

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (11): 2287.doi: 10.7503/cjcu20200404
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
收稿日期:2020-06-30
出版日期:2020-11-10
发布日期:2020-11-06
通讯作者:
鄢红
E-mail:yanhong@mail.buct.edu.cn
基金资助:
ZHU Yuquan, ZHAO Xiaojie, ZHONG Yuan, CHEN Ziru, YAN Hong( ), DUAN Xue
), DUAN Xue
Received:2020-06-30
Online:2020-11-10
Published:2020-11-06
Contact:
YAN Hong
E-mail:yanhong@mail.buct.edu.cn
Supported by:摘要:
因主体层板和层间客体具有丰富的可调性, 类水滑石材料(LDHs)在催化、 吸附、 生物医药及光、 电、 磁等方面展现出了广阔的应用前景. 近年来理论研究已成为揭示LDHs微观结构和性质的重要手段, 本文系统综述了LDHs材料主体结构、 客体结构以及主客体相互作用3个方面的理论研究工作进展, 及其在作为光驱动催化剂方面应用的理论研究. 从主体元素构成、 元素比例、 电荷分布、 拓扑结构转变、 能带结构、 态密度、 层间阴离子组成、 离子交换性能、 主客体作用力、 能量性质及光催化性能等方面, 在原子、 电子尺度上揭示了LDHs材料结构-性能之间的构效关系, 为以其为材料平台构筑一系列基于超分子插层结构主客体间相互作用的新型功能材料、 扩展材料的功能性提供了丰富的理论信息和有益指导.
中图分类号:
TrendMD:
朱玉荃, 赵晓婕, 钟嫄, 陈子茹, 鄢红, 段雪. 类水滑石材料主客体插层结构的构筑及特性的理论研究. 高等学校化学学报, 2020, 41(11): 2287.
ZHU Yuquan, ZHAO Xiaojie, ZHONG Yuan, CHEN Ziru, YAN Hong, DUAN Xue. Theoretical Study on the Construction and Characteristics of the Host-guest Intercalated Structure of Layered Double Hydroxides. Chem. J. Chinese Universities, 2020, 41(11): 2287.
| LDHs | Cell parameter/? | Mg(II) | Ca(II) | Co(II) | Ni(II) | Cu(II) | Zn(II) |
|---|---|---|---|---|---|---|---|
| M2Al?LDHs | a, b | 3.08[ | 5.75[ | 3.10[ | 3.03[ | 2.93[ | 3.08[ |
| c | 7.86[ | 23.49[ | 22.47[ | 25.94[ | 26.10[ | 7.75[ | |
| M2Cr?LDHs | a, b | 3.01[ | 2.99[ | 3.55[ | 3.58[ | 3.57[ | |
| c | 22.80[ | 22.80[ | 22.80[ | ||||
| M2Mn?LDHs | a, b | 3.05[ | 3.06[ | ||||
| c | |||||||
| M2Fe?LDHs | a, b | 3.11[ | 5.86[ | 3.13[ | 3.03[ | 3.03[ | |
| c | 23.19[ | 23.31[ | 24.92[ | 22.18[ | 23.58[ | ||
| M2Ga?LDHs | a, b | 3.09[ | 5.83[ | 3.08[ | 3.06[ | 3.20[ | |
| c | 23.42[ | 22.80[ | 22.98[ | 5.20[ | |||
| M2Ti?LDHs | a, b | 2.92[ | 3.02[ | 3.07[ | 3.06[ | ||
| c | 24.33[ | 21.43[ | 22.16[ | 22.15[ |
Table 1 Experimental cell parameters of the reported binary MⅡ2M(Ⅲ/Ⅳ)?Cl?LDHs(polytype: 3R)
| LDHs | Cell parameter/? | Mg(II) | Ca(II) | Co(II) | Ni(II) | Cu(II) | Zn(II) |
|---|---|---|---|---|---|---|---|
| M2Al?LDHs | a, b | 3.08[ | 5.75[ | 3.10[ | 3.03[ | 2.93[ | 3.08[ |
| c | 7.86[ | 23.49[ | 22.47[ | 25.94[ | 26.10[ | 7.75[ | |
| M2Cr?LDHs | a, b | 3.01[ | 2.99[ | 3.55[ | 3.58[ | 3.57[ | |
| c | 22.80[ | 22.80[ | 22.80[ | ||||
| M2Mn?LDHs | a, b | 3.05[ | 3.06[ | ||||
| c | |||||||
| M2Fe?LDHs | a, b | 3.11[ | 5.86[ | 3.13[ | 3.03[ | 3.03[ | |
| c | 23.19[ | 23.31[ | 24.92[ | 22.18[ | 23.58[ | ||
| M2Ga?LDHs | a, b | 3.09[ | 5.83[ | 3.08[ | 3.06[ | 3.20[ | |
| c | 23.42[ | 22.80[ | 22.98[ | 5.20[ | |||
| M2Ti?LDHs | a, b | 2.92[ | 3.02[ | 3.07[ | 3.06[ | ||
| c | 24.33[ | 21.43[ | 22.16[ | 22.15[ |
| LDHs | Exchange?correlation functional | Cutoff energy/eV | k?Points mesh | Unit cell parameter | |||
|---|---|---|---|---|---|---|---|
| a/? | b/? | c/? | V/?3 | ||||
| ZnAl?Cl?LDH | GGA/PBE[ | 408 | 3×3×2 | 3.13 | 3.13 | 23.47 | |
| (supercell: | GGA/revPBE?vdW[ | 408 | 3×3×2 | 3.18 | 3.18 | 23.87 | |
| Experimental[ | 3.08 | 3.08 | 23.35 | ||||
| MgFe?CO3?LDH | GGA/PW91[ | 480 | 2×2×2 | 3.07 | 3.08 | 22.81 | 186.90 |
| (supercell: | GGA spin polarized[ | 480 | 2×2×2 | 3.10 | 3.11 | 22.82 | 190.38 |
