

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (1): 145.doi: 10.7503/cjcu20190430
邓安强1,2,3,罗永春1,2,*( ),夏元华4,彭思慧2,马伟旗2,赵旭东2,杨洋2,侯晓东2
),夏元华4,彭思慧2,马伟旗2,赵旭东2,杨洋2,侯晓东2
收稿日期:2019-07-31
出版日期:2020-01-10
发布日期:2019-11-04
通讯作者:
罗永春
E-mail:luoyc@lut.cn
基金资助:
DENG Anqiang1,2,3,LUO Yongchun1,2,*( ),XIA Yuanhua4,PENG Sihui2,MA Weiqin2,ZHAO Xudong2,YANG Yang2,HOU Xiaodong2
),XIA Yuanhua4,PENG Sihui2,MA Weiqin2,ZHAO Xudong2,YANG Yang2,HOU Xiaodong2
Received:2019-07-31
Online:2020-01-10
Published:2019-11-04
Contact:
Yongchun LUO
E-mail:luoyc@lut.cn
Supported by:摘要:
通过电弧熔炼制备了无镁La-Y-Ni系A2B7型Y0.7La0.3Ni3.25Al0.1Mn0.15合金, 并在高纯0.2 MPa Ar气氛下分别对合金进行850~1050 ℃真空24 h退火热处理. 通过X射线衍射(XRD)、 中子衍射(ND)、 扫描电子显微镜/能量分散谱(SEM/EDS)和电化学测试方法研究了退火温度对合金结构和性能的影响. 结构分析表明, 铸态合金由CaCu5, Ce5Co19, Gd2Co7, Ce2Ni7多相构成, 随着退火温度升高, CaCu5, Ce5Co19, Gd2Co7相逐步减少直至消失, Ce2Ni7主相相丰度逐步增加. 900~950 ℃退火时, 合金为单相Ce2Ni7结构. 退火温度继续升高, 合金中出现少量PuNi3相. 合金电极的最大放电容量随着退火温度的升高先增加后降低. 从铸态的307.6 mA·h/g增加到900 ℃退火时的最大值393.1 mA·h/g, 后又降到1050 ℃退火时的366.4 mA·h/g. 合金电极的电化学循环稳定性随退火温度的升高而升高, 循环100次后电化学容量保持率(S100)从铸态的66%上升到1050 ℃退火后的88.5%, 900~950 ℃退火时, 合金电极具有较好的综合电化学性能.
中图分类号:
TrendMD:
邓安强,罗永春,夏元华,彭思慧,马伟旗,赵旭东,杨洋,侯晓东. 退火处理对新型无镁Y0.7La0.3Ni3.25Al0.1Mn0.15储氢合金结构和电化学性能的影响. 高等学校化学学报, 2020, 41(1): 145.
DENG Anqiang,LUO Yongchun,XIA Yuanhua,PENG Sihui,MA Weiqin,ZHAO Xudong,YANG Yang,HOU Xiaodong. Effect of Annealing Treatment on Structure and Electrochemical Properties of New Mg-free Y0.7La0.3Ni3.25Al0.1Mn0.15 Hydrogen Storage Alloys †. Chem. J. Chinese Universities, 2020, 41(1): 145.
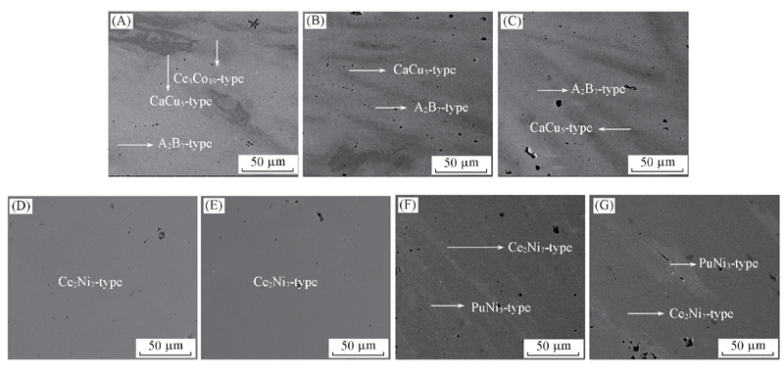
Fig.1 Backscattered SEM images of the as-cast(A) and annealed(B—G) alloys Y0.7La0.3Ni3.25Al0.1Mn0.15 Annealing temperature/℃: (B) 800; (C) 850; (D) 900; (E) 950; (F) 1000; (G) 1050.
