 | 0.0011 | 0.0019 | |
| O8 | 0.0307 | 0.0996 | C17 | 0.0009 | 0.0019 | |
| C9 | 0.0110 | 0.0198 | C18 | 0.0008 | 0.0013 | |
| PCAE3 | C1 | 0.0012 | 0.0721 | C11 | 0.1019 | 0.0033 |
| N2 | 0.0011 | 0.1005 | C12 | 0.0609 | 0.0011 | |
| N3 | 0.0024 | 0.1186 | C13 | 0.0609 | 0.0011 | |
| C4 | 0.0020 | 0.0885 | C14 | 0.1019 | 0.0033 | |
| C5 | 0.0008 | 0.0414 | C15 | 0.0489 | 0.0017 | |
| C6 | 0.0015 | 0.0736 | C16 | 0.0635 | 0.0019 | |
| O7 | 0.0020 | 0.2170 | C17 | 0.0635 | 0.0019 | |
| O8 | 0.0049 | 0.0964 | C18 | 0.0489 | 0.0017 | |
| C9 | 0.0123 | 0.0195 | C19 | 0.1315 | 0.0054 | |
| N10 | 0.0217 | 0.0023 | C20 | 0.1314 | 0.0053 |
| 1 | Li Z., Ren Y. H., Li Z. T., Zhang J. R., Fan Y. Q., Jiang G. M., Xu D. K., Gu T. Y., Wang F. H., Adv. Funct. Mater., 2024, 34(13), 2313120 |
| 2 | Kokalj A., Peljhan S., Finšgar M., Milošev I., J. Am. Chem. Soc., 2010, 132(46), 16657—16668 |
| 3 | Dong Q. C., Zhang G. H., Zhang W. B., Liu J., Chem. J. Chinese Universities, 2019, 40(10), 2195—2204 |
| 董秋辰, 张光华, 张万斌, 刘晶. 高等学校化学学报, 2019, 40(10), 2195—2204 | |
| 4 | An W. Z., Yao X. C., Hu L. H., Wang Z., Wang J., Zhang L., Corrosion Protection, 2021, 42(7), 14—19 |
| 安维峥, 姚星城, 胡丽华, 王竹, 王晶, 张雷. 腐蚀与防护, 2021, 42(7), 14—19 | |
| 5 | Kim S., Ahn K., Kim G., Song S. W., Corros. Sci., 2023, 218, 111176 |
| 6 | Mrani S. A., Ech⁃Chihbi E., Salim R., Daoui S., Benchat N., Saffaj T., Zarrouk A., Taleb M., J. Mol. Liq., 2023, 382, 122043 |
| 7 | Luo W., Lin Q. Y., Ran X., Li W. P., Tan B. C., Fu A. Q., Zhang S. T., J. Mol. Liq., 2021, 341, 117370 |
| 8 | Quadri T. W., Olasunkanmi L. O., Akpan E. D., Fayemi O. E., Lee H. S., Lgaz H., Verma C., Guo L., Kaya S., Ebenso E. E., Mater. Today Commun., 2022, 30, 103163 |
| 9 | El Assiri E., Driouch M., Lazrak J., Bensouda Z., Elhaloui A., Sfaira M., Saffaj T., Taleb M., Heliyon, 2020, 6(10), e05067 |
| 10 | Luo W., Li W. P., Tan J., Liu J., Tan B. C., Zuo X. L., Wang Z. Y., Zhang X., J. Mol. Liq., 2020, 314, 113630 |
| 11 | Olasunkanmi L. O., Mashuga M. E., Ebenso E. E., Surfaces Interfaces, 2018, 12, 8—19 |
| 12 | Beniken M., Daoui S., Mrani S. A., Benhiba F., Benchat N., Zarrouk A., Taleb M., Colloid. Surface. A, 2023, 673, 131699 |
