

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (4): 20210807.doi: 10.7503/cjcu20210807
收稿日期:2021-11-29
出版日期:2022-04-10
发布日期:2022-02-17
通讯作者:
郭谨昌
E-mail:guojc@sxu.edu.cn
基金资助:Received:2021-11-29
Online:2022-04-10
Published:2022-02-17
Contact:
GUO Jinchang
E-mail:guojc@sxu.edu.cn
Supported by:摘要:
采用密度泛函理论PBE0方法, 在aug-cc-pVTZ水平上理论预测了含平面五配位硅和锗原子的XBe5H6 (X=Si, Ge)团簇. 势能面系统搜索及高精度量化计算表明, 它们均为全局极小结构. XBe5H6(X=Si, Ge)团簇整体呈完美的扇形结构: Si/Ge原子被5个金属Be原子配位; 4个H原子以桥基方式与Be原子相键连, 剩余的2个 H原子以端基方式与两端的Be原子成键. 化学键分析表明, XBe5H6(X=Si, Ge) 团簇中XBe5单元具有完全离域的1个π及3个σ键, 外围铍氢间形成4个Be—H—Be 三中心二电子(3c-2e)键及2个定域的Be—H键. XBe5单元上离域的2π及6σ电子赋予体系π和σ双重芳香性, 并使Si/Ge原子满足八隅律(或八电子规则). 能量分解-化学价自然轨道分析揭示, Si/Ge和Be5H6之间主要为电子共享键.
中图分类号:
TrendMD:
郭谨昌, 刘芳林. 平面五配位硅、 锗XBe5H6(X=Si, Ge)团簇. 高等学校化学学报, 2022, 43(4): 20210807.
GUO Jinchang, LIU Fanglin. Planar Pentacoordinate Silicon and Germanium in XBe5H6(X=Si, Ge) Clusters. Chem. J. Chinese Universities, 2022, 43(4): 20210807.
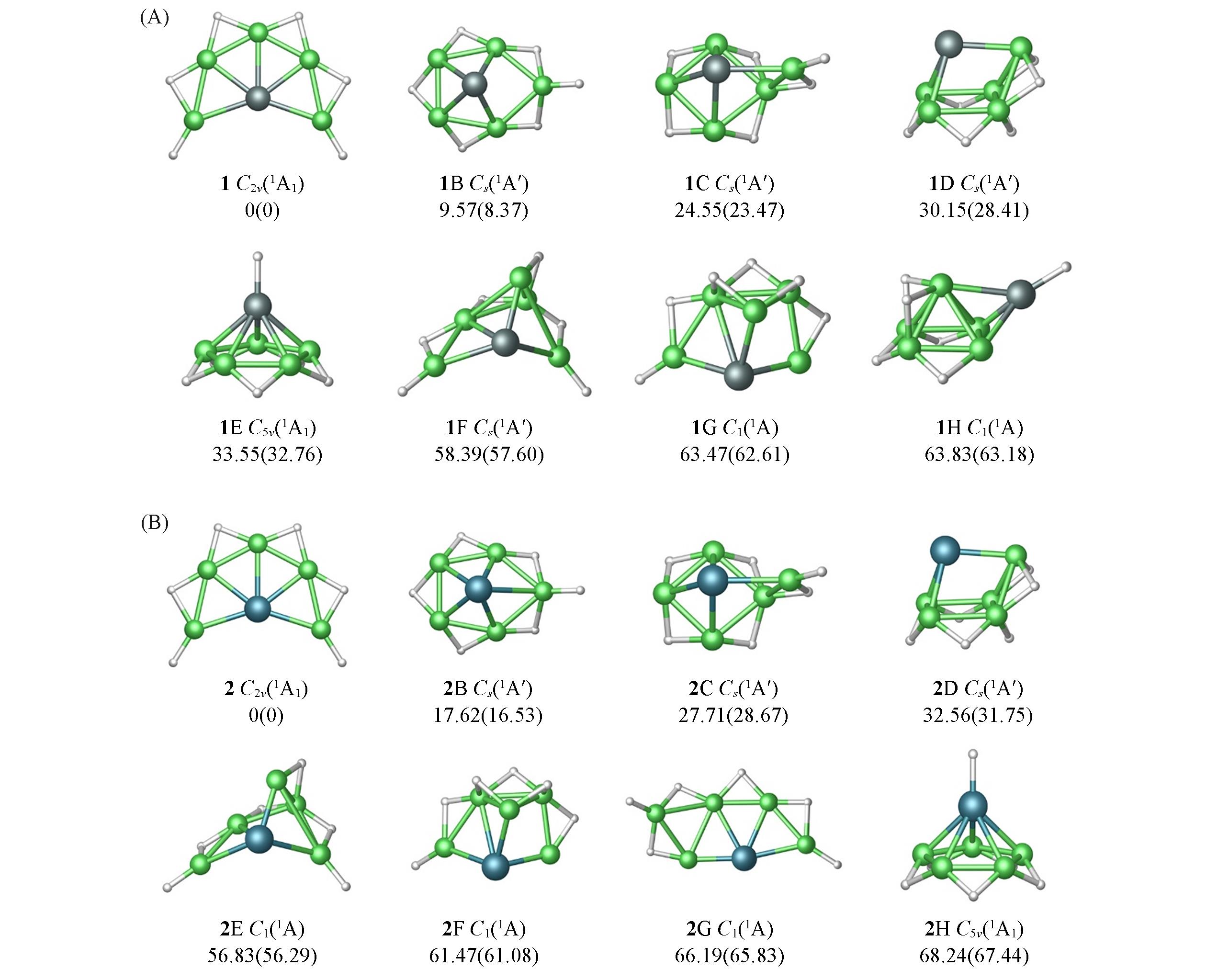