| Sample | Phase | Atomic fraction(%) | Stoichiometric B/A | ||||
|---|---|---|---|---|---|---|---|
| La | Y | Ni | Al | Mn | |||
| As-cast | | 0.3106 | 0.6894 | 3.2298 | 0.1021 | 0.1560 | 3.49 |
| As-cast | A2B7 | 0.2806 | 0.7194 | 3.2273 | 0.0711 | 0.1302 | 3.43 |
| Ce5Co19 | 0.2525 | 0.7475 | 3.5112 | 0.1121 | 0.1595 | 3.78 | |
| CaCu5 | 0.2147 | 0.7853 | 4.6011 | 0.1791 | 0.1638 | 4.94 | |
| Annealed at 800 ℃ | A2B7 | 0.3359 | 0.6641 | 3.1878 | 0.0804 | 0.1329 | 3.40 |
| CaCu5 | 0.2021 | 0.7979 | 4.3549 | 0.2176 | 0.1536 | 4.73 | |
| Annealed at 850 ℃ | A2B7 | 0.3020 | 0.6980 | 3.2045 | 0.0943 | 0.1388 | 3.44 |
| CaCu5 | 0.2090 | 0.7910 | 4.3423 | 0.2736 | 0.1642 | 4.78 | |
| Annealed at 900 ℃ | Ce2Ni7 | 0.2596 | 0.7404 | 3.1830 | 0.1106 | 0.1617 | 3.46 |
| Annealed at 950 ℃ | Ce2Ni7 | 0.3075 | 0.6925 | 3.1879 | 0.0961 | 0.1423 | 3.43 |
| Annealed at 1000 ℃ | Ce2Ni7 | 0.2958 | 0.7042 | 3.1633 | 0.1175 | 0.1317 | 3.41 |
| PuNi3 | 0.3704 | 0.6296 | 2.7189 | 0.1099 | 0.1164 | 2.95 | |
| Annealed at 1050 ℃ | Ce2Ni7 | 0.2600 | 0.7400 | 3.1340 | 0.1080 | 0.1280 | 3.37 |
| PuNi3 | 0.3312 | 0.6688 | 2.7239 | 0.0942 | 0.1051 | 2.92 | |
Table 1 Chemical composition of the alloys determined by ICP and micro-area composition by EDS analysis
| Sample | Phase | Atomic fraction(%) | Stoichiometric B/A | ||||
|---|---|---|---|---|---|---|---|
| La | Y | Ni | Al | Mn | |||
| As-cast | | 0.3106 | 0.6894 | 3.2298 | 0.1021 | 0.1560 | 3.49 |
| As-cast | A2B7 | 0.2806 | 0.7194 | 3.2273 | 0.0711 | 0.1302 | 3.43 |
| Ce5Co19 | 0.2525 | 0.7475 | 3.5112 | 0.1121 | 0.1595 | 3.78 | |
| CaCu5 | 0.2147 | 0.7853 | 4.6011 | 0.1791 | 0.1638 | 4.94 | |
| Annealed at 800 ℃ | A2B7 | 0.3359 | 0.6641 | 3.1878 | 0.0804 | 0.1329 | 3.40 |
| CaCu5 | 0.2021 | 0.7979 | 4.3549 | 0.2176 | 0.1536 | 4.73 | |
| Annealed at 850 ℃ | A2B7 | 0.3020 | 0.6980 | 3.2045 | 0.0943 | 0.1388 | 3.44 |
| CaCu5 | 0.2090 | 0.7910 | 4.3423 | 0.2736 | 0.1642 | 4.78 | |
| Annealed at 900 ℃ | Ce2Ni7 | 0.2596 | 0.7404 | 3.1830 | 0.1106 | 0.1617 | 3.46 |
| Annealed at 950 ℃ | Ce2Ni7 | 0.3075 | 0.6925 | 3.1879 | 0.0961 | 0.1423 | 3.43 |
| Annealed at 1000 ℃ | Ce2Ni7 | 0.2958 | 0.7042 | 3.1633 | 0.1175 | 0.1317 | 3.41 |
| PuNi3 | 0.3704 | 0.6296 | 2.7189 | 0.1099 | 0.1164 | 2.95 | |
| Annealed at 1050 ℃ | Ce2Ni7 | 0.2600 | 0.7400 | 3.1340 | 0.1080 | 0.1280 | 3.37 |
