

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (12): 20220320.doi: 10.7503/cjcu20220320
马小飞1, 胡山1, 李俊彬1, 杨盛2, 谌委菊1, 卿志和1( ), 周怡波1(
), 周怡波1( ), 杨荣华2(
), 杨荣华2( )
)
收稿日期:2022-05-10
出版日期:2022-12-10
发布日期:2022-05-30
通讯作者:
卿志和,周怡波,杨荣华
E-mail:qingzhihe@hnu.edu.cn;yibozhou@163.com;yangrh@pku.edu.cn
基金资助:
MA Xiaofei1, HU Shan1, LI Junbin1, YANG Sheng2, CHEN Weiju1, QING Zhihe1( ), ZHOU Yibo1(
), ZHOU Yibo1( ), YANG Ronghua2(
), YANG Ronghua2( )
)
Received:2022-05-10
Online:2022-12-10
Published:2022-05-30
Contact:
QING Zhihe, ZHOU Yibo, YANG Ronghua
E-mail:qingzhihe@hnu.edu.cn;yibozhou@163.com;yangrh@pku.edu.cn
Supported by:摘要:
细胞内原位信号放大策略是检测低丰度内源性目标物的有效手段, 但多数信号放大策略依赖于外源性辅助物, 不可避免地改变细胞内微环境, 进而对机体造成一定干扰. 针对此问题, 可利用细胞内源性物质(如金属离子、 核酸、 蛋白酶等)实现原位荧光信号放大, 对不同生物标志物进行荧光成像, 此方法对低丰度靶分子检测及成像具有重要意义. 本文对内源性物质辅助信号放大及细胞内荧光成像相关研究进行了归纳整理, 介绍了内源性核酸、 酶、 蛋白质、 三磷酸腺苷(ATP)和金属离子辅助信号放大策略, 并探讨了其信号放大机理; 总结了内源性物质辅助信号放大探针在低丰度物质检测及成像方面的研究进展; 最后展望了该策略在细胞成像方面的优势及应用前景.
中图分类号:
TrendMD:
马小飞, 胡山, 李俊彬, 杨盛, 谌委菊, 卿志和, 周怡波, 杨荣华. 细胞内源性分子辅助荧光信号放大策略及细胞成像. 高等学校化学学报, 2022, 43(12): 20220320.
MA Xiaofei, HU Shan, LI Junbin, YANG Sheng, CHEN Weiju, QING Zhihe, ZHOU Yibo, YANG Ronghua. Cellular Endogenous Molecule-assisted Fluorescence Signal Amplification Strategy and the Application of Cell Imaging. Chem. J. Chinese Universities, 2022, 43(12): 20220320.
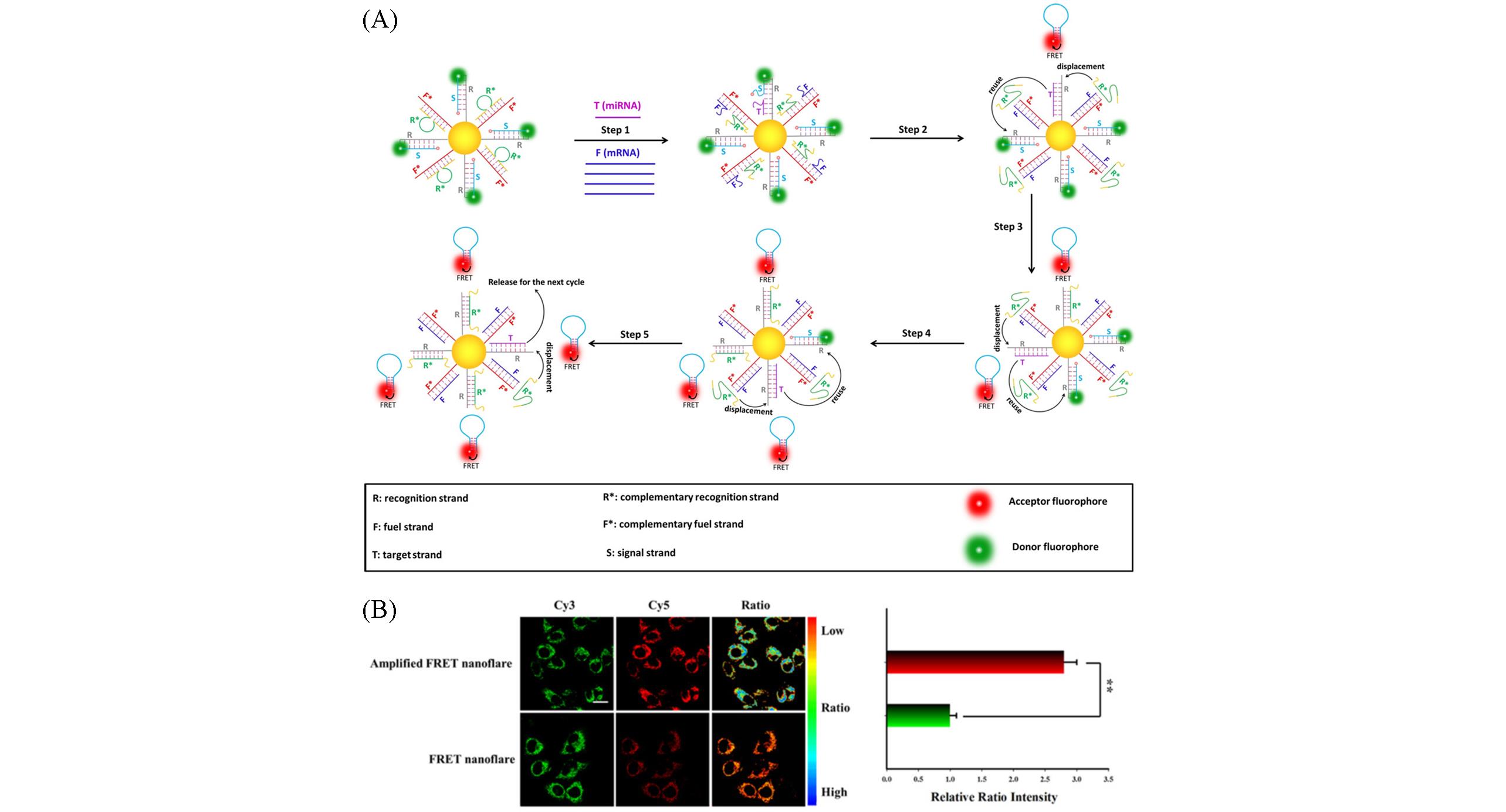
Fig.1 An endogenous mRNA⁃powered nanoflare image of intracellular microRNA in living cells[14](A)Schematic of working principle of mRNA-driven FRET nanoflares; (B)the amplification of imaging of miRNA in MCF-7 cells. Copyright 2020, Wiley‐VCH.