Fig.1 Optimized global minimum(GM) structures of SiBe5H6(1) and GeBe5H6(2) at the PBE0/aug?cc?pVTZ level along with seven lowest?lying isomer(nB—nH)Relative energies are listed in kJ/mol at the single?point CCSD(T) level using their PBE0 geometries, with zero?point energy(ZPE) corrections at PBE0. Values in parentheses are the relative energetics at single?point CCSD(T)/aug?cc?pVTZ//B3LYP/aug?cc?pVTZ level, including ZPE corrections at B3LYP.
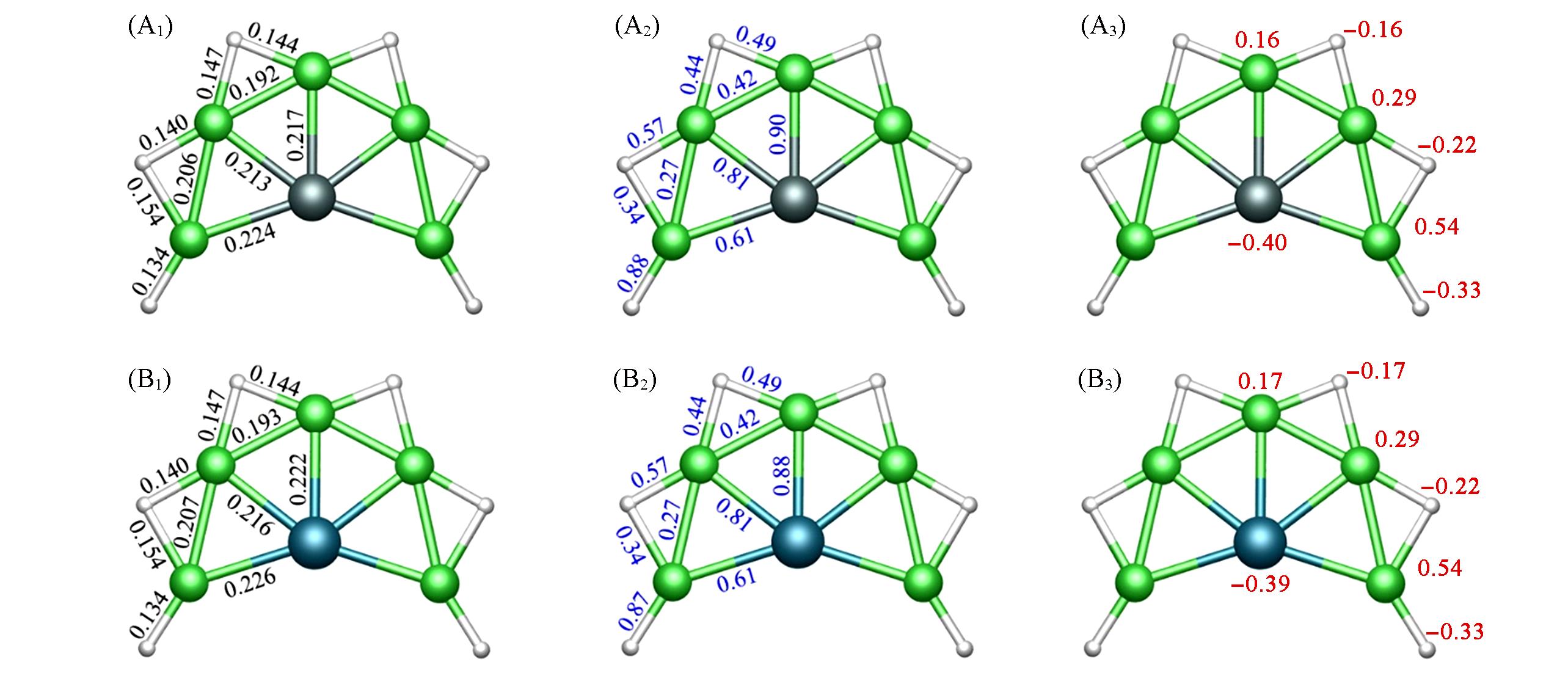
Fig.2 Bond distances(nm, A1, B1), Wiberg bond indices(WBIs, A2, B2), and natural atomic charges(|e|, A3, B3) of SiBe5H6(1)(A1—A3) and GeBe5H6(2)(B1—B3) clusters at the PBE0/aug?cc?pVTZ level
| System | Interaction | ΔEint/ (kJ·mol-1) | ΔEPauli/ (kJ·mol-1) | ΔEelstata / (kJ·mol-1) | ΔEorba / (kJ·mol-1) | ΔEorb(1)b / (kJ·mol-1) | ΔEorb(2)b / (kJ·mol-1) | ΔEorb(3)b / (kJ·mol-1) | ΔEorb(4)b / (kJ·mol-1) | ΔEorb(rest)b / (kJ·mol-1) |