| GGA+U[ | 480 | 2×2×2 | 3.14 | 3.14 | 22.80 | 194.77 | |
| Experimental[ | 3.10 | 3.10 | 23.17 | 192.83 | |||
Table 2 Calculated and experimental cell parameters of the optimized LDHs structure[58,59]
| LDHs | Exchange?correlation functional | Cutoff energy/eV | k?Points mesh | Unit cell parameter | |||
|---|---|---|---|---|---|---|---|
| a/? | b/? | c/? | V/?3 | ||||
| ZnAl?Cl?LDH | GGA/PBE[ | 408 | 3×3×2 | 3.13 | 3.13 | 23.47 | |
| (supercell: | GGA/revPBE?vdW[ | 408 | 3×3×2 | 3.18 | 3.18 | 23.87 | |
| Experimental[ | 3.08 | 3.08 | 23.35 | ||||
| MgFe?CO3?LDH | GGA/PW91[ | 480 | 2×2×2 | 3.07 | 3.08 | 22.81 | 186.90 |
| (supercell: | GGA spin polarized[ | 480 | 2×2×2 | 3.10 | 3.11 | 22.82 | 190.38 |
| GGA+U[ | 480 | 2×2×2 | 3.14 | 3.14 | 22.80 | 194.77 | |
| Experimental[ | 3.10 | 3.10 | 23.17 | 192.83 | |||
| Metal cation | Ueff/eV | Metal cation | Ueff/eV | Metal cation | Ueff/eV | Metal cation | Ueff/eV |
|---|---|---|---|---|---|---|---|
| Co2+ | 3.52[ | Cu2+ | 3.6[ | Mn3+ | 3.54[ | Ti4+ | 6.0[ |
| Ni2+ | 3.8[ | Cr3+ | 3.2[ | Fe3+ | 4.3[ |
Table 3 Ueff of the transition metal cations of LDHs
| Metal cation | Ueff/eV | Metal cation | Ueff/eV | Metal cation | Ueff/eV | Metal cation | Ueff/eV |
|---|---|---|---|---|---|---|---|
| Co2+ | 3.52[ | Cu2+ | 3.6[ | Mn3+ | 3.54[ | Ti4+ | 6.0[ |
| Ni2+ | 3.8[ | Cr3+ | 3.2[ | Fe3+ | 4.3[ |
| Bond length and bond angle | M=Mg2+ | M=Ca2+ | ||
|---|---|---|---|---|
| 6?31G(d) | LANL2DZ/6?31G(d) | 6?31G(d) | LANL2DZ/6?31G(d) | |
| M—O(in ?)a | 2.202 | 2.191 | 2.510 | 2.505 |
| Al—O3(in ?) | 1.884 | 1.885 | 1.874 | 1.877 |
| M1???M2(in ?) | 3.150 | 3.139 | 3.774 | 3.743 |
| M???Al(in ?)b | 3.042 | 3.037 | 3.365 | 3.366 |
| O3—H(in ?) | 0.968 | 0.968 | 0.968 | 0.969 |
| O—M—O(in degree)c | 76.70 | 76.81 | 71.81 | 71.85 |
| O—Al—O(in degree)d | 86.56 | 86.36 | 90.43 | 90.34 |
Table 4 Optimized Geometries of [M2Al(OH2)9(OH)4]3+[27]
| Bond length and bond angle | M=Mg2+ | M=Ca2+ | ||
|---|---|---|---|---|
| 6?31G(d) | LANL2DZ/6?31G(d) | 6?31G(d) | LANL2DZ/6?31G(d) | |
| M—O(in ?)a | 2.202 | 2.191 | 2.510 | 2.505 |
| Al—O3(in ?) | 1.884 | 1.885 | 1.874 | 1.877 |
| M1???M2(in ?) | 3.150 | 3.139 | 3.774 | 3.743 |
| M???Al(in ?)b | 3.042 | 3.037 | 3.365 | 3.366 |
| O3—H(in ?) | 0.968 | 0.968 | 0.968 | 0.969 |
| O—M—O(in degree)c | 76.70 | 76.81 | 71.81 | 71.85 |
| O—Al—O(in degree)d | 86.56 | 86.36 | 90.43 | 90.34 |
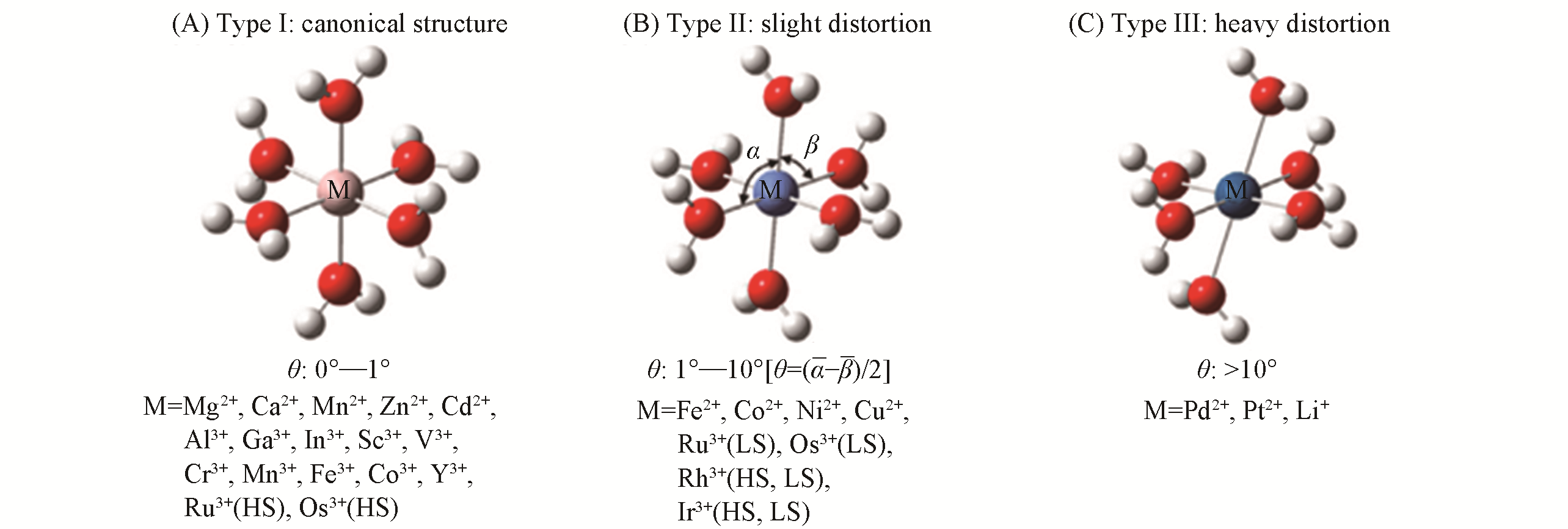
Fig.3 Optimized structure of three types of [M(OH2)6]n+ clusters[88]Canonical structure(θ=0°—1°); slight distortion(θ=1°—10°); heavy distortion(θ >10°). Copyright 2008, Elsevier.
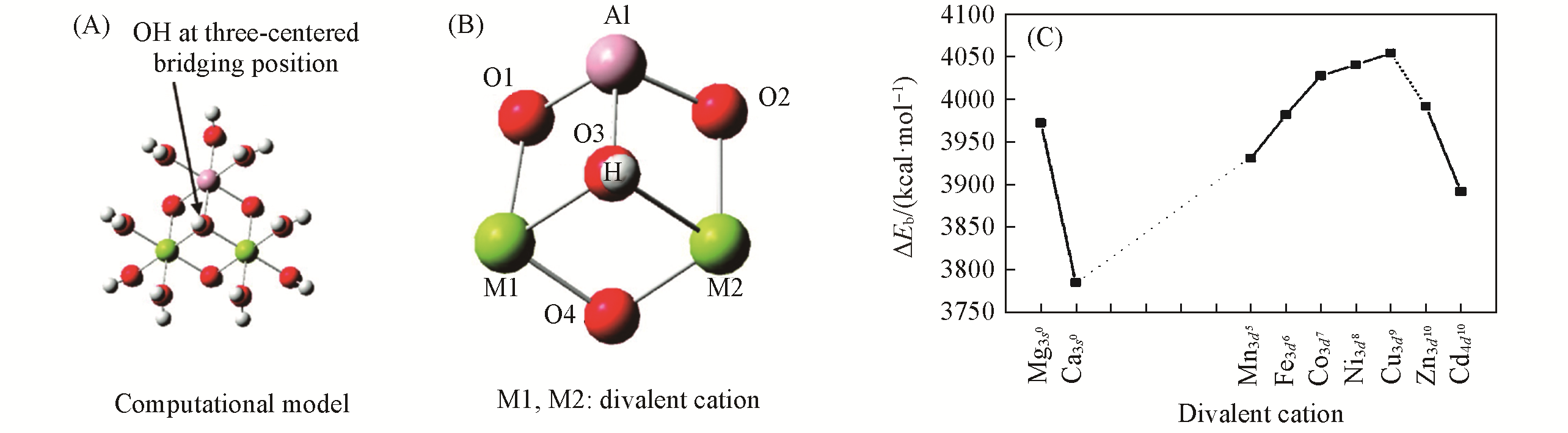
Fig.4 [MII2Al(OH2)9(OH)4]3+ cluster model(A), the linkage around the three?centered bridging OH group(B), the relationship between zero?point corrected binding energy (ZPE) ΔEb(1 kcal=4.19 kJ) of the clusters and the atomic number of MII(C)[27]Copyright 2009, American Chemical Society.
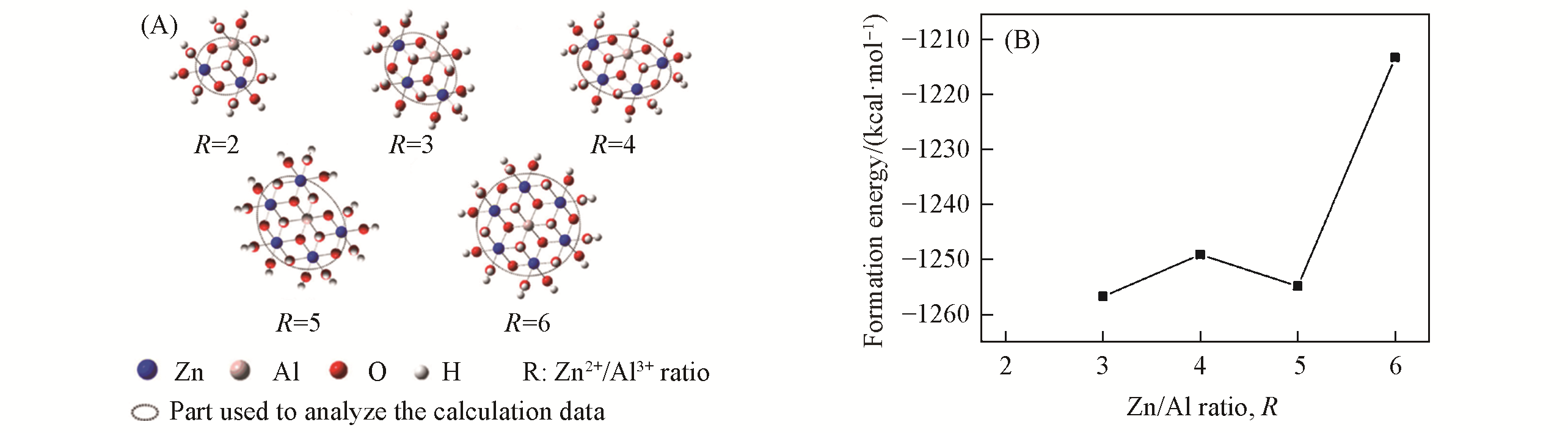
Fig.5 ZnAl?LDHs clusters with R(Zn2+/Al3+) in the range of 2—6(A) and relationship between the formation energy Ef(1 kcal=4.19 kJ) of the ZnRAl?LDHs(R=3—6) clusters and R(B)[69]Copyright 2010, Elsevier.