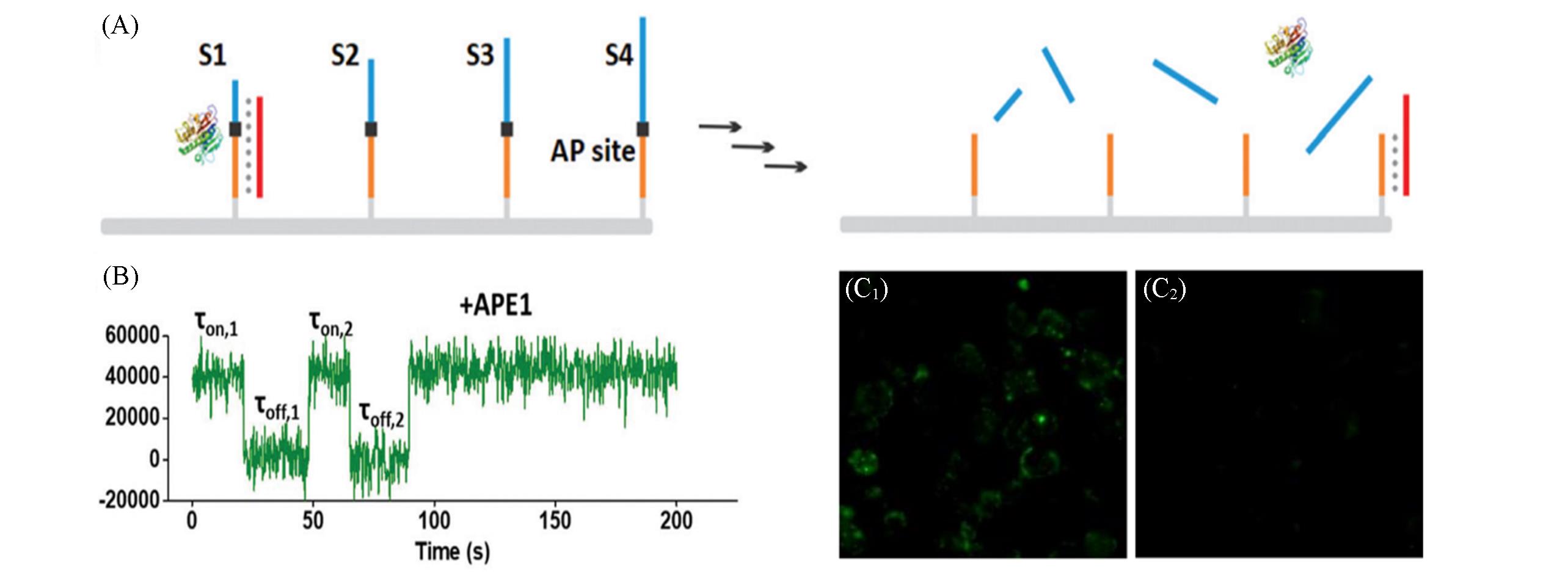
Fig.2 Intracellular endonuclease⁃driven DNA motors for discrimination of cancer cells and normal cells[21](A) Schematic of the APE1-driven burning “bridge” DNA motors; (B)single-molecule fluorescence trajectories of DNA motors in the presence of APE1 enzyme; (C1, C2) fluorescence images of living cells incubated with DNA motor. Copyright 2019, the Royal Society of Chemistry.
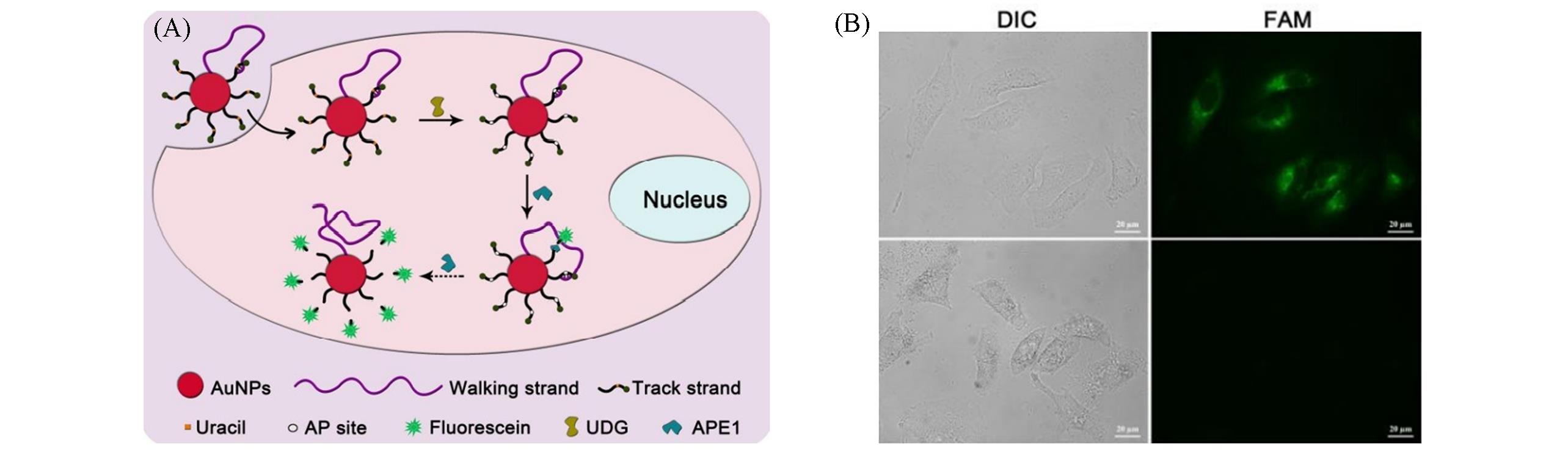
Fig.3 Endogenous enzyme⁃driven DNA walker image of uracil⁃DNA glycosylase in living cells[22](A) Schematic of the DNA walker powered by APE1 enzyme; (B) fluorescence images of HeLa cells treated with the DNA walker. Copyright 2013, the Royal Society of Chemistry.