|---|---|---|---|---|---|---|---|---|---|---|
| SiBe5H6 | — | -1438.1 | 719.91 | -1137.3 | -1020.7 | -14.4 | ||||
| (52.7%) | (47.3%) | (1.4%) | ||||||||
| X?Be5H6 electron? | -173.3 | |||||||||
| sharing π bond | (17.0%) | |||||||||
| X?Be5H6 electron? | -288.0 | -268.1 | -276.9 | |||||||
| sharing σ bond | (28.2%) | (26.3%) | (27.1%) | |||||||
| GeBe5H6 | — | -1503.4 | 873.2 | -1293.2 | -1083.4 | -20.3 | ||||
| (54.4%) | (45.6%) | (2.0%) | ||||||||
| X?Be5H6 electron? | -174.6 | |||||||||
| sharing π bond | (16.1%) | |||||||||
| X?Be5H6 electron? | -298.2 | -268.1 | -322.2 | |||||||
| sharing σ bond | (27.5%) | (24.7%) | (29.7%) |
Table 1 EDA-NOCV results of XBe5H6(X=Si, Ge) cluster at the PBE0/TZ2P//PBE0/aug-cc-pVTZ level, using X(3s13px13py13pz1) and Be5H6(Quintet) as interacting fragments
| System | Interaction | ΔEint/ (kJ·mol-1) | ΔEPauli/ (kJ·mol-1) | ΔEelstata / (kJ·mol-1) | ΔEorba / (kJ·mol-1) | ΔEorb(1)b / (kJ·mol-1) | ΔEorb(2)b / (kJ·mol-1) | ΔEorb(3)b / (kJ·mol-1) | ΔEorb(4)b / (kJ·mol-1) | ΔEorb(rest)b / (kJ·mol-1) |