| 13 | Fan D. B., The Study on Synthesis of Pyridazine and 4⁃Methyl⁃pyridazine, Zhejiang University, Hangzhou, 2007 |
| 樊东波. 哒嗪类化合物的合成研究, 杭州: 浙江大学, 2007 | |
| 14 | Yu X. K., Zhang S. Q., Fu S. K., Tan B. C., Zhou D., Zeng J., Wang Z. P., Li W. P., Hu C. G., Adv. Funct. Mater., 2024, 34(30), 2316140 |
| 15 | Xia S. W., Zhang G. L., Yu L. M., Periodical of Ocean University of China(Natural Sciences), 2020, 50(3), 54—60 |
| 夏树伟, 张广龙, 于良民. 中国海洋大学学报(自然科学版), 2020, 50(3), 54—60 | |
| 16 | Guo R., Li Y. P., Tu R. X., Song B., Guo Y., Chem. J. Chinese Universities, 2018, 39(5), 1018—1025 |
| 郭睿, 李云鹏, 土瑞香, 宋博, 郭煜. 高等学校化学学报, 2018, 39(5), 1018—1025 | |
| 17 | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Petersson G. A., Nakatsuji H., Li X., Caricato M., Marenich A. V., Bloino J., Janesko B. G., Gomperts R., Mennucci B., Hratchian H. P., Ortiz J. V., Izmaylov A. F., Sonnenberg J. L., Williams⁃Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V. G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J. J., Brothers E. N., Kudin K. N., Staroverov V. N., Keith T. A., Kobayashi R., Normand J., Raghavachari K., Rendell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Millam J. M., Klene M., Adamo C., Cammi R., Ochterski J. W., Martin R. L., Morokuma K., Farkas O., Foresman J. B., Fox D. J., Gaussian 16, Revision C.01, Gaussian Inc., Wallingford CT, 2016 |
| 18 | Yang W., Mortier W. J., J. Am. Chem. Soc., 1986, 108(19), 5708—5711 |
| 19 | Khan G., Basirun W. J., Kazi S. N., Ahmed P., Magaji L., Ahmed S. M., Khan G. M., Rehman M. A., J. Colloid Interf. Sci., 2017, 502, 134—145 |
| 20 | Rao X. X., Wang X., Wang Y. J., Xia S. W., Yu L. M., Surface Technology, 2020, 49(11), 252—261 |
| 饶兴兴, 王璇, 王言建, 夏树伟, 于良民. 表面技术, 2020, 49(11), 252—261 | |
| 21 | Swenson H., Stadie N. P., Langmuir, 2019, 35(16), 5409—5426 |
| 22 | Qiang Y. J., Guo L., Li H., Lan X. J., Chem. Eng. J., 2021, 406, 126863 |
| 23 | Dong Q. C., Zhang G. H., Zhang W. B., Zhang X., Liu J., Chem. J. Chinese Universities, 2019, 40(12), 2556—2565 |
| 董秋辰, 张光华, 张万斌, 张雪, 刘晶. 高等学校化学学报, 2019, 40(12), 2556—2565 | |
| 24 | Ozcan M., Karadag F., Dehri I., Acta Physico⁃Chimica Sinica, 2008, 24(8), 1387—1392 |
| 25 | Shang D. N., Wu S. H., Meng Y. J., Liu C., Wang J. K., Ma L. W., Develop. Appl. Mater., 2023, 38(4), 98—110 |