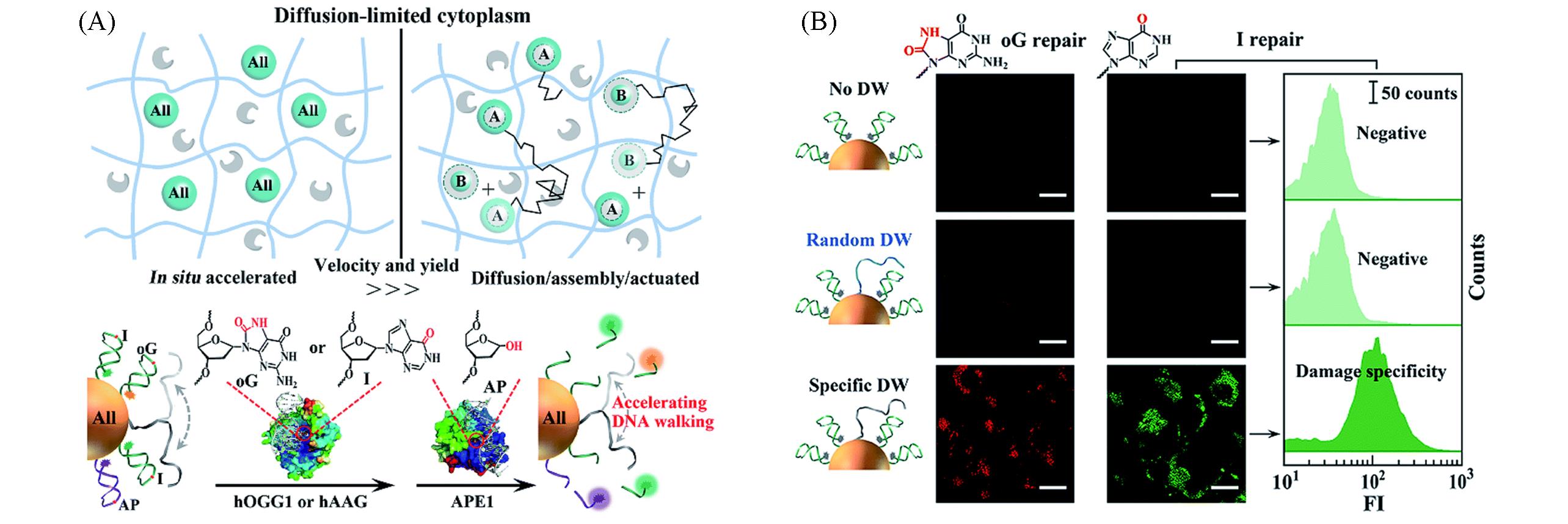
Fig.4 DNA walkers image of diffusion⁃restricted microenvironments in cells[27](A) Schematic of nanomotors powered by endogenous enzymes; (B) the DNA walkers monitor intracellular DNA damage repair pathways in living cells.Copyright 2019, the Royal Society of Chemistry.
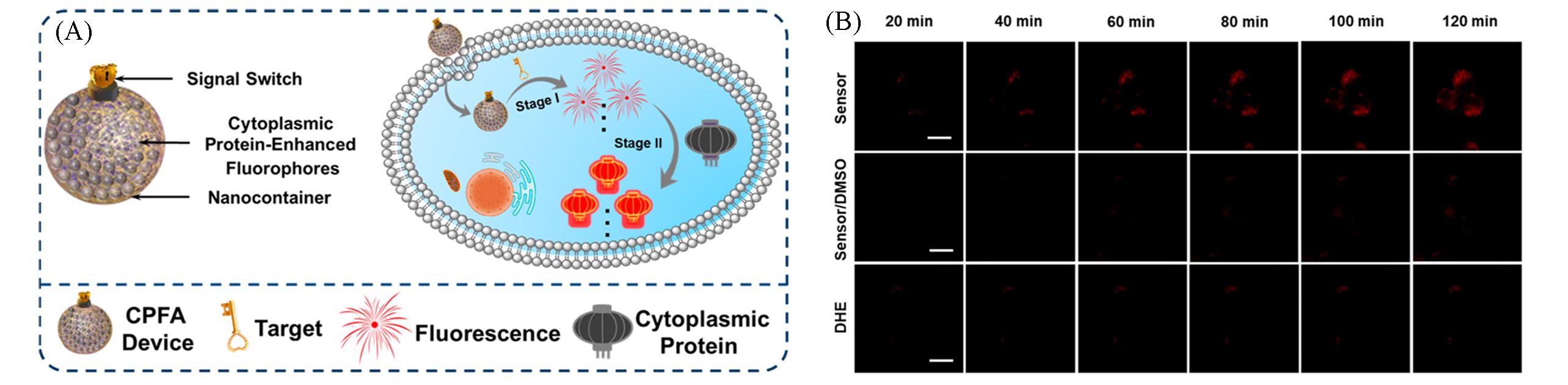
Fig.5 Nanosensor based on cytoplasmic protein⁃assisted signal amplification for imaging of ·OH[41](A) Schematic of cytoplasmic proteins-powered fluorescence amplification image of low abundance of target; (B) fluorescence images of RAW264.7 cells treated with nanosensor under different conditions.Copyright 2019, American Chemical Society.
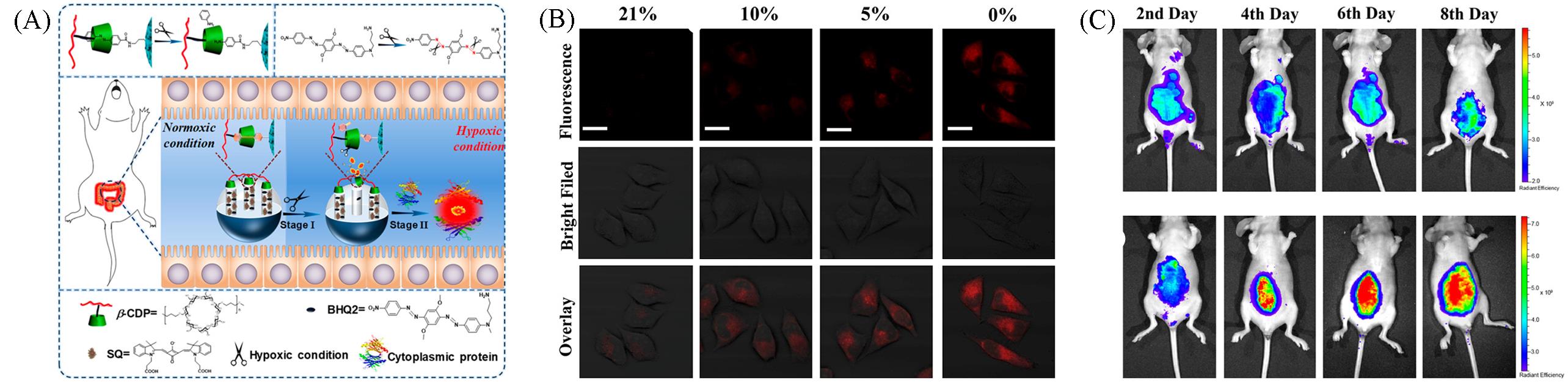
Fig.6 Nanoprobe based on cytoplasmic protein⁃assisted signal amplification image of hypoxia associated with acute colitis[42](A) Schematic of nanoprobe image of hypoxia associated with acute colitis; (B) fluorescence images of different hypoxic conditions in living cells; (C) mice of acute colitis model treated with nanoprobe.Copyright 2020, American Chemical Society.