|---|---|---|---|---|---|---|---|---|---|---|
| SiBe5H6 | — | -1438.1 | 719.91 | -1137.3 | -1020.7 | -14.4 | ||||
| (52.7%) | (47.3%) | (1.4%) | ||||||||
| X?Be5H6 electron? | -173.3 | |||||||||
| sharing π bond | (17.0%) | |||||||||
| X?Be5H6 electron? | -288.0 | -268.1 | -276.9 | |||||||
| sharing σ bond | (28.2%) | (26.3%) | (27.1%) | |||||||
| GeBe5H6 | — | -1503.4 | 873.2 | -1293.2 | -1083.4 | -20.3 | ||||
| (54.4%) | (45.6%) | (2.0%) | ||||||||
| X?Be5H6 electron? | -174.6 | |||||||||
| sharing π bond | (16.1%) | |||||||||
| X?Be5H6 electron? | -298.2 | -268.1 | -322.2 | |||||||
| sharing σ bond | (27.5%) | (24.7%) | (29.7%) |
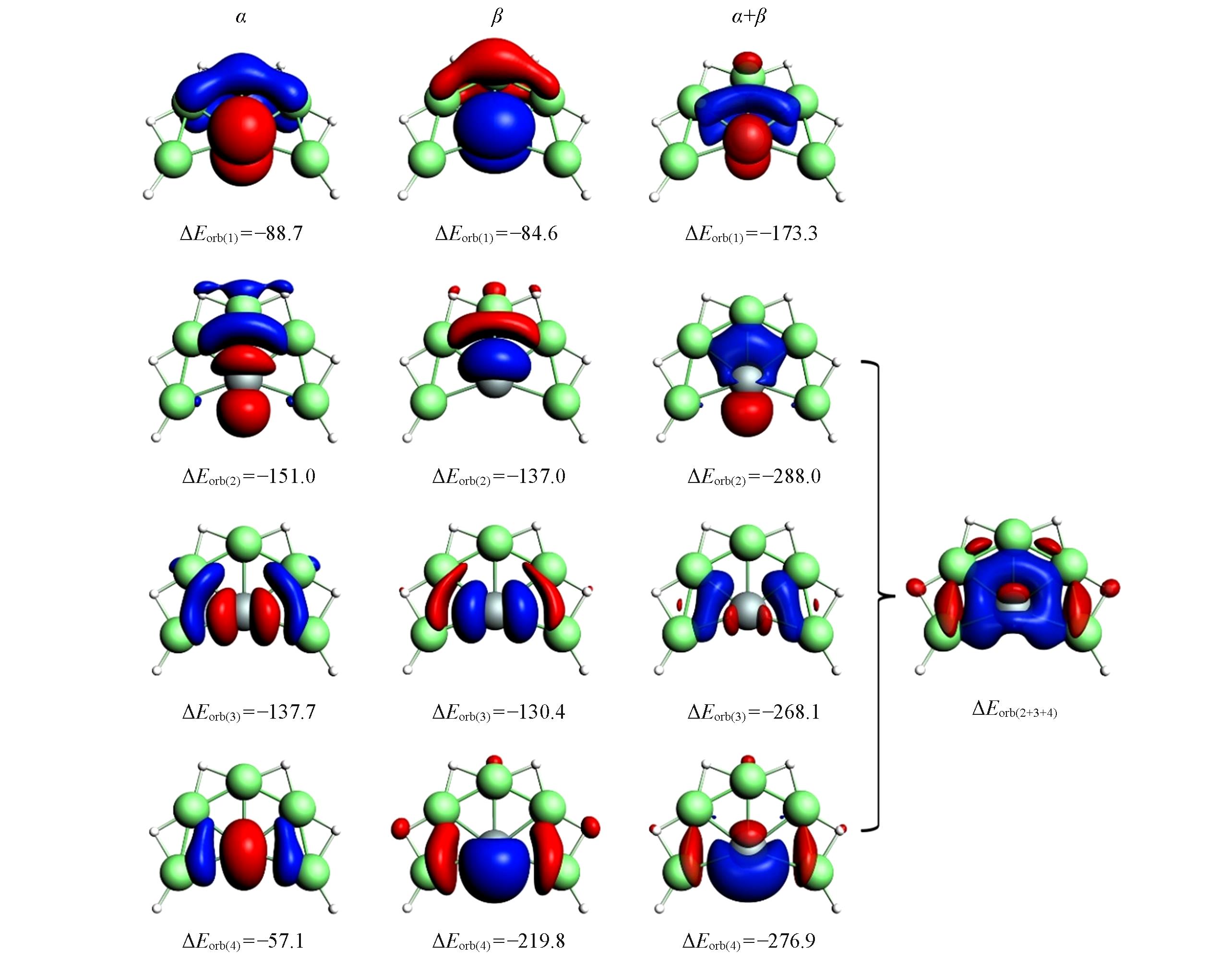
Fig.5 Plot of the deformation densities(Δρ) for EDA?NOCV analysis of SiBe5H6(1)The isovalues of the surfaces are 0.002. The direction of electron flow is from red to blue. Energy values are given in kJ/mol.