| PuNi3 | 0.3312 | 0.6688 | 2.7239 | 0.0942 | 0.1051 | 2.92 | |
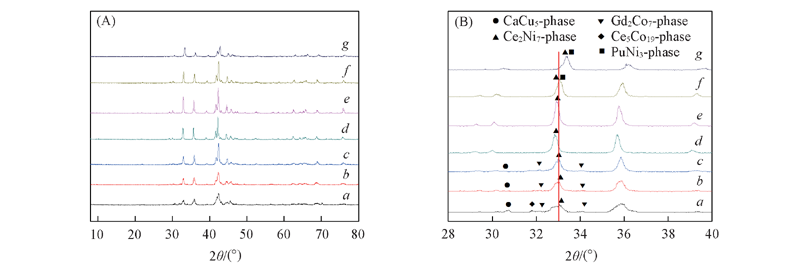
Fig.2 XRD patterns of as-cast and annealed alloys(A) and its partial magnification(B) a. As-cast; b. 800 ℃; c. 850 ℃; d. 900 ℃; e. 950 ℃; f. 1000 ℃; g. 1050 ℃.
| Condition | Phase | Space group(No.) | Phase abundance(%) | Lattice constant | V/nm3 | Strain(%) | Ce2Ni7 | |
|---|---|---|---|---|---|---|---|---|
| a/nm | c/nm | |||||||
| As-cast | Ce2Ni7 | P63/mmc(194) | 65.78 | 0.50173 | 2.43745 | 0.53138 | 1.29 | 1.674 |
| Gd2Co7 | R | 15.80 | 0.44902 | 3.93293 | 0.68672 | |||
| Ce5Co19 | R | 8.37 | 0.50579 | 4.80588 | 1.06470 | |||
| CaCu5 | P6/mmm(191) | 10.05 | 0.49621 | 0.40003 | 0.08530 | |||
| Annealed at 800 ℃ | Ce2Ni7 | P63/mmc(194) | 82.03 | 0.50191 | 2.43675 | 0.53161 | 0.80 | 1.247 |
| Gd2Co7 | R | 9.01 | 0.44832 | 3.96713 | 0.69054 | |||
| CaCu5 | P6/mmm(191) | 8.96 | 0.50048 | 0.40145 | 0.08708 | |||
| Annealed at 850 ℃ | Ce2Ni7 | P63/mmc(194) | 89.16 | 0.50172 | 2.44092 | 0.53211 | 0.55 | 1.116 |
| Gd2Co7 | R | 6.45 | 0.44669 | 4.04017 | 0.69813 | |||
| CaCu5 | P6/mmm(191) | 4.39 | 0.49996 | 0.40513 | 0.08770 | |||
| Annealed at 900 ℃ | Ce2Ni7 | P63/mmc(194) | 100.00 | 0.50281 | 2.44133 | 0.53451 | 0.17 | 1.030 |
| Annealed at 950 ℃ | Ce2Ni7 | P63/mmc(194) | 100.00 | 0.50202 | 2.43837 | 0.53219 | 0.20 | 1.030 |
| Annealed at 1000 ℃ | Ce2Ni7 | P63/mmc(194) | 89.92 | 0.50161 | 2.43729 | 0.53108 | 0.22 | 1.028 |
| PuNi3 | R | 10.08 | 0.49521 | 2.43776 | 0.51772 | |||
| Annealed at 1050 ℃ | Ce2Ni7 | P63/mmc(194) | 84.68 | 0.50158 | 2.43659 | 0.53087 | 0.24 | 1.020 |
| PuNi3 | R | 15.32 | 0.49448 | 2.43004 | 0.51456 | |||
Table 2 Structure parameters of various phases for different annealed alloys*
| Condition | Phase | Space group(No.) | Phase abundance(%) | Lattice constant | V/nm3 | Strain(%) | Ce2Ni7 | |
|---|---|---|---|---|---|---|---|---|
| a/nm | c/nm | |||||||
| As-cast | Ce2Ni7 | P63/mmc(194) | 65.78 | 0.50173 | 2.43745 | 0.53138 | 1.29 | 1.674 |
| Gd2Co7 | R | 15.80 | 0.44902 | 3.93293 | 0.68672 | |||
| Ce5Co19 | R | 8.37 | 0.50579 | 4.80588 | 1.06470 | |||
| CaCu5 | P6/mmm(191) | 10.05 | 0.49621 | 0.40003 | 0.08530 | |||
| Annealed at 800 ℃ | Ce2Ni7 | P63/mmc(194) | 82.03 | 0.50191 | 2.43675 | 0.53161 | 0.80 | 1.247 |