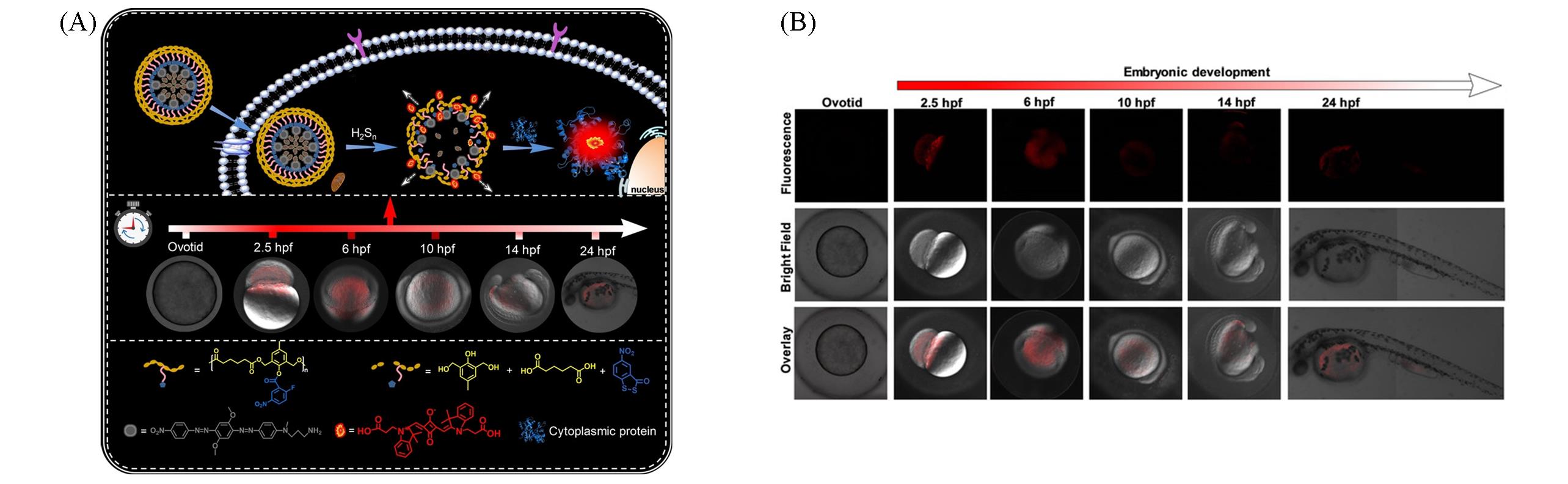
Fig.7 Monitoring the fluctuation of hydrogen polysulfides during fertilization and embryonic development with polymeric nanobeacon[43](A) Illustration of the polymeric nanobeacon in the presence of endogenous H2S n; (B) fluorescence imaging of H2S n during fertilization and embryonic development with the nanobeacon.Copyright 2022, Wiley‐VCH.
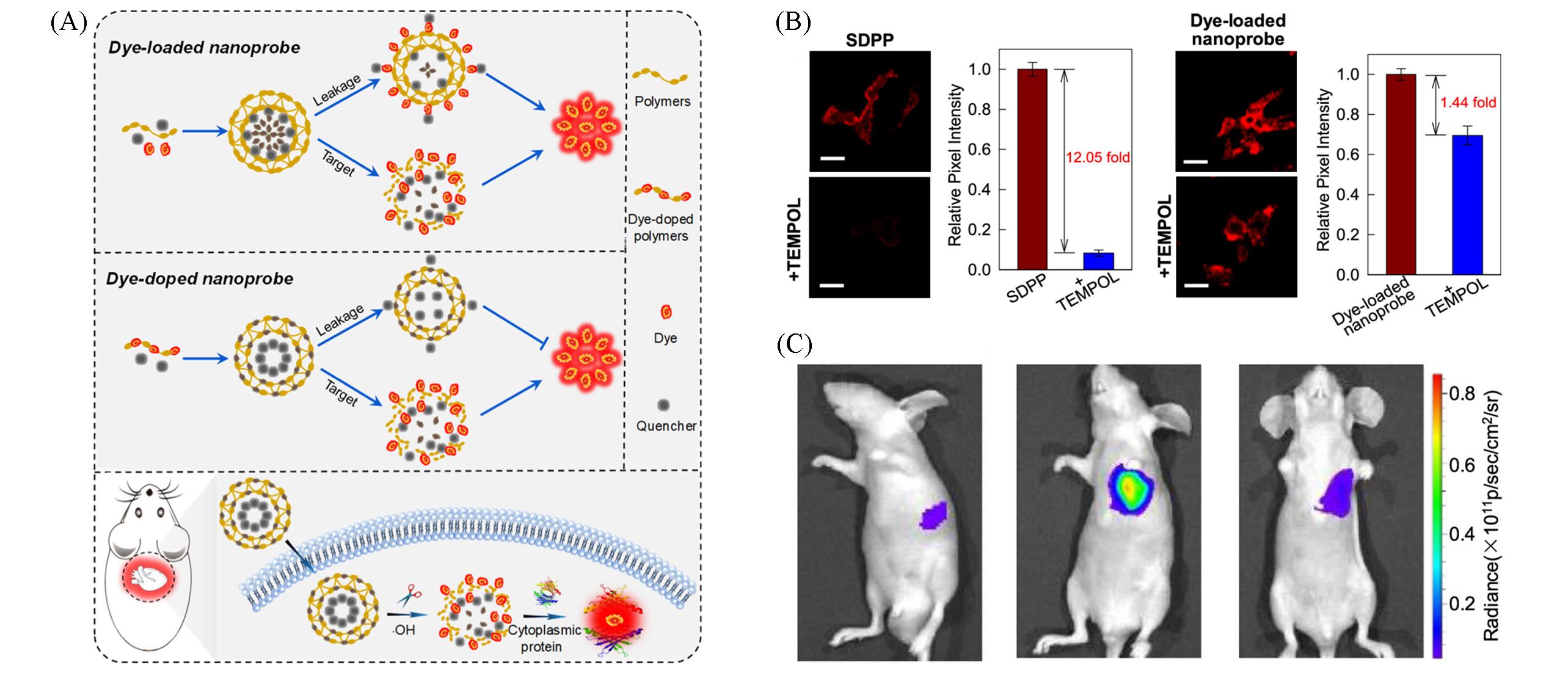
Fig.8 Self⁃immolative dye⁃doped polymeric probe image of ·OH by avoiding leakage[44](A) Schematic of the working mechanism of self-immolative dye-doped polymeric probe; (B) fluorescence images of living cells treated with SDPP and dye-loaded nanoprobe; (C) myocarditic nude mice treated with SDPP.Copyright 2021, American Chemical Society.