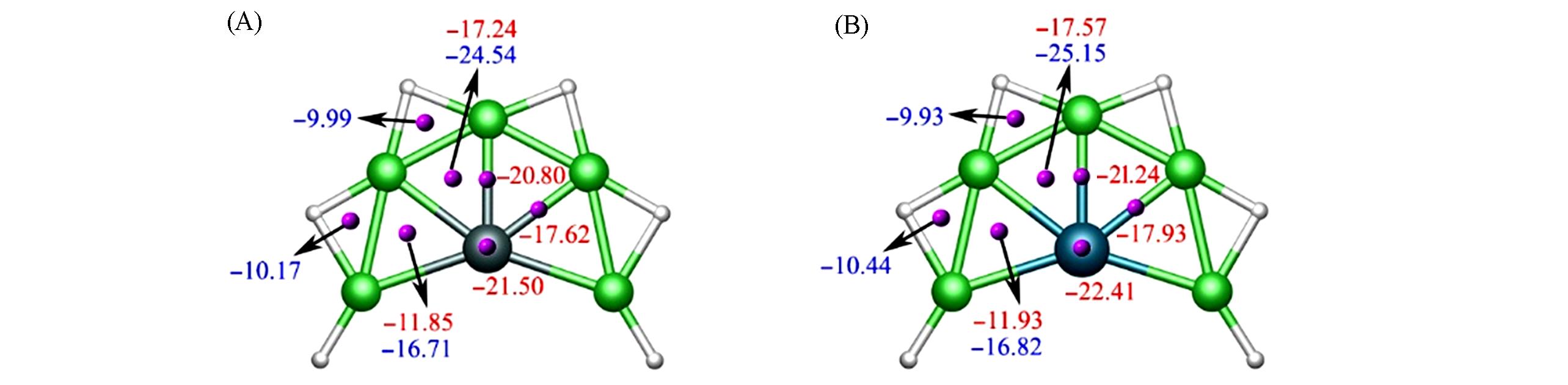
Fig.6 Nucleus independent chemical shifts(NICSs) for SiBe5H6(1)(A) and GeBe5H6(2)(B) clustersNICS(0)(blue color) is calculated at the center of a triangle, whereas NICS(0.1)(red color) is calculated at 0.1 nm above the X atom, Be—X and Be—X—Be triangle centers.
| 1 | Hoffmann R., Alder R. W., Wilcox C. F., J. Am. Chem. Soc., 1970, 92(16), 4992—4993 |
| 2 | Collins J. B., Dill J. D., Jemmis E. D., Apeloig Y., Schleyer P. V. R., Seeger R., Pople J. A., J. Am. Chem. Soc., 1976, 98(18), 5419—5427 |
| 3 | Schleyer P. V. R., Boldyrev A. I., J. Chem. Soc. Chem. Commun., 1991, 1991(21), 1536—1538 |
| 4 | Siebert W., Gunale A., Chem. Soc. Rev., 1999, 28(6), 367—371 |
| 5 | Minkin V. I., Minyaev R. M., Hoffmann R., Russ. Chem. Rev., 2002, 71(11), 869—892 |
| 6 | Keese R., Chem. Rev., 2006, 106(12), 4787—4808 |
| 7 | Merino G., Mendez⁃Rojas M. A., Vela A., Heine T., J. Comput. Chem., 2007, 28(1), 362—372 |
| 8 | Yang L. M., Ganz E., Chen Z., Wang Z. X., Schleyer P. V. R., Angew. Chem. Int. Ed., 2015, 54(33), 9468—9501 |
| 9 | Vassilev⁃Galindo V., Pan S., Donald K. J., Merino G., Nat. Rev. Chem., 2018, 2, 0114 |
| 10 | Li X., Wang L. S., Boldyrev A. I., Simons J., J. Am. Chem. Soc., 1999, 121(25), 6033—6038 |
| 11 | Wang L. S., Boldyrev A. I., Li X., Simons J., J. Am. Chem. Soc., 2000, 122(32), 7681—7687 |
| 12 | Li X., Zhang H. F., Wang L. S., Geske G. D., Boldyrev A. I., Angew. Chem. Int. Ed., 2000, 39(20), 3630—3632 |
| 13 | Xu J., Zhang X. X., Yu S., Ding Y. H., Bowen K. H., J. Phys. Chem. Lett., 2017, 8(10), 2263—2267 |
| 14 | Zhang X. X., Wang Y. Q., Geng Y., Zhang M., Su Z. M., Chem. J. Chinese Universities, 2018, 39(11), 2485—2491 |
| 张醒醒, 王艺桥, 耿允, 张珉, 苏忠民. 高等学校化学学报, 2018, 39(11), 2485—2491 | |
| 15 | Wang Z. X., Schleyer P. V. R., Science, 2001, 292(5526), 2465—2469 |
| 16 | Pei Y., An W., Ito K., Schleyer P. V. R., Zeng X. C., J. Am. Chem. Soc., 2008, 130(31), 10394—10400 |
| 17 | Guo J. C., Feng L. Y., Barroso J., Merino G., Zhai H. J., Chem. Commun., 2020, 56(59), 8305—8308 |
| 18 | Guo J. C., Tian W. J., Wang Y J., Zhao X. F., Wu Y. B., Zhai H. J., Li S. D., J. Chem. Phys., 2016, 144(24), 244303 |