| 商丹妮, 吴尚浩, 孟怡君, 刘畅, 王金科, 马菱薇. 材料开发与应用, 2023, 38(4), 98—110 | |
| 26 | Liu Q., Liu J. Y., Wang J., Zhong Y., Surface Technology, 2022, 51(10), 250—259 |
| 刘倩, 刘金彦, 王佳, 种瑶. 表面技术, 2022, 51(10), 250—259 | |
| 27 | Wang J. Q., Ma Y. G., Chem. J. Chinese Universities, 2022, 43(4), 20210856 |
| 王剑桥, 马於光. 高等学校化学学报, 2022, 43(4), 20210856 | |
| 28 | Hotop H., Lineberger W. C., J. Phys. Chem. Ref. Data, 1985, 14(3), 731—750 |
| 29 | Hu S. Q., Hu J. C., Fan C. C., Mi S. Q., Zhang J., Guo W. Y., Acta Physico⁃Chimica Sinica, 2010, 26(8), 2163—2170 |
| 胡松青, 胡建春, 范成成, 米思奇, 张军, 郭文跃. 物理化学学报, 2010, 26(8), 2163—2170 | |
| 30 | Huang Y. K., Qiu X. H., University Chemistry, 2016, 31(11), 45—50 |
| 黄一珂, 邱晓航. 大学化学, 2016, 31(11), 45—50 |
| [1] | 汤茹, 但琪, 叶荣兴, 兰越景, 王劲川, 谌晓洪, 宋江锋, 周琳森. 水分子在ZrCo(110)表面聚集与解离的理论研究[J]. 高等学校化学学报, 2024, 45(8): 20240186. |
| [2] | 刘星雨, 张晓晶, 向婷婷, 陈士欣, 马硕, 邵慧敏, 崔博, 夏春龙, 李岩, 布乃顺, 李丛. 含硫多孔芳香框架材料的制备及超灵敏检测同步去除汞的双重性能[J]. 高等学校化学学报, 2024, 45(7): 20240138. |
| [3] | 张苡宁, 卫来, 石彤非, 徐林. 界面吸附对高分子液体膜去润湿动力学的影响[J]. 高等学校化学学报, 2024, 45(5): 20240046. |
| [4] | 刘智, 谷俊红, 李宁宁, 刘志生, 刘斌, 李阳雪. 新型镓基金属有机凝胶的快速室温制备及吸附四环素性能[J]. 高等学校化学学报, 2024, 45(5): 20230485. |
| [5] | 余谟鑫, 史文旭, 孙宇航, 张晨, 王晓婷. P掺杂煤沥青基多孔炭的制备及对废水中广谱抗生素的吸附性能[J]. 高等学校化学学报, 2024, 45(4): 20230481. |
| [6] | 陈荣, 温良英, 岳东, 杨仲卿. Cl2和O2在TiC(100)表面共吸附行为的密度泛函理论分析[J]. 高等学校化学学报, 2024, 45(4): 20230497. |
| [7] | 田振华, 高盼盼, 于若泓, 赵文杰. 基于含铬革屑制备吸附-降解型胶原/ZnO复合材料[J]. 高等学校化学学报, 2024, 45(3): 20230416. |
| [8] | 童大银, 赵耀林, 王禹齐, 韩子彤, 王杰, 张俊, 喻晨曦. 气态碘在COF-103上吸附的理论研究[J]. 高等学校化学学报, 2024, 45(1): 20230401. |
| [9] | 杨玉婷, 丛明晓, 景晓飞, 刘佳. 低成本季铵盐类多孔材料的合成及氨气吸附性能[J]. 高等学校化学学报, 2024, 45(1): 20230438. |
| [10] | 刘金露, 郭嘉禹, 华佳, 李光华, 施展, 冯守华. 一种酰胺基Cu-MOF的构筑与吸附性能研究[J]. 高等学校化学学报, 2023, 44(6): 20220746. |
| [11] | 陈佳琪, 程晚亭, 温秋慧, 韩静茹, 马福秋, 颜永得, 薛云. 活性炭电极的改性及对Co2+, Mn2+和Ni2+的电吸附性能[J]. 高等学校化学学报, 2023, 44(4): 20220598. |
| [12] | 王兆宇, 陈益宾, 程锦添, 张明文. Ni-Co/TiO2增强CO2加氢反应性能的研究[J]. 高等学校化学学报, 2023, 44(11): 20230308. |
| [13] | 于先超, 亓文帅, 邓全花, 侯万国. 水-短链醇二元溶液的表面吸附[J]. 高等学校化学学报, 2023, 44(11): 20230316. |
| [14] | 廖首维, 刘炎昌, 石泽南, 赵道辉, 魏嫣莹, 李理波. 水/石墨烯界面离子吸附的分子动力学模拟: 力场参数优化与吸附机制[J]. 高等学校化学学报, 2023, 44(10): 20230155. |
| [15] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||