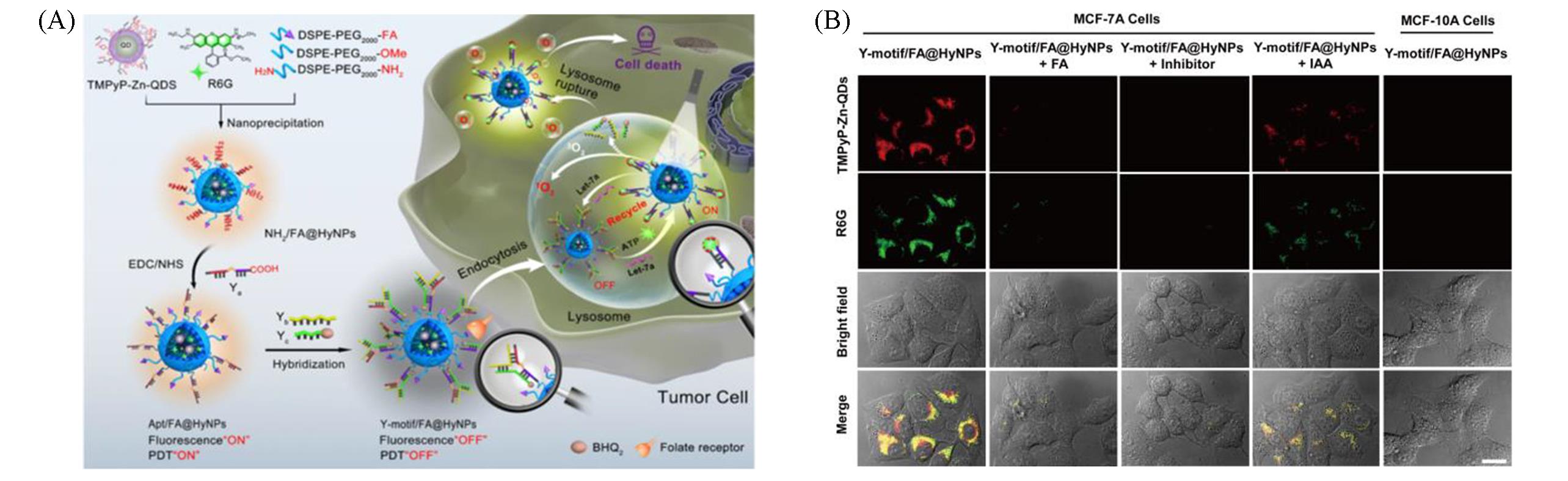
Fig.9 ATP⁃driven photosensitizers image of microRNA in cells[48](A) Schematic of the intracellular ATP-driven strand displacement reaction; (B) fluorescence images of cells incubated with photosensitizers under different conditions.Copyright 2019, American Chemical Society.
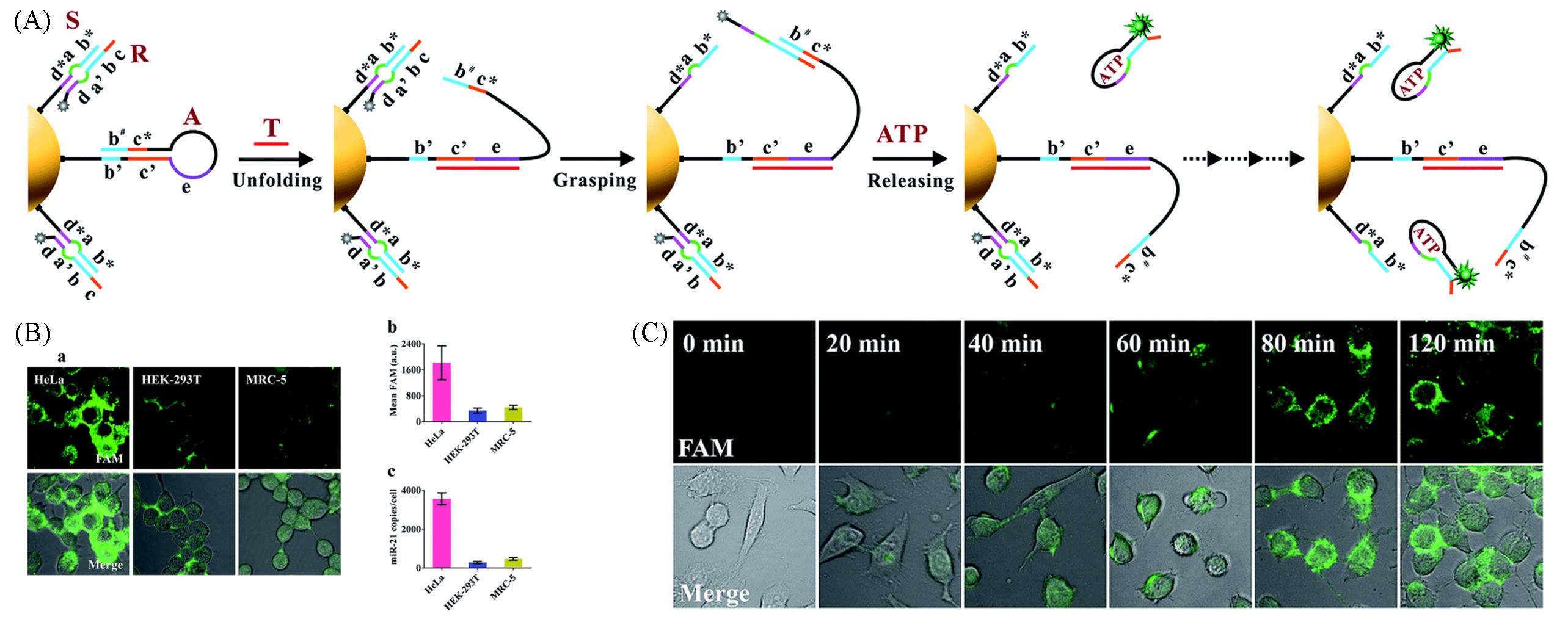
Fig.10 DNA nanomachine powered by endogenous ATP sense target in living cells[49](A) Schematic of the motion of proposed ATP-powered DNA nanomachine; (B) detection of miR-21 in living cells; (C) real-time monitoring the operation of nanomachine in HeLa cells. Copyright 2018, Royal Society of Chemistry.