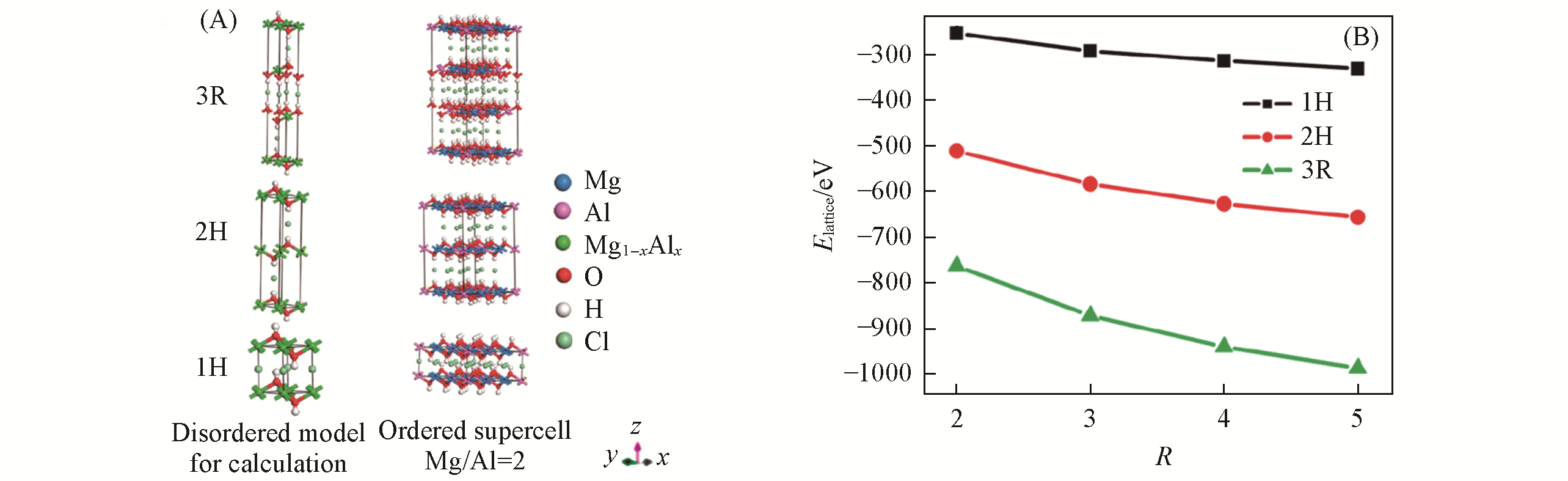
Fig.6 Periodic disordered computational model formulated as Mg1-xAlx(OH)2Clx and ordered 3×3×1 supercells (Mg/Al ratio=2) for MgAl?Cl?LDHs with different stacking sequences(3R, 2H and 1H)(A) and lattice energy of the disordered MgAl?Cl?LDHs model stacking in different sequences as a function of R(B)[57]Copyright 2010, American Chemical Society.
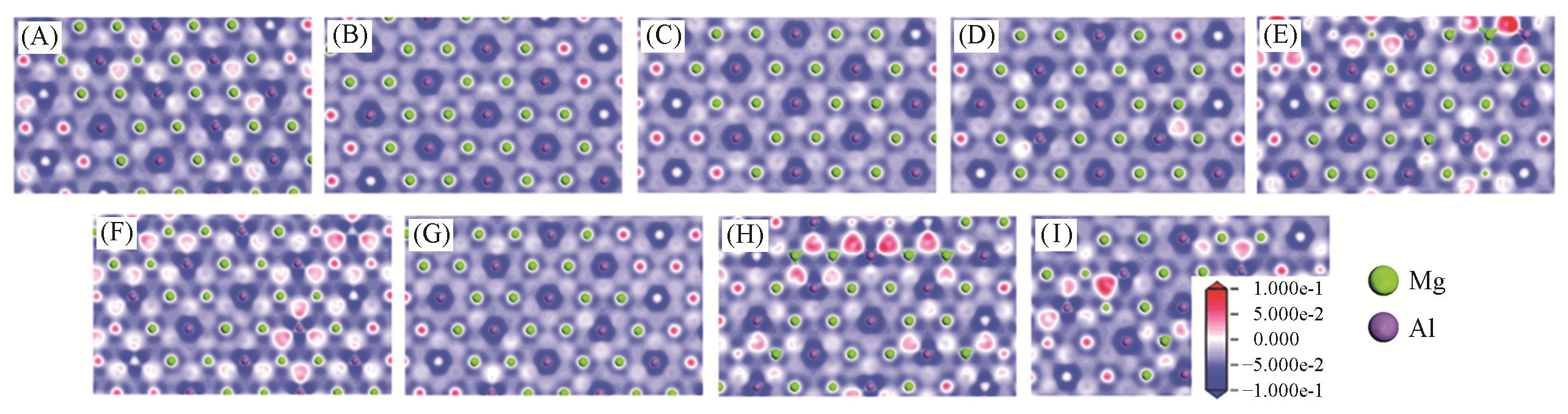
Fig.7 Electron density difference of layer in Mg2Al?A?LDHs[34](A) Mg2Al?F?LDHs (B) Mg2Al?Cl?LDHs; (B) Mg2Al?Br?LDHs; (D) Mg2Al?I?LDHs; (E) Mg2Al?OH?LDHs; (F) Mg2Al?NO3?LDHs; (G) Mg2Al?CO3?LDHs; (H) Mg2Al?SO4?LDHs; (I) Mg2Al?PO4?LDHs. The blue region presents the depletion of electronic density, and the red region indicates the increase of electronic density. Copyright 2020, Royal Society of Chemistry.
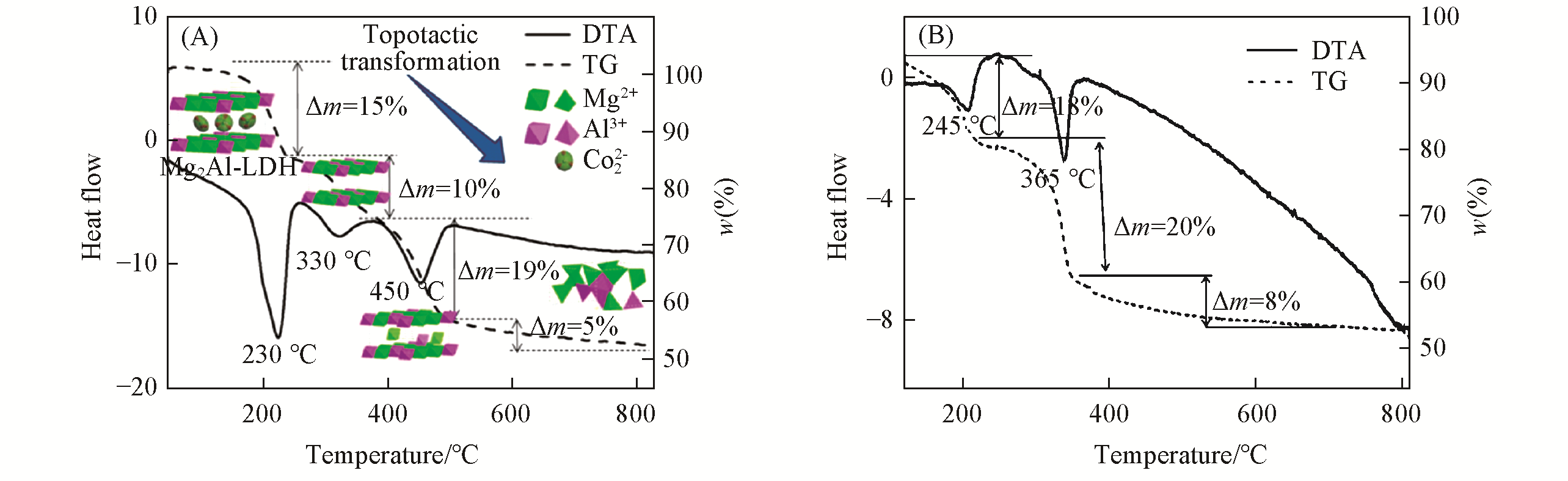
Fig.8 Simulation of the thermal decomposition process of the MgAl?LDHs(A)[94] and NiAl?LDHs(B)[95]Copyright 2016, John Wiley & Sons, Inc. Copyright 2017, Informa UK Limited.