| 19 | Exner K., Schleyer P. V. R., Science, 2000, 290(5498), 1937—1940 |
| 20 | Wu Y. B., Duan Y., Lu G., Lu H. G., Yang P., Schleyer P. V. R., Merino G., Islasef R., Wang Z. X., Phys. Chem. Chem. Phys., 2012, 14(43), 14760—14763 |
| 21 | Leyva⁃Parra L., Diego L., Yañez O., Inostroza D., Barroso J., Vasquez⁃Espinal A., Merino G., Tiznado W., Angew. Chem. Int. Ed., 2021, 60(16), 8700—8704 |
| 22 | Li S. D., Ren G. M., Miao C. Q., Li D. D., Chem. J. Chinese Universities, 2007, 28(1), 129—131 |
| 李思殿, 任光明, 苗常青, 李栋东. 高等学校化学学报, 2007, 28(1), 129—131 | |
| 23 | Boldyrev A. I., Li X., Wang L. S., Angew. Chem. Int. Ed., 2000, 39(18), 3307—3310 |
| 24 | Li S. D., Miao C. Q., Guo J. C., Ren G. M., J. Am. Chem. Soc., 2004, 126(49), 16227—16231 |
| 25 | Li S. D., Miao C. Q., J. Phys. Chem. A, 2005, 109(33), 7594—7597 |
| 26 | Islas R., Heine T., Ito K., Schleyer P. V. R., Merino G., J. Am. Chem. Soc., 2007, 129(47), 14767—14774 |
| 27 | Liu F. L., Jalbout A. F., J. Mol. Graphics Modell., 2008, 26, 1327—1332 |
| 28 | Alexandrova A. N., Nayhouse M. J., Huynh M. T., Kuo J. L., Melkonian A. V., Chavez G., Hernando N. M., Kowal M. D., Li C. P., Phys. Chem. Chem. Phys., 2012, 14(43), 14815—14821 |
| 29 | Guo J. C., Miao C. Q., Ren G. M., Comput. Theor. Chem., 2014, 1032, 7—11 |
| 30 | Guo J. C., Wu H. X., Ren G. M., Miao C. Q., Li Y. X., Comput. Theor. Chem., 2016, 1083, 1—6 |
| 31 | Sui J. J., Xu J., Ding Y. H., Dalton Trans., 2016, 45(1), 56—60 |
| 32 | Xu J., Ding Y. H., J. Comput. Chem., 2015, 36(6), 355—360 |
| 33 | Wang M. H., Dong X., Cui Z. H., Orozco⁃Ic M., Ding Y. H., Barroso J., Merino G., Chem. Commun., 2020, 56(89), 13772—13775 |
| 34 | Guo J. C., Ren G. M., Miao C. Q., Tian W. J., Wu Y. B., Wang X., J. Phys. Chem. A, 2015, 119(52), 13101—13106 |
| 35 | Guo J. C., Feng L. Y., Dong C., Zhai H. J., Phys. Chem. Chem. Phys., 2019, 21(39), 22048—22056 |
| 36 | Sergeeva A. P., Averkiev B. B., Zhai H. J., Boldyrev A. I., Wang L. S., J. Chem. Phys., 2011, 134(22), 224304 |
| 37 | Saunders M., J. Comput. Chem., 2004, 25(5), 621—626 |
| 38 | Bera P. P., Sattelmeyer K. W., Saunders M., Schaefer III H. F., Schleyer P. V. R., J. Phys. Chem. A, 2006, 110(13), 4287—4290 |
| 39 | Adamo C., Barone V., J. Chem. Phys., 1999, 110(13), 6158—6170 |