| Gd2Co7 | R | 9.01 | 0.44832 | 3.96713 | 0.69054 | |||
| CaCu5 | P6/mmm(191) | 8.96 | 0.50048 | 0.40145 | 0.08708 | |||
| Annealed at 850 ℃ | Ce2Ni7 | P63/mmc(194) | 89.16 | 0.50172 | 2.44092 | 0.53211 | 0.55 | 1.116 |
| Gd2Co7 | R | 6.45 | 0.44669 | 4.04017 | 0.69813 | |||
| CaCu5 | P6/mmm(191) | 4.39 | 0.49996 | 0.40513 | 0.08770 | |||
| Annealed at 900 ℃ | Ce2Ni7 | P63/mmc(194) | 100.00 | 0.50281 | 2.44133 | 0.53451 | 0.17 | 1.030 |
| Annealed at 950 ℃ | Ce2Ni7 | P63/mmc(194) | 100.00 | 0.50202 | 2.43837 | 0.53219 | 0.20 | 1.030 |
| Annealed at 1000 ℃ | Ce2Ni7 | P63/mmc(194) | 89.92 | 0.50161 | 2.43729 | 0.53108 | 0.22 | 1.028 |
| PuNi3 | R | 10.08 | 0.49521 | 2.43776 | 0.51772 | |||
| Annealed at 1050 ℃ | Ce2Ni7 | P63/mmc(194) | 84.68 | 0.50158 | 2.43659 | 0.53087 | 0.24 | 1.020 |
| PuNi3 | R | 15.32 | 0.49448 | 2.43004 | 0.51456 | |||
| Subunit | Atom | Site | x | y | z | 102Biso/nm2 | Occ |
|---|---|---|---|---|---|---|---|
| Laves | Y | 4f | 0.33333 | 0.66667 | 0.02776 | 1.24 | 1.00 |
| CaCu5 | Y | 4f | 0.33333 | 0.66667 | 0.16953 | 0.78 | 0.40 |
| La | 4f | 0.33333 | 0.66667 | 0.16953 | 0.78 | 0.60 | |
| Laves | Ni | 2a | 0 | 0 | 0 | 0.66 | 0.92 |
| Al | 2a | 0 | 0 | 0 | 0.66 | 0.02 | |
| Mn | 2a | 0 | 0 | 0 | 0.66 | 0.06 | |
| CaCu5 | Ni | 4e | 0 | 0 | 0.17051 | 1.60 | 0.83 |
| Al | 4e | 0 | 0 | 0.17051 | 1.60 | 0.08 | |
| Mn | 4e | 0 | 0 | 0.17051 | 1.60 | 0.09 | |
| CaCu5 | Ni | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.90 |
| Al | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.05 | |
| Mn | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.06 | |
| CaCu5 | Ni | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.77 |
| Al | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.10 | |
| Mn | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.13 | |
| Laves/CaCu5 | Ni | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.81 |
| Al | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.06 | |
| Mn | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.12 |
Table 3 Crystallographic parameters for the alloy annealed at 900 ℃*
| Subunit | Atom | Site | x | y | z | 102Biso/nm2 | Occ |
|---|---|---|---|---|---|---|---|
| Laves | Y | 4f | 0.33333 | 0.66667 | 0.02776 | 1.24 | 1.00 |
| CaCu5 | Y | 4f | 0.33333 | 0.66667 | 0.16953 | 0.78 | 0.40 |