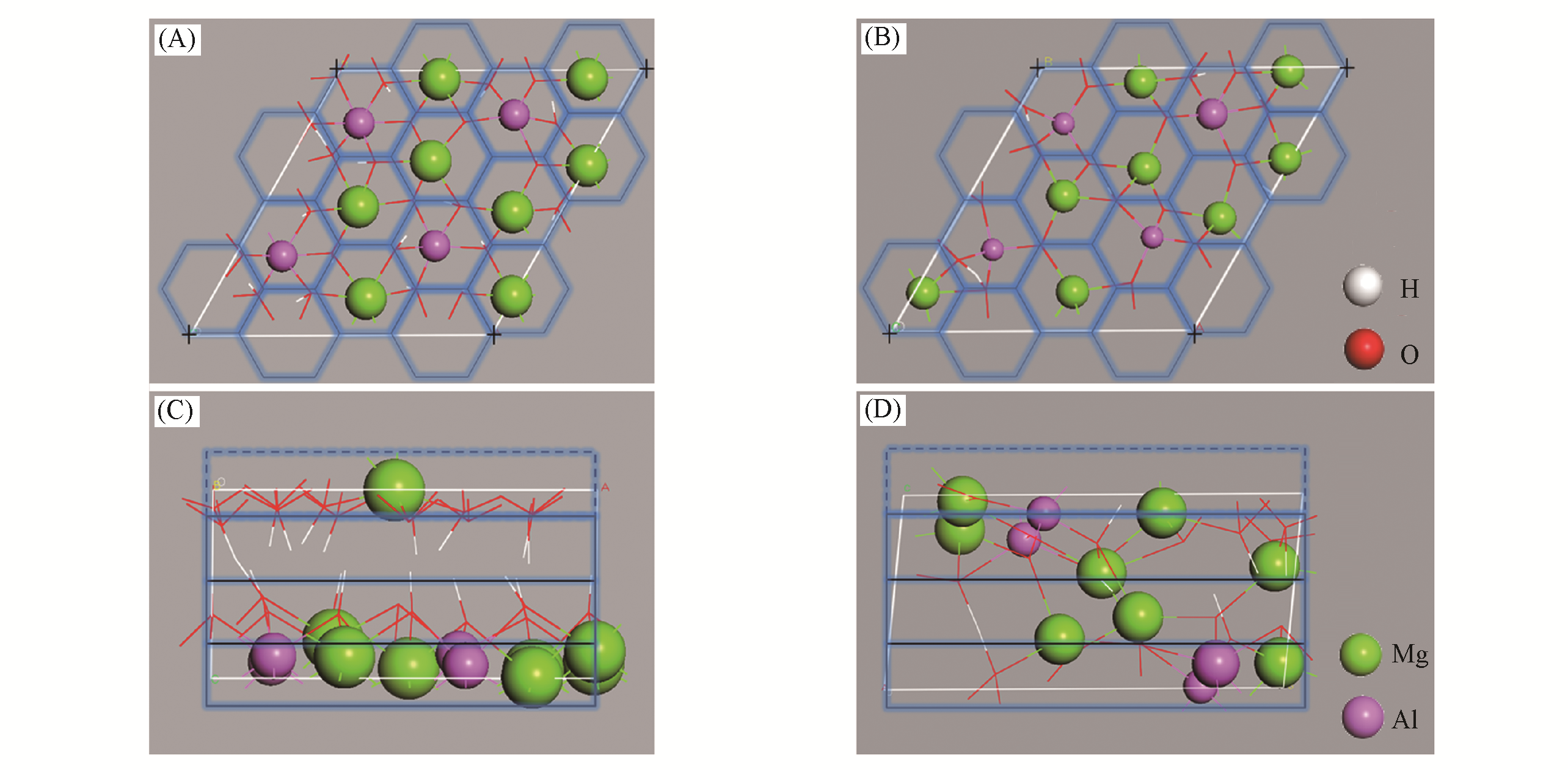
Fig.9 Top?view of CLDH?1(A) and CLDH?6 (B); side?view of CLDH?1 (C) and CLDH?6(D)[94]The cell used in calculating the values of σ is defined by the blue line, and the blue dashed line represents the imaginary neighbor cell. Copyright 2016, John Wiley & Sons, Inc.
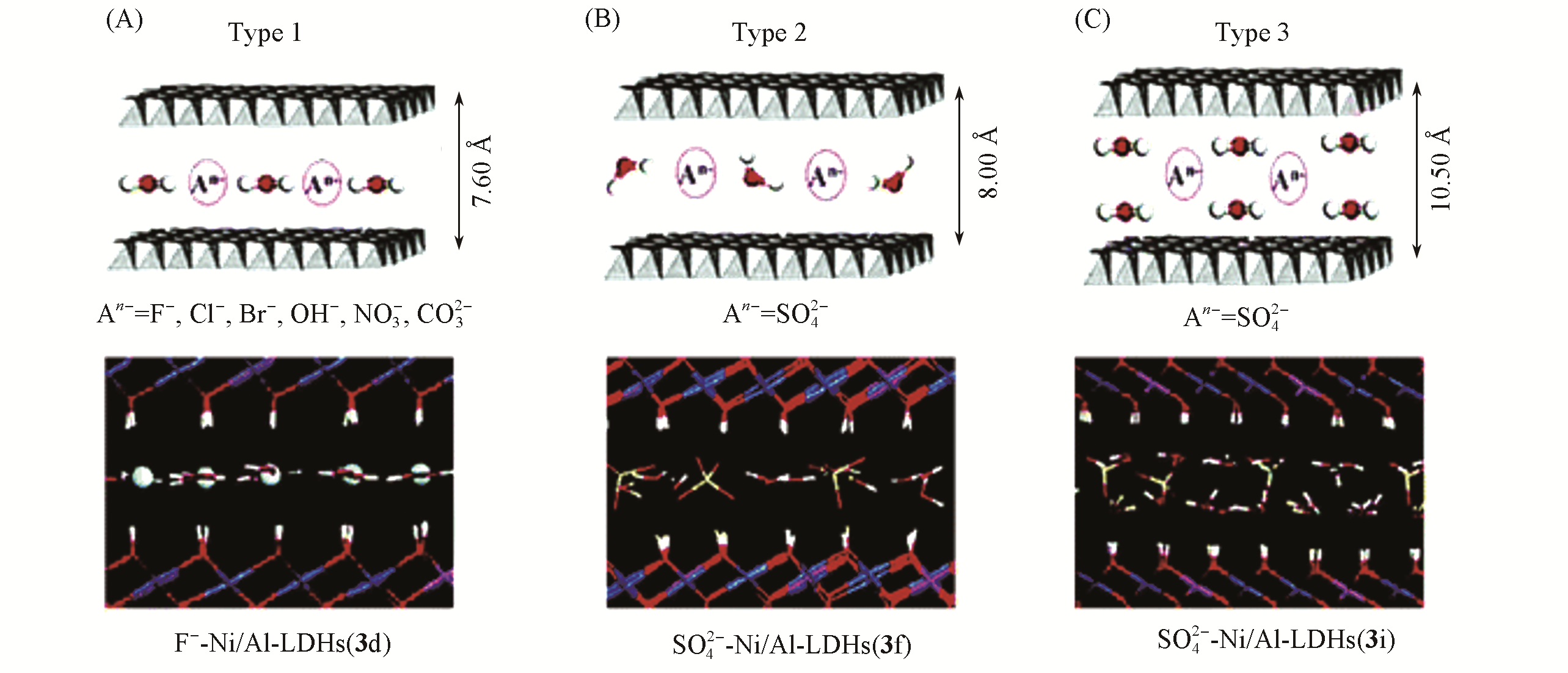
Fig.10 Possible packing styles of interlayer water molecules of α?Ni(OH)2 and Ni/Al?LDHs[100]The snapshots obtained at 50 ps for F-?Ni/Al?LDHs(A), SO42-?Ni/Al?LDHs(B), and SO42-?Ni/Al?LDHs(C) are also given for illustration. 1 ?=0.1 nm. Copyright 2006, American Chemical Society.
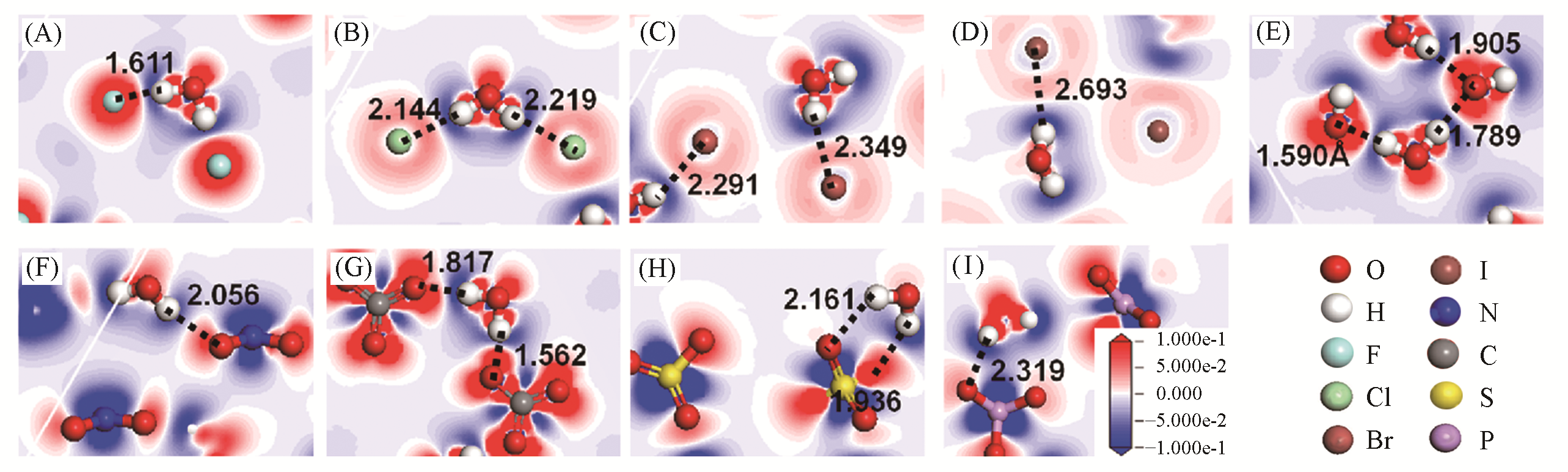
Fig.11 Electronic density difference of interlayer species of Mg2Al?A?LDHs interlayered with different anions(A=F-, Cl-, Br-, I-, OH-, NO3-, CO32-, SO42- and PO43-) under the top view, along with the distances between the H atom (H) of water and halogen anions(X)(dX···H) or O atoms within oxygen acid anions(dO···H) (in ?, 1 ?=0.1 nm)[34](A) Mg2Al?F?LDHs; (B) Mg2Al?Cl?LDHs; (B) Mg2Al?Br?LDHs; (D) Mg2Al?I?LDHs; (E) Mg2Al?OH?LDHs; (F) Mg2Al? NO3?LDHs; (G) Mg2Al?CO3?LDHs; (H) Mg2Al?SO4?LDHs; (I) Mg2Al?PO4?LDHs. The blue region presents the depletion of electronic density, and the red region indicates the increase of electronic density. Copyright 2020, Royal Society of Chemistry.