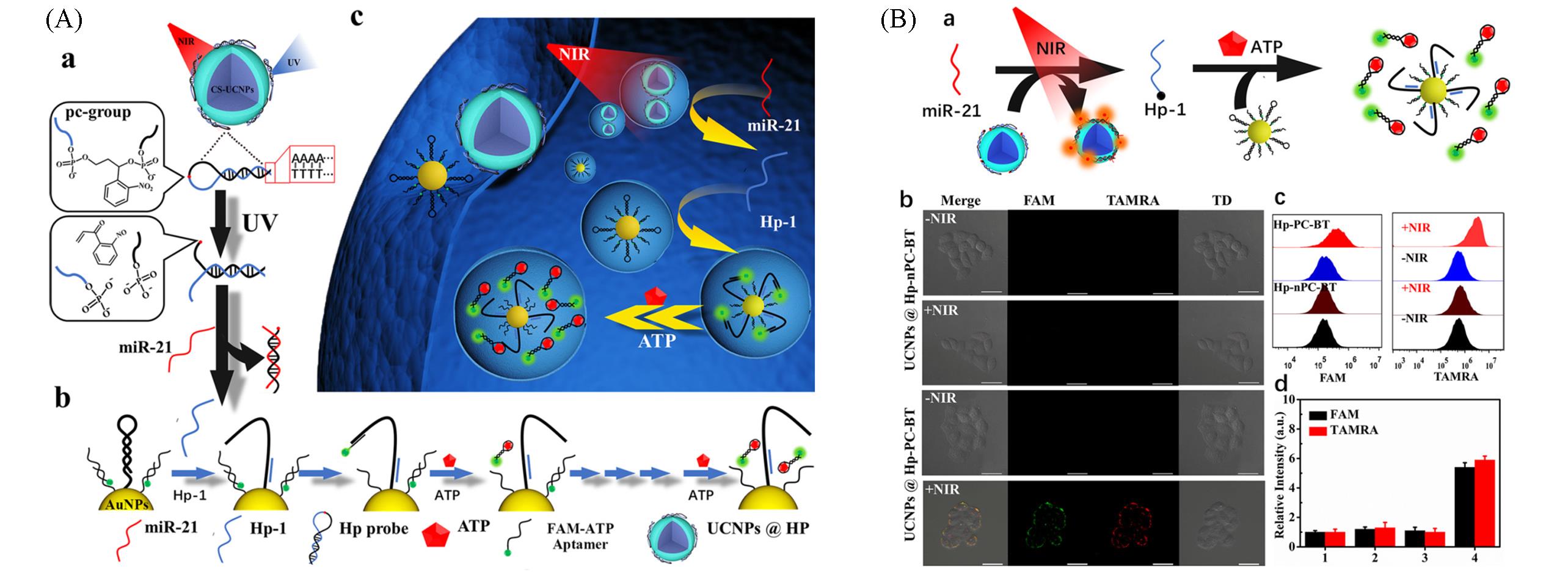
Fig.11 Imaging of intracellular microRNA with near⁃infrared light controllable DNA walker driven by ATP[50](A) Schematic diagram of light-activated DNA walker system for spatiotemporally sensing miRNA; (B) HeLa cells fluorescence imaging.Copyright 2021, American Chemical Society.
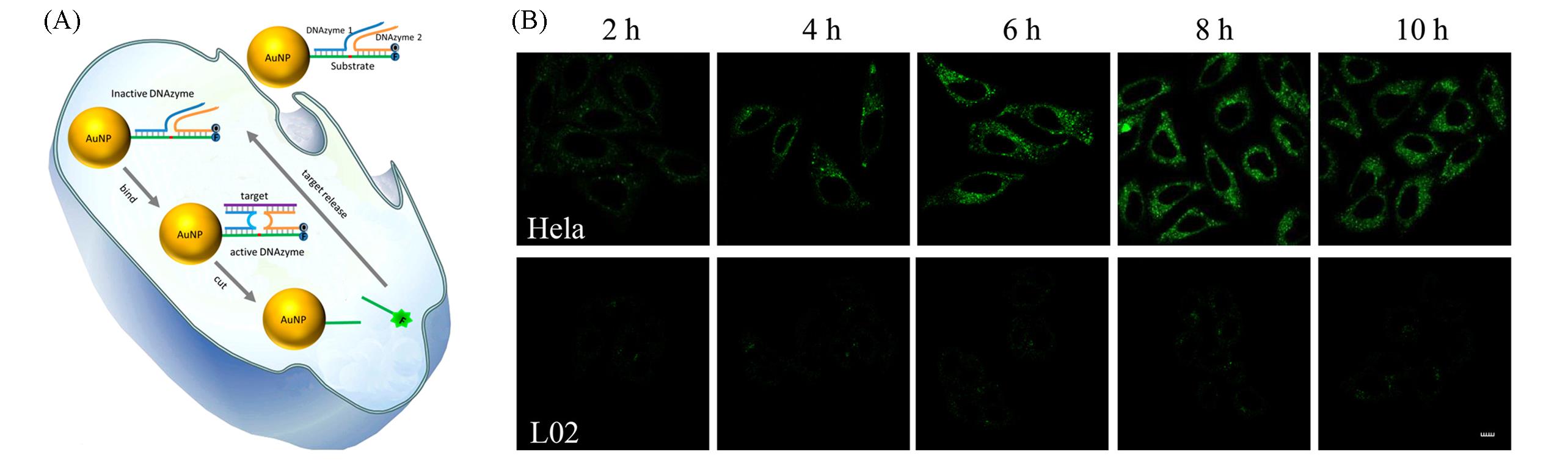
Fig.12 Nanoprobe based on metal ion⁃assisted signal amplification for miRNA imaging[53](A) Schematic of DNAzyme probe for amplified miRNA detection in live cells; (B) real-time fluorescence imaging of intracellular miR-21.Copyright 2017, American Chemical Society.