| La | 4f | 0.33333 | 0.66667 | 0.16953 | 0.78 | 0.60 | |
| Laves | Ni | 2a | 0 | 0 | 0 | 0.66 | 0.92 |
| Al | 2a | 0 | 0 | 0 | 0.66 | 0.02 | |
| Mn | 2a | 0 | 0 | 0 | 0.66 | 0.06 | |
| CaCu5 | Ni | 4e | 0 | 0 | 0.17051 | 1.60 | 0.83 |
| Al | 4e | 0 | 0 | 0.17051 | 1.60 | 0.08 | |
| Mn | 4e | 0 | 0 | 0.17051 | 1.60 | 0.09 | |
| CaCu5 | Ni | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.90 |
| Al | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.05 | |
| Mn | 4f | 0.66667 | 0.33333 | 0.1681 | 0.39 | 0.06 | |
| CaCu5 | Ni | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.77 |
| Al | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.10 | |
| Mn | 6h | 0.1692 | 0.33838 | 0.75 | 0.35 | 0.13 | |
| Laves/CaCu5 | Ni | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.81 |
| Al | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.06 | |
| Mn | 12k | 0.167 | 0.33406 | 0.91513 | 0.21 | 0.12 |
| Alloy | Na | Cmax/(mA·h·g-1) | S100(%) | Ecorr/V | icorr/(mA·cm-2) |
|---|---|---|---|---|---|
| As-cast | 2 | 307.6 | 66.0 | -0.933 | 21.34 |
| Annealed at 800 ℃ | 5 | 347.3 | 68.1 | -0.925 | 18.56 |
| Annealed at 850 ℃ | 3 | 372.8 | 79.2 | -0.921 | 18.25 |
| Annealed at 900 ℃ | 2 | 393.1 | 80.0 | -0.906 | 14.06 |
| Annealed at 950 ℃ | 3 | 371.8 | 88.0 | -0.913 | 14.71 |
| Annealed at 1000 ℃ | 3 | 366.4 | 87.5 | -0.917 | 14.71 |
| Annealed at 1050 ℃ | 6 | 366.4 | 88.5 | -0.92 | 14.84 |
Table 4 Electrochemical properties for different annealed alloy electrodes*
| Alloy | Na | Cmax/(mA·h·g-1) | S100(%) | Ecorr/V | icorr/(mA·cm-2) |
|---|---|---|---|---|---|
| As-cast | 2 | 307.6 | 66.0 | -0.933 | 21.34 |
| Annealed at 800 ℃ | 5 | 347.3 | 68.1 | -0.925 | 18.56 |
| Annealed at 850 ℃ | 3 | 372.8 | 79.2 | -0.921 | 18.25 |
| Annealed at 900 ℃ | 2 | 393.1 | 80.0 | -0.906 | 14.06 |
| Annealed at 950 ℃ | 3 | 371.8 | 88.0 | -0.913 | 14.71 |
| Annealed at 1000 ℃ | 3 | 366.4 | 87.5 | -0.917 | 14.71 |
| Annealed at 1050 ℃ | 6 | 366.4 | 88.5 | -0.92 | 14.84 |
| [1] |
Kadir K., Sakai T., Uehara I ., J. Alloys Compd., 1997,257(1/2), 115— 121
doi: 10.1016/S0925-8388(96)03132-5 URL |
| [2] |
Zhao Y. M ., Wang W. F.,Han S. M.,Guo W.,Li Y.,Zhang L.,Xu G. C., J. Alloys Compd., 2019,775, 259— 269
doi: 10.1016/j.jallcom.2018.10.079 URL |
| [3] |