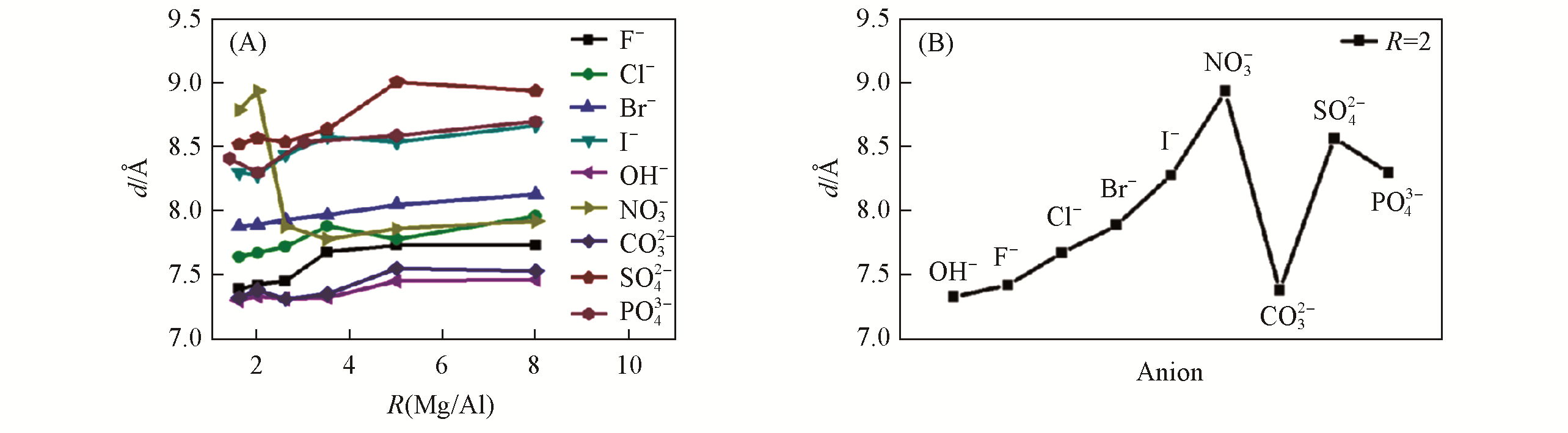
Fig.12 Interlayer distance (d) of MgAl?A?LDHs with different anions and different R(Mg2+/Al3+)(A) and Mg2Al?A?LDHs with different anions(B)[34]Copyright 2020, Royal Society of Chemistry.
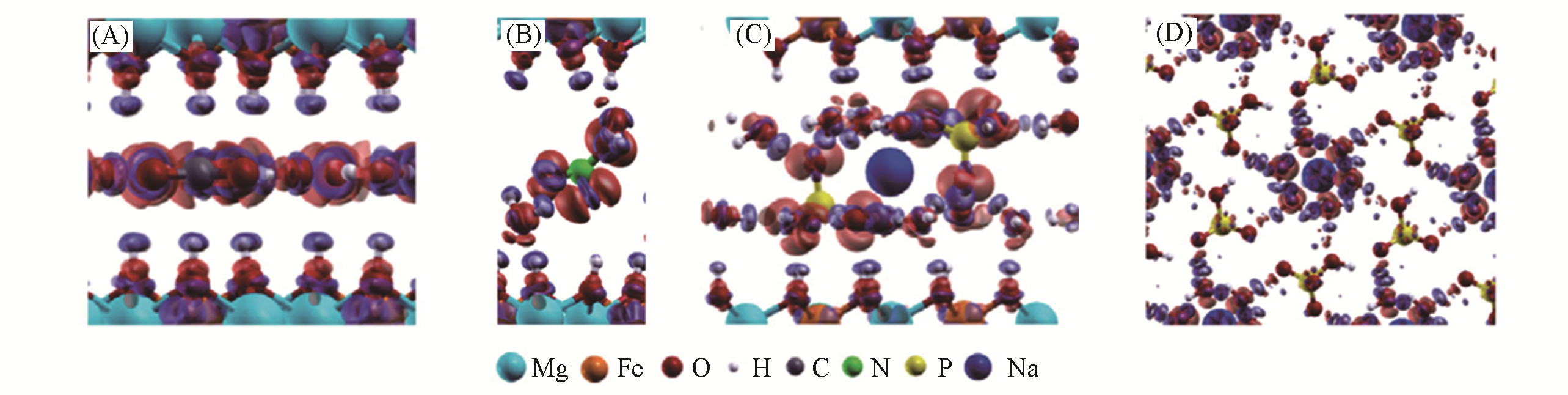
Fig.13 Electronic density difference of [Mg?Fe?CO3] (A), [Mg?Fe?NO3] (B), [Mg?Fe?HPO4, Na] (C), and hexahydrated sodium(D) in the interlayer region of [Mg?Fe?HPO4, Na] with the HPO42- anion, using GGA calculation[59]The blue region presents the depletion of electronic density, and the red region indicates the increase of electronic density. Copyright 2016, American Chemical Society.
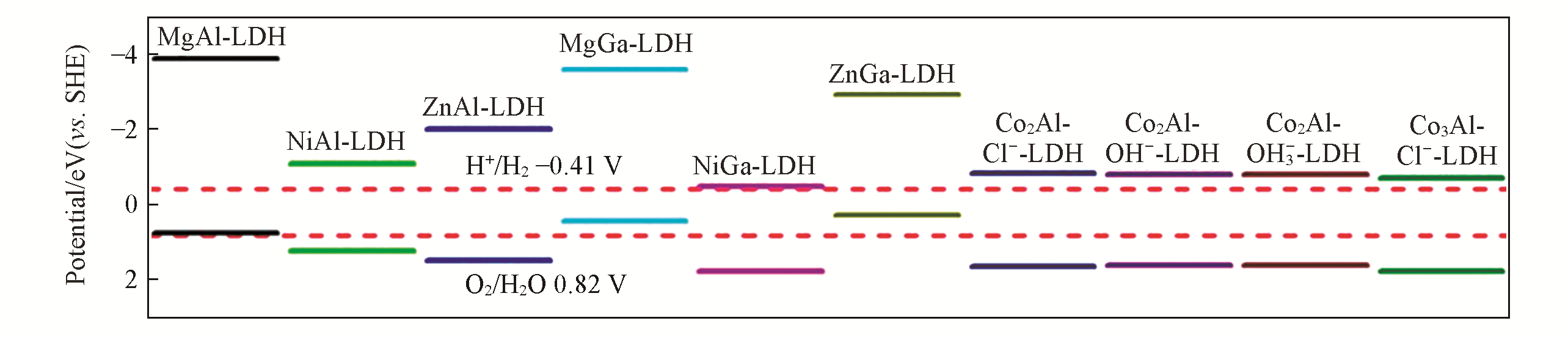
Fig.14 Band edge placements of MIIMII?LDHs(MII=Mg, Co, Ni, Zn; MIII=Al and Ga)[28]The two dashed red lines(-0.41 and 0.82 V vs. SHE) represent the reduction potential of H2 and the oxidation potential of O2 at pH=7. Copyright 2015, American Chemical Society.