| 1 | Achilefu S., Chem. Rev., 2010, 110(5), 2575—2578 |
| 2 | Cheng X. H., Jia H. Z., Long T., Feng J., Qin J. G., Li Z., Chem. Commun., 2011, 47(43), 11978—11980 |
| 3 | Kobayashi H., Ogawa M., Alford R., Choyke P. L., Urano Y., Chem. Rev., 2010, 110(5), 2620—2640 |
| 4 | Lei J. P., Ju H. X., Chem. Soc. Rev., 2012, 41, 2122—2134 |
| 5 | Choi H. M. T., Chang J. Y., Trinh L. A., Padilla J. E., Fraser S. E., Pierce N. A., Nat. Biotechnol., 2010, 28(11), 1208—1212 |
| 6 | Wu C. C., Cansiz S., Zhang L. Q., Teng I. T., Qiu L. P., Li J., Liu Y., Zhou C. S., Hu R., Zhang T., Cui C., Cui L., Tan W. H., J. Am. Chem. Soc., 2015, 137(15), 4900—4903 |
| 7 | Wu Z., Liu G. Q., Yang X. L., Jiang J. H., J. Am. Chem. Soc., 2015, 137(21), 6829—6836 |
| 8 | Deng R. J., Tang L. H., Tian Q. Q., Wang Y., Lin L., Li J. H., Angew. Chem. Int. Ed., 2014, 53(9), 2389—2393 |
| 9 | Ge J., Zhang L. L., Liu S. J., Yu R. Q., Chu X., Anal. Chem., 2014, 86(3), 1808—1815 |
| 10 | Cheng Y., Zhang X., Li Z., Jiao X., Wang Y., Zhang Y., Angew. Chem. Int. Ed., 2009, 48(18), 3268—3272 |
| 11 | Wu C., Cansiz S., Zhang L., Teng I. T., Qiu L., Li J., Liu Y., Zhou C., Hu R., Zhang T., Cui C., Cui L., Tan W., J. Am. Chem. Soc., 2015, 137(15), 4900—4903 |
| 12 | Cheglakov Z., Cronin T. M., He C., Weizmann Y., J. Am. Chem. Soc., 2015, 137(19), 6116—6119 |
| 13 | Zheng F. F., Meng T. T., Jiang D. F., Sun J. M., Yao H. Y., Zhu J. J., Min Q. H., Angew. Chem. Int. Ed., 2021, 60(39), 21565—21574 |
| 14 | Li J., Wang J. L., Liu S. Y., Xie N. L., Quan K., Yang Y. J., Yang X. H., Huang J., Wang K. M., Angew. Chem. Int. Ed., 2020, 59(45), 20104—20111 |
| 15 | Li J., Liu S. Y., Wang J. L., Liu R. T., Yang X. H., Wang K. M., Huang J., Nucleic Acids Res., 2021, 50(7), e40 |
| 16 | Chen Y., Wang Q., Xu J., Xiang Y., Yuan R., Chai Y. Q., Chem. Commun., 2013, 49(20), 2052—2054 |
| 17 | Chen G. Y., Chen R., Ding S., Li M., Wang J. Y., Zou J. W., Du F., Dong J., Cui X., Huang X., Deng Y., Tang Z., Analyst, 2020, 145(2), 440—444 |
| 18 | Walker G. T., Fraiser M. S., Schram J. L., Little M. C., Nadeau J. G., Malinowski D. P., Nucleic Acids Res., 1992, 20(7), 1691—1696 |
| 19 | Zhao W. A., Ali M. M., Brook M. A., Li Y. F., Angew. Chem. Int. Ed., 2008, 47(34), 6330—6337 |
| 20 | Ali M. M., Li F., Zhang Z. Q., Zhang K. X., Kang D. K., Ankrum J. A., Le X. C., Zhao W. A., Chem. Soc. Rev., 2014, 43(10), 3324—3341 |
| 21 | Li L. D., Li N., Fu S. N., Deng Y. N., Yu C. Y., Su X., Nanoscale, 2019, 11(3), 1343—1350 |
| 22 | Xu X. W., Zhang P. P., Zhang R. Y., Zhang N., Jiang W., Chem. Commun., 2019, 55(43), 6026—6029 |
| 23 | Etoc F., Balloul E., Vicario C., Normanno D., Liße D., Sittner A., Piehler J., Dahan M., Coppey M., Nat. Mater., 2018, 17(8), 740—746 |
| 24 | Delarue M., Brittingham G. P., Pfeffer S., Surovtsev I. V., Pinglay S., Kennedy K. J., Schaffer M., Gutierrez J. I., Sang D., Poterewicz G., Chung J. K., Plitzko J. M., Groves J. T., Jacobs⁃Wagner C., Engel B. D., Holt L. J., Cell, 2018, 174(2), 338—349 |
| 25 | Lukacs G. L., Haggie P., Seksek O., Lechardeur D., Freedman N., Verkman A. S., J. Biol. Chem., 2000, 275(3), 1625—1629 |
| 26 | Verma P. K., Kundu A., Ha J. H., Cho M., J. Am. Chem. Soc., 2016, 138(49), 16081—16088 |
| 27 | Chen F., Xue J., Bai M., Qin J., Zhao Y. X., Chem. Sci., 2019, 10(10), 3103—3109 |
| 28 | Liu Z. L., Luo X. Y., Li Z., Huang Y., Nie Z., Wang H. H., Yao S. Z., Anal. Chem., 2017, 89(3), 1892—1899 |
| 29 | Hu Q. Y., Sun W. J., Lu Y., Bomba H. N., Ye Y., Jiang T. Y., Isaacson A. J., Gu Z., Nano Lett., 2016, 16(2), 1118—1126 |
| 30 | Biswas A., Joo K. I., Liu J., Zhao M. X., Fan G. P., Wang P., Gu Z., Tang Y., ACS Nano, 2011, 5(2), 1385—1394 |
| 31 | Wei Z. Y., Chen Y., Zhang B., Ren Y. L., Qiu L. J., Plant Sci., 2020, 294, 110423—110433 |
| 32 | Kao C. Y., Yang P. M., Wu M. H., Huang C. C., Lee Y. C., Lee K. H., Peer J., 2016, 4, e1683—e1703 |
| 33 | Imbeault P., Vidal H., Tremblay A., Vega N., Nadeau A., Després J. P., Mauriège P., J. Clin. Endocrinol. Metab., 2001, 86(2), 828—833 |