Denys R. V ., Riabov A. B.,Yartys V. A.,Sato M.,Delaplane R. G., J. Solid State Chem., 2008,181(4), 812— 821
doi: 10.1016/j.jssc.2007.12.041 URL |
| [4] |
Gal L., Charbonnier V., Zhang J., Goubault L., Int. J. Hydrogen Energy, 2015,40(47), 17017— 17020
doi: 10.1016/j.ijhydene.2015.06.068 URL |
| [5] |
Deng A. Q., Fan J. B.,Qian K. N.,Luo Y. C., Acta Phys. Chim. Sin., 2011,27(1), 103— 107
doi: 10.3866/PKU.WHXB20110133 URL |
|
( 邓安强, 樊静波, 钱克农, 罗永春. 物理化学学报, 2011,27(1), 103— 107)
doi: 10.3866/PKU.WHXB20110133 URL |
|
| [6] |
Baddour-Hadjean R., Meyer L., Pereira-Ramos J. P ., Latroche M.,Percheron-Guegan A., Electrochim. Acta, 2001,46(15), 2385— 2393
doi: 10.1016/S0013-4686(01)00440-6 URL |
| [7] |
Aoki K., Masumoto T ., J. Alloys Compd., 1995,231(1/2), 20— 28
doi: 10.1016/0925-8388(95)01832-8 URL |
| [8] |
Charbonnier V., Zhang J., Monnier J., Goubault L., Bernard P., Magén C., Serin V., Latroche M ., J. Phys. Chem. C, 2015,119(22), 12218— 12225
doi: 10.1021/acs.jpcc.5b03096 URL |
| [9] |
Berezovets’ V. V ., Denys R. V.,Ryabov O. B.,Zavalii I. Y., Mater. Sci., 2007,43(4), 499— 507
doi: 10.1007/s11003-007-0058-4 URL |
| [10] |
Lartigue C., Percheron-Guégan A., Achard J. C ., Tasset F., J. Less. Common. Met., 1980,75(11), 23— 29
doi: 10.1016/0022-5088(80)90365-3 URL |
| [11] |
Takeshita T., Gschneidner K. A ., Lakner J. F., J. Less. Common. Met., 1981,78(1), 43— 47
doi: 10.1016/0022-5088(81)90142-9 URL |
| [12] |
Subramanian P. R ., Smith J. F., Metall. Trans. B, 1985,16(3), 577— 584
doi: 10.1007/BF02654856 URL |
| [13] | Roisnel T., Rodríquez-Carvajal J., Mater. Sci. Forum., 2001, 378—381(1), 118— 123 |
| [14] | Will G ., Powder Diffraction: the Rietveld Method and the Two-stage Method to Determine and Refine Crystal Structures from Powder Diffraction Data, Springer,. Berlin, 2006 |
| [15] |
Latroche M., Baddour-Hadjean R., Percheron-Guégan A ., J. Solid State Chem., 2003,173(1), 236— 243
doi: 10.1016/S0022-4596(03)00038-0 URL |
| [16] |
Yasuoka S., Ishida J. Kai T., Kajiwara T., Doi S.,Yamazaki T.,Kishida K.,Inui H., Int. J. Hydrogen Energy, 2017, 42(16), 11574—11583
doi: 10.1016/j.ijhydene.2017.02.150 URL |
| [17] |
Denys R. V., Riabov B., Yartys V. A., Delaplane R. .,Sato M., J. Alloys Compd., 2007,446/447(1), 166— 172
doi: 10.1016/j.jallcom.2006.12.137 URL |
| [18] |