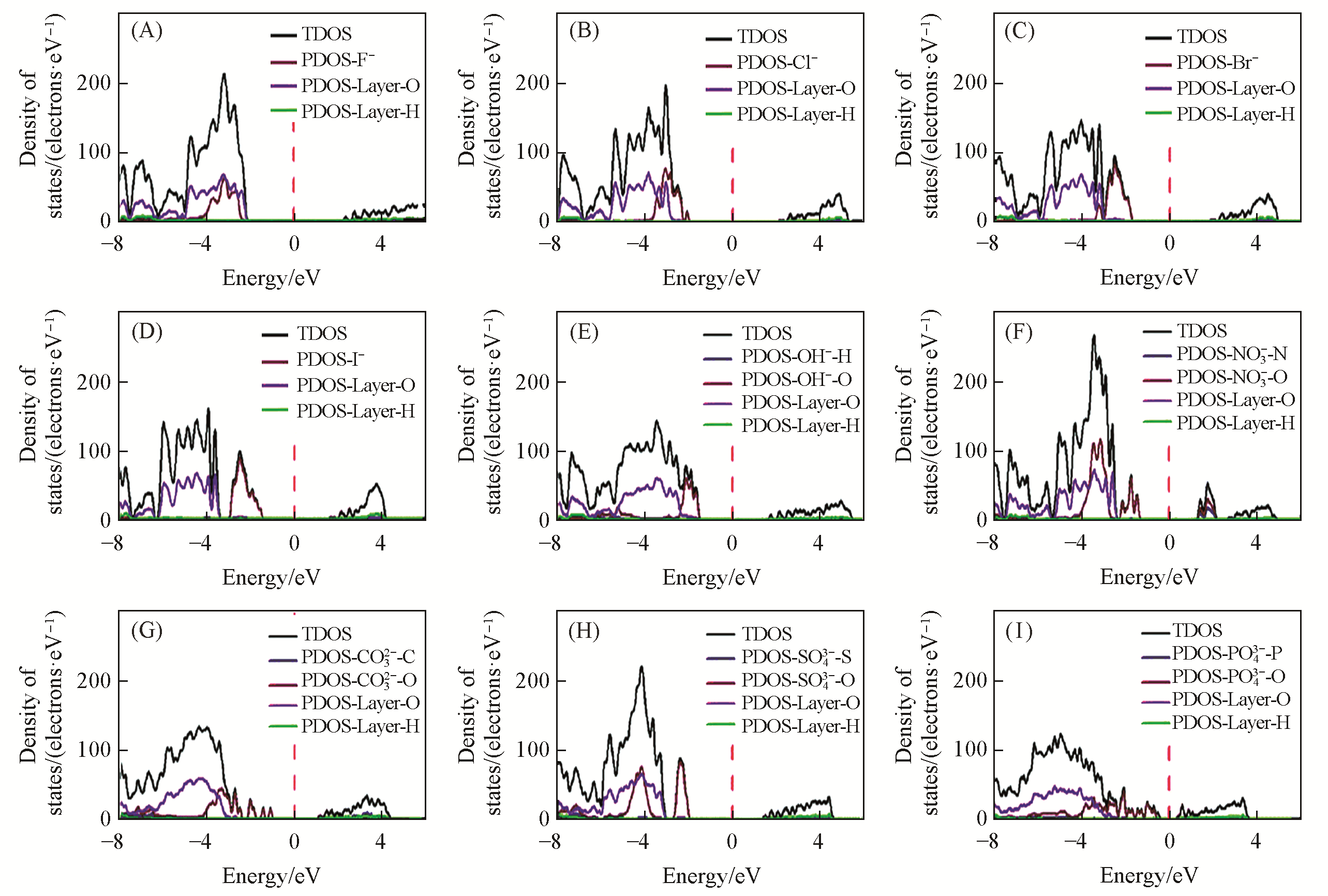
Fig.15 Total density of states (TDOS) and partial density of states (PDOS) for the Mg2Al?A?LDHs intercalated with different interlayer anions[34]The Fermi energy is shown as a dashed vertical line, and has been set as 0 eV. (A) Mg2Al?F?LDHs(4.431 eV); (B) Mg2Al?Cl?LDHs(4.141 eV); (C) Mg2Al?Br?LDHs(3.607 eV); (D) Mg2Al?I?LDHs(3.090 eV); (E) Mg2Al?OH?LDHs(3.208 eV); (F) Mg2Al?NO3?LDHs(2.800 eV); (G) Mg2Al?CO3?LDHs(2.197 eV); (H) Mg2Al?SO4?LDHs(3.505 eV); (I) Mg2Al?PO4?LDHs(1.263 eV). Copyright 2020, Royal Society of Chemistry.
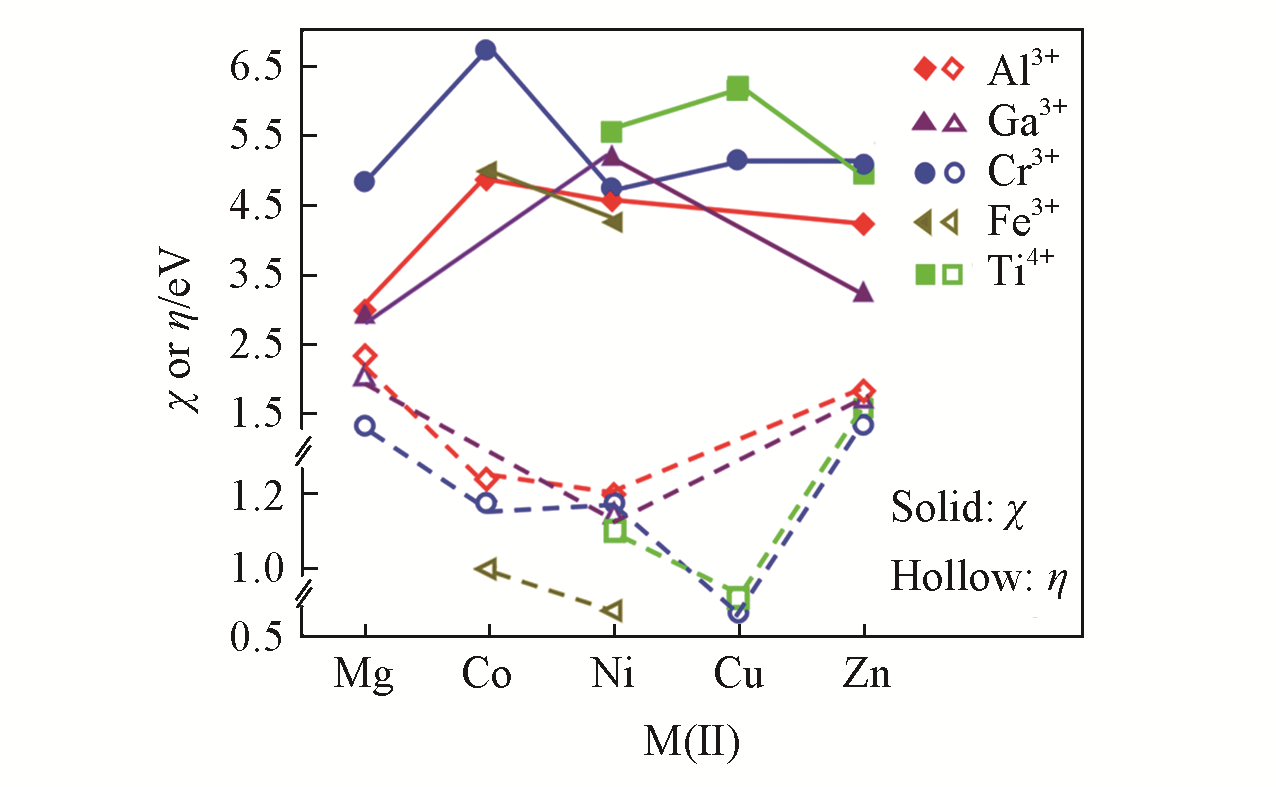
Fig.16 Mulliken electronegativities(χ) and hardness(η) of M(Ⅱ)M(Ⅲ/Ⅳ)?Cl?LDHs as a function of the atomic numbers of divalent cations[89]Copyright 2019, Springer?Nature Switzerland AG.