| 34 | Gupta A., Pillai V. S., Chittela R. K., J. Biosci., 2019, 44(6), 139—147 |
| 35 | Lee Y. C., Zhou Q., Chen J. J., Yuan J. S., Curr. Biol., 2016, 26(24), 3257—3268 |
| 36 | Chen W., Wang B., Gruber J. D., Zhang Y. M., Davies C., Front. Microbiol., 2018, 9, 2244—2256 |
| 37 | Hashimoto S., Kishimoto T., Semin. Cancer Biol., 2022, doi. 10.1016/j. semcancer. 2022.03.017 |
| 38 | Ihara F., Fereig R. M., Himori Y., Kameyama K., Umeda K., Tanaka S., Ikeda R., Yamamoto M., Nishikawa Y., Front. Immunol., 2020, 11, 1709—1726 |
| 39 | Huang F. J., Lin M. H., Duan R. L., Lou X. D., Xia F., Willner I., Nano Lett., 2018, 18(8), 5116—5123 |
| 40 | Zhou W. J., Liang W. B., Li D, X., Yuan R., Xiang Y., Biosens. Bioelectron., 2016, 85, 573—579 |
| 41 | Zhou Y. B., Yang S., Xiao Y., Zou Z., Qing Z. H., Liu J. W., Yang R. H., Anal. Chem., 2019, 91(23), 15179—15186 |
| 42 | Zhou Y. B., Yang S., Guo J. R., Dong H., Yin K. Y., Huang W. T., Yang R. H., Anal. Chem., 2020, 92(8), 5787—5794 |
| 43 | Zhou Y. B., Gu Z. X., Liu C. H., Yang S., Ma X. F., Chen Q. S., Lei Y. L., Quan K., Liu J. W., Qing Z. H., Yang R. H., Angew. Chem. Int. Ed., 2022, 61(16), e202114504 |
| 44 | Zhou Y. B., Dong H., Gu Z. X., Yang S., Ouyang M., Qing Z. H., Ma X. F., Hu S., Li J. B., Yang R. H., Anal. Chem., 2021, 93(38), 12944—12953 |
| 45 | Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B., Nat. Neurosci., 2005, 8(6), 752—758 |
| 46 | Burnstock G., Trends Pharmacol. Sci., 2006, 27(3), 166—176 |
| 47 | Ashcroft F. M., Gribble F. M., Diabetologia, 1999, 42(8), 903—919 |
| 48 | Shen Y. Z., Wu T. T., Tian Q., Mao Y., Hu J. J., Luo X. L., Ye Y. W., Chen H. Y., Xu J. J., Anal. Chem., 2019, 91(12), 7879—7886 |
| 49 | Ma P. Q., Liang C. P., Zhang H. H., Yin B. C., Ye B. C., Chem. Sci., 2018, 9(13), 3299—3304 |
| 50 | Ye M. Q., Kong Y. J., Zhang C. L., Lv Y. F., Cheng S. S., Hou D. Y., Xian Y. Z., ACS Nano, 2021, 15(9), 14253—14262 |
| 51 | Ryazanova L. V., Rondon L. J., Zierler S., Hu Z., Galli J., Yamaguchi T. P., Mazur A., Fleig A., Ryazanov A. G., Nat. Commun., 2010, 1(1), 109—117 |
| 52 | Quamme G. A., Am. J. Physiol., 2010, 298(3), C407—C429 |
| 53 | Wu Y. N., Huang J., Yang X. H., Yang Y. J., Quan K., Xie N. L., Li J., Ma C. B., Wang K. M., Anal. Chem., 2017, 89(16), 8377—8383 |
| [1] | 王学斌, 薛源, 茆华女, 项艳鑫, 包春燕. 光/还原双重响应水凝胶微球的制备及在细胞三维(3D)培养中的应用[J]. 高等学校化学学报, 2022, 43(8): 20220116. |
| [2] | 姬发, 刘玲, 余林玲, 孙彦. 黏惰化及酸敏感修饰对纳米粒子黏膜穿透的影响[J]. 高等学校化学学报, 2022, 43(6): 20210837. |
| [3] | 曾晛阳, 赵熹, 黄旭日. 细胞松弛素B对葡萄糖/质子共转运蛋白GlcPSe的抑制机理[J]. 高等学校化学学报, 2022, 43(4): 20210822. |
| [4] | 王雪丽, 宋相伟, 解颜宁, 杜妮阳, 王振新. 部分还原氧化石墨烯的制备、 表征及对人宫颈癌HeLa细胞的体外杀伤作用[J]. 高等学校化学学报, 2022, 43(2): 20210595. |
| [5] | 张恩东, 吕凤婷, 黄一鸣, 白昊天, 王树. 细胞内原位纳米催化在疾病诊断与治疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220676. |
| [6] | 常通航, 程震. 整合荧光成像和化疗的有机小分子诊疗探针的研究进展[J]. 高等学校化学学报, 2022, 43(12): 20220430. |
| [7] | 陈尚钰, 沈清明, 孙鹏飞, 范曲立. 小分子基温敏聚合物纳米粒子的制备及在近红外二区荧光成像与光热治疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220392. |
| [8] | 汪诗琪, 罗博文, 俞计成, 顾臻. 近红外二区活体荧光成像在肿瘤诊疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220577. |
| [9] | 赵雪琪, 赵越, 薛静, 白敏, 陈锋, 孙颖, 宋大千, 赵永席. 单细胞核酸编码扩增成像分析[J]. 高等学校化学学报, 2022, 43(12): 20220572. |
| [10] | 张钤, 刘雅薇, 王帆, 刘凯, 张洪杰. 稀土纳米材料在高分辨活体成像及诊疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220552. |
| [11] | 邵文惠, 胡欣, 尚静, 林峰, 金黎明, 权春善, 张艳梅, 李军. 高效广谱复合光催化抗菌剂Ag-AgVO3/BiVO4的设计合成及抗菌机制[J]. 高等学校化学学报, 2022, 43(10): 20220132. |
| [12] | 刘苗, 刘瑞波, 刘巴蒂, 钱鹰. 溶酶体靶向吲哚氟硼二吡咯光敏剂的合成、 双光子荧光成像及光动力治疗[J]. 高等学校化学学报, 2022, 43(10): 20220326. |
| [13] | 李安然, 赵冰, 阚伟, 宋天舒, 孔祥东, 卜凡强, 孙立, 殷广明, 王丽艳. 基于菲并咪唑的ON⁃OFF⁃ON双比色荧光探针及细胞成像[J]. 高等学校化学学报, 2021, 42(8): 2403. |
| [14] | 温炜, 黄达锭, 鲍劲霄, 张增辉. PD-1与单克隆抗体残基特异性结合机制的计算丙氨酸扫描研究[J]. 高等学校化学学报, 2021, 42(7): 2161. |
| [15] | 杨依然, 姚华, 闫江红, 孙志恒, 张余, 房雪晴, 李绪文, 金永日. 薤中新的甾体皂苷类化学成分[J]. 高等学校化学学报, 2021, 42(6): 1742. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||