| 40 | Kendall R. A., Dunning T. H., Harrison R.J., J. Chem. Phys., 1992, 96(9), 6796—6806 |
| 41 | Pople J. A., Head⁃Gordon M., Raghavachari K., J. Chem. Phys., 1987, 87(10), 5968—5975 |
| 42 | Scuseria G. E., Schaefer III H. F., J. Chem. Phys., 1989, 90(7), 3700—3703 |
| 43 | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5659 |
| 44 | Lee, C., Yang, W., Parr R. G., Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785—791 |
| 45 | Millam J. M., Bakken V., Chen W., Hase W. L., Schlegel H. B., J. Chem. Phys., 1999, 111, 3800—3805 |
| 46 | Reed A. E., Curtiss L. A., Weinhold F. A., Chem. Rev., 1988, 88(6), 899—926 |
| 47 | Zubarev D. Y., Boldyrev A. I., Phys. Chem. Chem. Phys., 2008, 10(34), 5207—5217 |
| 48 | Lu T., Chen F., J. Comput. Chem., 2012, 33(5), 580—592 |
| 49 | Baerends E. J., Ziegler T., Autschbach J., Bashford D., Bérces A., Bickelhaupt F. M., Bo C., Boerrigter P. M., Cavallo L., Chong D. P., Deng L., Dickson R. M., Ellis D. E., Van Faassen M., Fan L., Fischer T. H., Fonseca Guerra C., Franchini M., Ghysels A., Giammona A., Van Gisbergen S. J. A., GÖtz A.W., Groeneveld J. A., Gritsenko O. V., Grüning M., Gusarov S., Harris F. E., Van den Hoek P., Jacob C.R., Jacobsen H., Jensen L., Kaminski J. W., Van Kessel G., Kootstra F., Kovalenko A., Krykunov M. V., Van Lenthe E., McCormack D. A., Michalak A., Mitoraj M., Morton S. M., Neugebauer J., Nicu V. P., Noodleman L., Osinga V. P., Patchkovskii S., Pavanello M., Philipsen P. H. T., Post D., Pye C. C., Ravenek W., Rodríguez J. I., Ros P., Schipper P. R. T., Van Schoot H., Schreckenbach G., Seldenthuis J. S., Seth M., Snijders J. G., Solà M., Swart M., Swerhone D., Te Velde G., Vernooijs P., Versluis L., Visscher L., Visser O., Wang F., Wesolowski T. A., Van Wezenbeek E. M., Wiesenekker G., Wolff S. K., Woo T. K., Yakovlev A. L., ADF2021, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, http://www.scm.com |
| 50 | Ziegler T., Rauk A., Theor. Chim. Acta, 1977, 46, 1—10 |
| 51 | Mitoraj M., Michalak A., Organometallics, 2007, 26, 6576—6580 |
| 52 | Schleyer P. V. R., Maerker C., Dransfeld A., Jiao H. J., Hommes N. J. R. V. E., J. Am. Chem. Soc., 1996, 118(26), 6317—6318 |