Liu J. J ., Li Y.,Han D.,Yang S. Q.,Chen X. C.,Zhang L.,Han S. M., J. Power Sources, 2015,300(12), 77— 86
doi: 10.1016/j.jpowsour.2015.09.058 URL |
| [19] |
Senoh H., Takeichi N., Takeshita H. T ., Tanaka H.,Kiyobayashi T.,Kuriyama N.,. Materials Science and Engineering: B, 2004,108(1/2), 96— 99
doi: 10.1016/j.mseb.2003.10.055 URL |
| [20] |
Iwase K., Mori K., Hoshikawa A., Ishigaki T., Int. J. Hydrogen Energy, 2012,37(6), 5122— 5127
doi: 10.1016/j.ijhydene.2011.12.108 URL |
| [21] | Liu Y. R ., Yuan H. P.,Guo M.,Jiang L. J., Int.J. Hydrogen Energy, 2019,44(39), 22064— 22073 |
| [22] |
Fang F., Chen Z. L ., Wu D. Y.,Liu H.,Dong C. K.,Song Y.,Sun D. L., J. Power Sources, 2019,427(7), 145— 153
doi: 10.1016/j.jpowsour.2019.04.072 URL |
| [1] | 汪思聪, 庞贝贝, 刘潇康, 丁韬, 姚涛. XAFS技术在单原子电催化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220487. |
| [2] | 江博文, 陈敬轩, 成永华, 桑微, 寇宗魁. 单原子材料在电化学生物传感中的研究进展[J]. 高等学校化学学报, 2022, 43(9): 20220334. |
| [3] | 仵宇帅, 尚颖旭, 蒋乔, 丁宝全. 可控自组装DNA折纸结构作为药物载体的研究进展[J]. 高等学校化学学报, 2022, 43(8): 20220179. |
| [4] | 李琳, 齐丰莲, 邱丽莉, 孟子晖. 基于六边形磁纳米片构建动态非晶态光学结构图案[J]. 高等学校化学学报, 2022, 43(8): 20220123. |
| [5] | 贾洋刚, 邵霞, 程婕, 王朋朋, 冒爱琴. 赝电容控制型钙钛矿高熵氧化物La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3负极材料的制备及储锂性能[J]. 高等学校化学学报, 2022, 43(8): 20220157. |
| [6] | 杨丹, 刘旭, 戴翼虎, 祝艳, 杨艳辉. 金团簇电催化二氧化碳还原反应的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220198. |
| [7] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [8] | 陈玮琴, 吕佳敏, 余申, 刘湛, 李小云, 陈丽华, 苏宝连. 有机杂化介孔Beta分子筛的合成及在苯甲醇烷基化反应中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220086. |
| [9] | 刘情情, 王普, 王永帅, 赵曼, 董焕丽. 新型萘/苝酰亚胺取代丁二炔衍生物的合成及拓扑聚合[J]. 高等学校化学学报, 2022, 43(6): 20220091. |
| [10] | 施耐克, 张娅, SANSON Andrea, 王蕾, 陈骏. Zn(NCN)单轴的负热膨胀性及机理研究[J]. 高等学校化学学报, 2022, 43(6): 20220124. |
| [11] | 朱凯, 利婕, 武潇逸, 胡薇薇, 吴冬梅, 虞诚潇, 葛志伟, 叶兴乾, 陈士国. 基于多孔石墨化碳柱-四极杆-飞行时间质谱解析甜菜果胶精细结构[J]. 高等学校化学学报, 2022, 43(6): 20220023. |
| [12] | 夏天, 万家炜, 于然波. 异原子配位结构碳基单原子电催化剂结构与性能相关性的研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220162. |
| [13] | 谷雨, 奚宝娟, 李江潇, 熊胜林. 单原子催化剂在氧还原反应中的分子级调控[J]. 高等学校化学学报, 2022, 43(5): 20220036. |
| [14] | 徐斯然, 阴恒铂, 薛冬萍, 夏会聪, 赵舒琰, 闫文付, 木士春, 张佳楠. 应用于氧还原反应的非贵金属原子分散级金属-氮-碳催化剂的设计[J]. 高等学校化学学报, 2022, 43(5): 20220028. |
| [15] | 郭谨昌, 刘芳林. 平面五配位硅、 锗XBe5H6(X=Si, Ge)团簇[J]. 高等学校化学学报, 2022, 43(4): 20210807. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||