| 1 | Cavani F., Trifiro F., Vaccari A., Catal. Today, 1991, 11(2), 173—301 |
| 2 | Rives V., Layered Double Hydroxides: Present and Future, Nova Science Publishers, New York, 2001, 194—195 |
| 3 | Duan X., Evans D. G., Layered Double Hydroxides: Structure and Bonding, Springer⁃Verlag, Berlin, 2006, 41(4), 879 |
| 4 | Bhattacharyya A., Hall D. B., Inorg. Chem., 1992, 31(18), 3869—3870 |
| 5 | Maritin K. J., Pinnavaia T. J., J. Am. Chem. Soc., 1986, 108(3), 541—542 |
| 6 | Jones W., Chibwe M., Pillared Layered Structures: Current Trends and Applications, Elsevier, London, 1990, 67—77 |
| 7 | Dimotakis E. D., Pinnavaia T. J., Inog. Chem., 1990, 29(13), 2393—2394 |
| 8 | Rives V., Ulibarri M. A., Coord. Chem. Rev., 1999, 181(1), 61—120 |
| 9 | Chibwe K., Jones W., Chem. Mater., 1989, 1(5), 489—490 |
| 10 | Giannelis E. P., Nocera D. G., Pinnavaia T. J., Inorg. Chem., 1987, 26(1), 203—205 |
| 11 | Itaya K., Chang H. C., Uchida I., Inorg. Chem., 1987, 26(4), 624—626 |
| 12 | Lopez⁃Salinas E., Ono Y., Micropor. Mater., 1993, 1(1), 33—42 |
| 13 | Kwon T., Pinnavaia T. J., Chem. Mater., 1989, 1(4), 381—383 |
| 14 | Ulibarri M. A., Labajos F. M., Rives V., Trujllano R., Kagunga W., Jones W., Inorg. Chem., 1994, 33(12), 2592—2599 |
| 15 | Choy J. H., Kwak S. Y., Park J. S., Jeong Y. J., Portier J., J. Am. Chem. Soc., 1999, 121(6), 1399—1400 |
| 16 | Theiss F. L., Ayoko G. A., Frost A. R., Appl. Surf. Sci., 2016, 383, 200—213 |
| 17 | Bookin A. S., Drits V. A., Clays Clay Miner., 1993, 41(5), 551—557 |
| 18 | Guo L. F., Feng L., Evans D. G., Duan X., Chem. Soc. Rev., 2014, 43(20), 7040—7066 |
| 19 | Guo L., Wu Y. Y., Duan P., Zhang Z. H., Constr. Build Mater., 2020, 232, 117—256 |
| 20 | Yue X. J., Li J. X., Zhang T., Qiu F. X., Yang D. Y., Xue M. W., Chem. Eng. J., 2017, 328(15), 117—123 |
| 21 | Lee J. H., Rhee S. W., Jung D. Y., Chem. Commun., 2003, 9(21), 2740—2741 |
| 22 | Liu Z. P., Ma R. Z., Osada M., Iyi N., Ebina Y., Takada K., Sasaki T., J. Am. Chem. Soc., 2006, 128(14), 4872—4880 |
| 23 | Vaccari A., Appl. Clay Sci., 1999, 14(4), 161—198 |
| 24 | Vaccari A., Catal. Today, 1998, 41(1—3), 53—71 |
| 25 | Sels B. F., de Vos. D., Buntinx M., Pierard F., Mesmaeker A. K. D., Jacobs P., Nature, 1999, 400(26), 855—857 |
| 26 | Zhao Y. F., Jia X. D., Waterhouse G. I. N., Wu L. Z., Tung C. H., O’Hare D., Zhang T. R., Adv. Energy Mater., 2016, 6(6), 1501974 |
| 27 | Yan H., Wei M., Ma J., Li F., Evans D. G., Duan X., J. Phys. Chem. A, 2009, 113(21), 6133—6141 |
| 28 | Xu S. M., Pan T., Dou Y. B., Yan H., Zhang S. T., Ning F. Y., Shi W. Y., Wei M., J. Phys. Chem. C, 2015, 119(33), 18823— 18834 |
| 29 | Xu S. M., Yan H., Wei M., J. Phys. Chem. C, 2017, 121(5), 2683—2695 |
| 30 | Tsukanov A. A., Psakhie S. G., Sci Rep., 2016, 6(1), 19986 |
| 31 | Mohapatra L., Parida K. M., J. Mater. Chem. A, 2016, 4(28), 10744—10766 |
| 32 | Peterson R. C., Hill R. J., Gibbss G. V., Can. Mineral., 1979, 17(4), 703—711 |
| 33 | Sauer J., Chem. Rev., 1989, 89(1), 199—255 |
| 34 | Liu H. M., Zhao X. J., Zhu Y. Q., Yan H., Phys. Chem. Chem. Phys., 2020, 22, 2521—2529 |
| 35 | Dias A., Cunha L., Vieira A. C., Mater. Res. Bull., 2011, 46(9), 1346—1351 |
| 36 | Miyata S., Clays Clay Miner., 1983, 31, 305—311 |
| 37 | Rousselot I., Taviot⁃Guého C., Leroux F., Léone P., Palvadeau P., Besse J. P., J. Solid State Chem., 2002, 167(1), 137—144 |
| 38 | Han J., Dou Y., Zhao J., Wei M., Evans D. G., Duan X., Small, 2013, 9, 98—106 |
| 39 | Caravaggio G. A., Detellier C., Wronski Z., J. Mater. Chem., 2001, 11(3), 912—921 |
| 40 | Li M. , Li L., Lin S., Chin. Chem. Lett., 2020, 31(6), 1511—1515 |
| 41 | Aisawa S., Takahashi S., Ogasawara W., Umetsu Y., Narita E., J. Solid State Chem., 2001, 162(1), 52—62 |
| 42 | Israëli Y., Guého C.T., Besse J.P., Morel J.P., Desrosiers N. M., J. Chem. Soc., Dalton Trans., 2000, 5, 791—796 |
| 43 | Maeda K., Domen K., Phys. Chem. Lett., 2010, 1(18), 2655—2661 |
| 44 | Baliarsingh N., Parida K. M., Pradhan G. C., Ind. Eng. Chem. Res., 2014, 53(10), 3834—3841 |
| 45 | Jiratova K., Kovanda F., Ludvikova J., Balabanova J., Klempa J., Catal. Today, 2016, 277, 61—67 |
| 46 | Li M., Cheng J. P., Wang J., Liu F., Zhang X. B., Electrochim. Acta, 2016, 206, 108—115 |
| 47 | Zhang H., Qi R., Liu L. N., Duan X., Chin. J. Chem. Phys., 2003, 16(1), 45—50 |
| 48 | Al-Jaberi M., Naille S., Dossot M., Ruby C., J. Mol. Struct., 2015, 1102, 253—260 |
| 49 | Ma R., Liu Z., Takada K., Iyi N., Bando Y., Sasaki T., J. Am. Chem. Soc., 2007, 129(16), 5257—5263 |
| 50 | Allmann R., Jepsen H., Jhb. Miner. Mh., 1969, 12, 544—551 |
| 51 | Unal U., J. Sol. State Chem., 2007, 180(9), 2525—2533 |
| 52 | Zhang J., Dong C., Wang Z., Gao H., Niu J., Peng Z., Zhang Z., Small Methods, 2018, 3(2), 1800286 |
| 53 | Altuntasoglu O., Unal U., Ida S., Goto M., Matsumoto Y., J. Solid State Chem., 2008, 181(12), 3257—3263 |
| 54 | Yang J. H., Pei Y. R., Kim S. J., Choi G., Vinu A., Choy J. H., Ind. Eng. Chem. Res., 2008, 57(48), 16264—16271 |
| 55 | Chowdhury P. R., Bhattacharyya K. G., RSC Adv., 2015, 5(112), 92189—92206 |
| 56 | Shu X., Zhang W., He J., Gao F., Zhu Y., Solid State Sci., 2006, 8(6), 634—639 |
| 57 | Yan H., Wei M., Ma J., Evans D. G., Duan X., J. Phys. Chem. A, 2010, 114(27), 7369—7376 |
| 58 | Costa D. G., Rocha A. B., Souza W. F., Chiaro S. S. X., Leitão A. A., Appl. Clay Sci., 2012, 56, 16—22 |
| 59 | Moraes P. I. R., Tavares S. R., Vaiss V. S., Leitão A. A., J. Phys. Chem. C, 2016, 120(18), 9965—9974 |
| 60 | Radha A.V., Kamath P.V., Shivakumara C., J. Phys. Chem. B, 2007, 111(13), 3411—3418 |
| 61 | Manohara G. V., Prasanna S. V., Kamath P. V., Eur. J. Inorg. Chem., 2011, 16, 2624—2630 |
| 62 | Rohrbach A., Hafner J., Kresse G., J. Phys. Condens.Mat., 2003, 15(6), 979—996 |
| 63 | Bajdich M., García-Mota M., Vojvodic A., Nørskov J. K., Bell A. T., J. Am. Chem. Soc., 2013, 135(36), 13521—13530 |
| 64 | Liao P., Keith J. A., Carter E. A., J. Am. Chem. Soc., 2012, 134(32), 13296—13309 |
| 65 | Kronawitter C. X., Riplinger C., He X., Zahl P., Carter E. A., Sutter P., Koel B. E., J. Am. Chem. Soc., 2014, 136(38), 13283—13288 |
| 66 | Mosey N. J., Liao P., Carter E. A., J. Chem. Phys., 2008, 129(1), 014103 |
| 67 | Kanan D. K., Carter E. A., J. Phys. Chem. C, 2012, 116(18), 9876—9887 |
| 68 | Zhou F., Cococcioni M., Marianetti C. A., Morgan D., Ceder G., Phys. Rev. B, 2004, 70(23), 235121 |
| 69 | Yan H., Wei M., Ma J., Duan X., Particuology, 2010, 8, 212—220 |
| 70 | Hu S., Sun Y., Pu M., Yun R., Xiang X., Sep. Purif. Technol., 2019, 229, 115813 |
| 71 | Cygan R. T., Liang J. J., Kalinichev A. G., J. Phys. Chem. B, 2004, 108(4), 1255—1266 |
| 72 | Kim N., Harale A., Tsotsis T. T., Sahimi M., J. Chem. Phys., 2007, 127(22), 224701—224782 |