| 53 | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta E. J., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2009 |
| 54 | Legault C. Y., CYLview, 1.0b, Universite´ de Sherbrooke, 2009, http://www.cylview.org |
| 55 | Varetto U., Molekel 5.4.0.8, Swiss National Supercomputing Center, Manno, 2009 |
| 56 | Pyykkö P., J. Phys. Chem. A, 2015, 119(11), 2326—2337 |
| 57 | Guo J. C., Feng L. Y., Zhai H. J., Phys. Chem. Chem. Phys., 2018, 20, 6299—6306 |
| 58 | Guo J. C., Feng L. Y., Dong C., Zhai H. J., J. Phys. Chem. A, 2018, 122(42), 8370—8376 |
| 59 | Boldyrev A. I., Simons J., J. Am. Chem. Soc., 1998, 120(31), 7967—7972 |
| 60 | Wang M.H., Chen C., Pan S., Cui Z. H., Chem. Sci., 2021, 12, 15067—15076 |
| [1] | 李晓蕾, 孙云娇, 唐颖, 王长生. 醇及脱氧核糖与水分子间三体作用强度的快速准确计算[J]. 高等学校化学学报, 2021, 42(12): 3664. |
| [2] | 吴婷, 陈梦瑶, 肖凯霞, 周艳梅, 张庆友. 化学键的高选择性拓扑指数及应用[J]. 高等学校化学学报, 2019, 40(6): 1158. |
| [3] | 张醒醒, 王艺桥, 耿允, 张珉, 苏忠民. 硫铝桥键稳定平面CAl4结构的理论研究[J]. 高等学校化学学报, 2018, 39(11): 2485. |
| [4] | 张祺, 陆甦晖, 郑安呐, 管涌, 危大福, 黄添华, 李书召. 化学键合法制备长效抗菌聚对苯二甲酸乙二醇酯材料及其性能[J]. 高等学校化学学报, 2014, 35(4): 873. |
| [5] | 杨忠志, 闵芳, 赵东霞, 宫利东, 丁艳丽. 应用分子形貌理论研究类SN2反应 [J]. 高等学校化学学报, 2008, 29(12): 2381. |
| [6] | 师进生 ; 曲郸 ; 张思远 . Tb3+离子4f75d组态能级的电子云扩大效应[J]. 高等学校化学学报, 2006, 27(7): 1303. |
| [7] | 李俊杰, 曹培江, 郑伟涛, 吕宪义, 卞海蛟, 金曾孙. 轰击离子能量对CNx薄膜中sp3型C-N键含量的影响[J]. 高等学校化学学报, 2003, 24(5): 880. |
| [8] | 章永凡, 李俊篯, 丁开宁, 陈文凯, 周立新. TiC固体的相稳定性和高压相变的密度泛函研究[J]. 高等学校化学学报, 2003, 24(5): 863. |
| [9] | 武志坚, 杨明, 张思远. RBa2Cu4O8(R=Dy,Ho,Er,Tm,Yb)和Y2Ba4Cu7O14.3的化学键参数计算[J]. 高等学校化学学报, 2001, 22(4): 614. |
| [10] | 姜萍, 石静, 孙宏伟, 袁满雪, 赖城明. 有机金属化合物中二级化学键的理论研究(Ⅴ)──二级化学键生成的分子力学研究[J]. 高等学校化学学报, 2001, 22(1): 55. |
| [11] | 孙政, 郑世钧, 孟令鹏, 王殿勋. 杂氮硅三环类化合物的紫外光电子能谱及化学键的理论研究[J]. 高等学校化学学报, 1999, 20(8): 1285. |
| [12] | 袁满雪, 杨大军, 石静, 卜显和, 赖城明. 有机化合物中二级化学键的理论研究(Ⅳ)——喹啉基氮杂大环铜配合物中的二级化学键[J]. 高等学校化学学报, 1998, 19(4): 583. |
| [13] | 孟庆波, 武志坚, 张思远. LaX(X=N,P,As,Sb)晶体的能带结构和化学键性质研究[J]. 高等学校化学学报, 1998, 19(3): 429. |
| [14] | 章永凡, 吴立明, 李俊篯, 黄尊行, 胡建明, 周立新. 簇合物Mo2X4(X=S,O)电子结构和光谱性质的Ab Initio研究[J]. 高等学校化学学报, 1998, 19(10): 1659. |
| [15] | 赖城明, 唐珺, 袁满雪, 牛树强. 有机金属化合物中二级化学键的理论研究(Ⅲ)──金属原子簇化合物离子[M3(CO)9CCO」2-(M=Fe,Ru,Os)结构的特异性及二级化学键[J]. 高等学校化学学报, 1997, 18(2): 250. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