| 73 | Pisson J., Morel J. P., Morel-Desrosiers N., Taviot⁃Gueho C., Malfreyt P., J. Phys. Chem. B, 2008, 112(26), 7856—7864 |
| 74 | Aicken A. M., Bell I. S., Coveney P. V., Jones W., Adv. Mater., 1997, 9(6), 409—500 |
| 75 | Zhang S. T., Yan H., Wei M., Evans D. G., Duan X., J. Phys. Chem. C, 2012, 116(5), 3421—3431 |
| 76 | Pan T., Xu S. M., Dou Y. B., Liu X. X., Li Z. Z., Han J. B., Yan H., Wei M., J. Mater. Chem. A, 2015, 3(23), 12350—12356 |
| 77 | Dou Y. B., Xu S. M., Liu X. X., Han J. B., Yan H., Wei M., Evans D. G., Duan X., Adv. Funct. Mater., 2014, 24(4), 514—521 |
| 78 | Dou Y. B., Pan T., Xu S. M., Yan H., Han J. B., Wei M., Evans D. G., Duan X., Angew. Chem., 2015, 127(33), 9809—9814 |
| 79 | Kaassis A. Y. A., Xu S. M., Evans D. G., Williams G. R., Wei M., Duan X., J. Phys. Chem. C, 2015, 119(32), 18729—18740 |
| 80 | Xu S. M., Zhang S. T., Shi W. Y., Ning F. Y., Fu Y., Yan H., RSC Adv., 2014, 4(88), 47472—47480 |
| 81 | Lv K., Kang H., Zhang H., Yuan S., Colloid Surface A, 2012, 402, 108—116 |
| 82 | Wang N., Huang Z., Li X., Li J., Ji S., An Q. F., J. Mater. Chem. A, 2018, 35(6), 17148—17155 |
| 83 | Qian Y. T., Introduction to Crystal Chemistry, University of Science and Technology of China Press, Hefei, 2005(钱逸泰. 结晶化学导论, 合肥: 中国科学技术大学出版社, 2005) |
| 84 | Millange F., Walton R. I., Lei L., O’Hare D., Chem. Mater., 2000, 12(7), 1990—1994 |
| 85 | Vivhi F. M., Alves O. L., J. Mater. Chem., 1997, 7(8), 1631—1634 |
| 86 | Basile F., Fornasari G., Gazzano M., Vaccari A., Appl. Clay Sci., 2001, 18(1/2), 51—57 |
| 87 | Basile F., Formasari G., Gazzano M., Vaccari A., Appl. Clay Sci., 2000, 16(3/4), 185—200 |
| 88 | Yan H., Lu J., Wei M., Ma J., Li H., He J., Evans D. G., Duan X., J. Mol. Struc⁃Theochem., 2008, 866(1—3), 34—45 |
| 89 | Yan H., Zhao X. J., Zhu Y. Q., Wei M., Evans D. G., Duan X., Struct. Bond., 2019, 182, 89—120 |
| 90 | Wang X. R., Li Y., Tang L. P., Gan W., Zhou W., Zhao Y. F., Bai D. S., Chinese Chem. Lett., 2017, 28(2), 394—399 |
| 91 | Ding S., Du X., Yang Y., Wang P., Zhang Z., Hao X., Peng C., Guan G., Phys. Chem. Chem. Phys.,2018, 20(25), 17313—17323 |
| 92 | Hibino T., Tsunashima A., J. Mater. Sci Lett., 2000, 19, 1403—1405 |
| 93 | Carvalho H. W. P., Pulcinelli S. H., Santilli C. V., Leroux F., Meneau F., Briois V., Chem. Mater., 2013, 25(14), 2855—2867 |
| 94 | Zhang S. T., Dou Y., Zhou J., Pu M., Yan H., Wei M., Evans D. G., Duan X., Chem. Phys. Chem., 2016, 17(17), 2754—2766 |
| 95 | Meng Q. T., Yan H., Mol. Simulat., 2017, 43(13—16), 1338—1347 |
| 96 | Meng Q. T., Yan H., Sci. China Chem., 2017, 47(4), 493—502(孟庆婷, 鄢红. 中国科学: 化学, 2017, 47(4), 493—502) |
| 97 | Constantino V. R. L., Pinnavaia T. J., Catal. Lett., 1994, 23(3), 361—367 |
| 98 | Liu X., Zhao X. F., Zhu Y., Zhang F. Z., Appl. Catal. B, 2013, 140/141, 241—248 |
| 99 | Pisson J., Morel⁃Desrosiers N., Morel J. P., Roy A., Leroux F., Taviot⁃Guého C., Malfreyt P., Chem. Mater., 2011, 23(6), 1482—1490 |
| 100 | Li H., Ma J., Evans D. G., Zhou T., Li F., Duan X., Chem. Mater., 2006, 18(18), 4405—4414 |
| 101 | Kumar P. P., Kalinichev A. G., Kirkpatrick R. J., J. Phys. Chem. C, 2007, 111(36), 13517—13523 |
| 102 | Petrova N., Mizota T., Stanimirova T., Kirov G., Micropor. Mesopor. Mater., 2003, 63(1—3), 139—145 |
| 103 | Kumar P. P., Kalinichev A. G., Kirkpatrick R. J., J Phys. Chem. B, 2006, 110(9), 3841—3844 |
| 104 | Kalinichev A. G., Kumar P. P., Kirkpatrick R. J., Philos. Mag., 2010, 90, 2475—2488 |
| 105 | Wang J. W., Kalinichev A. G., Kirkpatrick R. J., Hou X. Q., Chem. Mater., 2001, 13(1), 145—150 |
| 106 | Costa D. G., Rocha A. B., Souza W. F., Chiaro S. S. X., Leitão A. A., J. Phys. Chem. B, 2011, 115(13), 3531—3537 |
| 107 | Arunan E., Desiraju G. R., Klein R. A., Sadlej J., Scheiner S., Alkorta I., Clary D. C., Crabtree R. H., Dannenberg J. J., Hobza P., Kjaergaard H. G., Legon A. C., Mennucci B., Nesbitt D. J., Pure Appl. Chem., 2011, 83(8), 1637—1641 |
| 108 | Xu Q., Ni Z. M., Yao P., Li Y., J. Mol. Struct., 2010, 977(1—3), 165—169 |
| 109 | Prasad B. E., Kamath P. V., Vijayamohanan K., Langmuir, 2011, 27(22), 13539—13543 |
| 110 | Rahman M. T., Kameda T., Miura T., Kumagai S., Yoshioka T., J. Mate. Cycles Waste Manage., 2019, 21(5), 1232—1241 |
| 111 | Li L., Zhao K. C., Liu P. F., Zhu K., RSC. Adv., 2014, 4(35), 18086—18093 |
| 112 | Costa D. G., Rocha A. B., Diniz R., Souza W. F., Chiaro S. S. X., Leitão A. A., J. Phys. Chem. C, 2010, 114(33), 14133—14140 |
| 113 | Nangoi I. M., Tavares S. R., Wypych F., Leitão A. A., Appl. Clay Sci., 2019, 179, 105153 |
| 114 | Yan D. P., Lu J., Wei M., Ma J., Evans D. G., Duan X., Chem. Commun., 2009, 42, 6358—6360 |
| 115 | Zhang G., Zhang X., Meng Y., Pan G., Ni Z., Xia S., Chem. Eng. J., 2020, 392, 123684 |
| 116 | Xia S., Qian M., Zhou X., Meng Y., Xue J., Ni Z., Mol. Catal., 2017, 435, 118—127 |
| 117 | Xia S., Zhang G., Meng Y., Yang C., Ni Z., Hu J., Appl. Catal. B-Environ., 2020, 278, 119266 |
| 118 | Wu M. J., Wu J. Z., Zhang J., Chen H., Zhou J. Z., Qian G. R., Xu Z. P., Du Z., Rao Q. L., Catal. Sci. Technol., 2018, 8(5), 1207—1228 |
| 119 | Li X., Xin M., Guo S., Cai T., Du D., Xing W., Zhao L., Guo W., Xue Q., Yan Z., Electrochim. Acta, 2017, 253, 302—310 |
| 120 | Lv M., Liu H., J. Solid State Chem., 2015, 227, 232—238 |
| [1] | 滕镇远, 张启涛, 苏陈良. 聚合物单原子光催化剂的载流子分离和表面反应机制[J]. 高等学校化学学报, 2022, 43(9): 20220325. |
| [2] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [3] | 林治, 彭志明, 贺韦清, 沈少华. 单原子与团簇光催化: 竞争与协同[J]. 高等学校化学学报, 2022, 43(9): 20220312. |
| [4] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [5] | 夏雾, 任颖异, 刘京, 王锋. 壳聚糖包裹CdSe量子点组装体的水相可见光催化CO2还原[J]. 高等学校化学学报, 2022, 43(7): 20220192. |
| [6] | 赵盈喆, 张建玲. 金属-有机框架基材料在二氧化碳光催化转化中的应用[J]. 高等学校化学学报, 2022, 43(7): 20220223. |
| [7] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [8] | 龚妍熹, 王建兵, 柴歩瑜, 韩元春, 马云飞, 贾超敏. 钾掺杂g-C3N4薄膜光阳极的制备及光电催化氧化降解水中双氯芬酸钠性能[J]. 高等学校化学学报, 2022, 43(6): 20220005. |
| [9] | 高志伟, 李军委, 史赛, 付强, 贾钧儒, 安海龙. 基于分子动力学模拟的TRPM8通道门控特性分析[J]. 高等学校化学学报, 2022, 43(6): 20220080. |
| [10] | 王广琦, 毕艺洋, 王嘉博, 石洪飞, 刘群, 张钰. 非贵金属三元复合Ni(PO3)2-Ni2P/CdS NPs异质结的构建及可见光高效催化产氢性能[J]. 高等学校化学学报, 2022, 43(6): 20220050. |
| [11] | 宋颖颖, 黄琳, 李庆森, 陈立妙. CuO/BiVO4光催化剂的制备及光催化CO2还原性能[J]. 高等学校化学学报, 2022, 43(6): 20220126. |
| [12] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [13] | 陶雨, 欧鸿辉, 雷永鹏, 熊禹. 单原子催化剂在光催化二氧化碳还原中的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220143. |
| [14] | 冯丽, 邵兰兴, 李思骏, 全文选, 庄金亮. 超薄Sm-MOF纳米片的合成及可见光催化降解芥子气模拟剂性能[J]. 高等学校化学学报, 2022, 43(4): 20210867. |
| [15] | 曾晛阳, 赵熹, 黄旭日. 细胞松弛素B对葡萄糖/质子共转运蛋白GlcPSe的抑制机理[J]. 高等学校化学学报, 2022, 43(4): 20210822. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||