

高等学校化学学报 ›› 2023, Vol. 44 ›› Issue (5): 20220775.doi: 10.7503/cjcu20220775
• 综合评述 • 上一篇
张小玉1, 曲干1, 薛冬萍1, 闫文付2( ), 张佳楠1(
), 张佳楠1( )
)
收稿日期:2022-12-30
出版日期:2023-05-10
发布日期:2023-02-24
通讯作者:
闫文付,张佳楠
E-mail:yanw@jlu.edu.cn;zjn@zzu.edu.cn
基金资助:
ZHANG Xiaoyu1, QU Gan1, XUE Dongping1, YAN Wenfu2( ), ZHANG Jianan1(
), ZHANG Jianan1( )
)
Received:2022-12-30
Online:2023-05-10
Published:2023-02-24
Contact:
YAN Wenfu, ZHANG Jianan
E-mail:yanw@jlu.edu.cn;zjn@zzu.edu.cn
Supported by:摘要:
过氧化氢(H2O2)作为一种多功能且环保的氧化剂, 在工业生产、 漂白、 消毒和废水处理等领域都发挥着重要作用. 传统的蒽醌工艺由于不环保、 不安全且流程复杂, 无法成为批量生产过氧化氢的最佳选择. 基于电化学氧还原反应(ORR)的合成方法是一种有价值的替代蒽醌生产的方法. 通常, H2O2可以通过2e‒ ORR过程合成. 碳基催化剂因储量丰富、 成本低、 结构可调和导电性好等优点, 被认为是用于2e‒ ORR的最佳催化剂之一. 本文综合评述了近年来碳基催化剂在电化学合成H2O2方面的研究进展. 首先, 介绍了2e‒ ORR过程的基本原理, 揭示了影响ORR路径的关键因素; 然后, 阐述了密度泛函理论(DFT)计算对揭示催化活性位点的关键作用, 并指明火山图是一种预测催化剂选择性的重要工具; 综合评述了促进H2O2产生的几种有效策略(优化金属单原子、 构建催化剂表面缺陷工程、 引入吡咯氮、 掺杂含氧官能团及掺杂其它杂原子); 介绍了批量生产H2O2的装置发展及其优缺点; 最后, 展望了电化学合成H2O2在未来发展中可能面临的机遇和挑战.
中图分类号:
TrendMD:
张小玉, 曲干, 薛冬萍, 闫文付, 张佳楠. 碳基催化剂用于电催化氧还原生产H2O2的研究进展: 策略、 计算及实际应用. 高等学校化学学报, 2023, 44(5): 20220775.
ZHANG Xiaoyu, QU Gan, XUE Dongping, YAN Wenfu, ZHANG Jianan. Recent Process of Carbon-based Catalysts for the Production of H2O2 by Electrocatalytic Oxygen Reduction: Strategies, Calculation and Practical Applications. Chem. J. Chinese Universities, 2023, 44(5): 20220775.
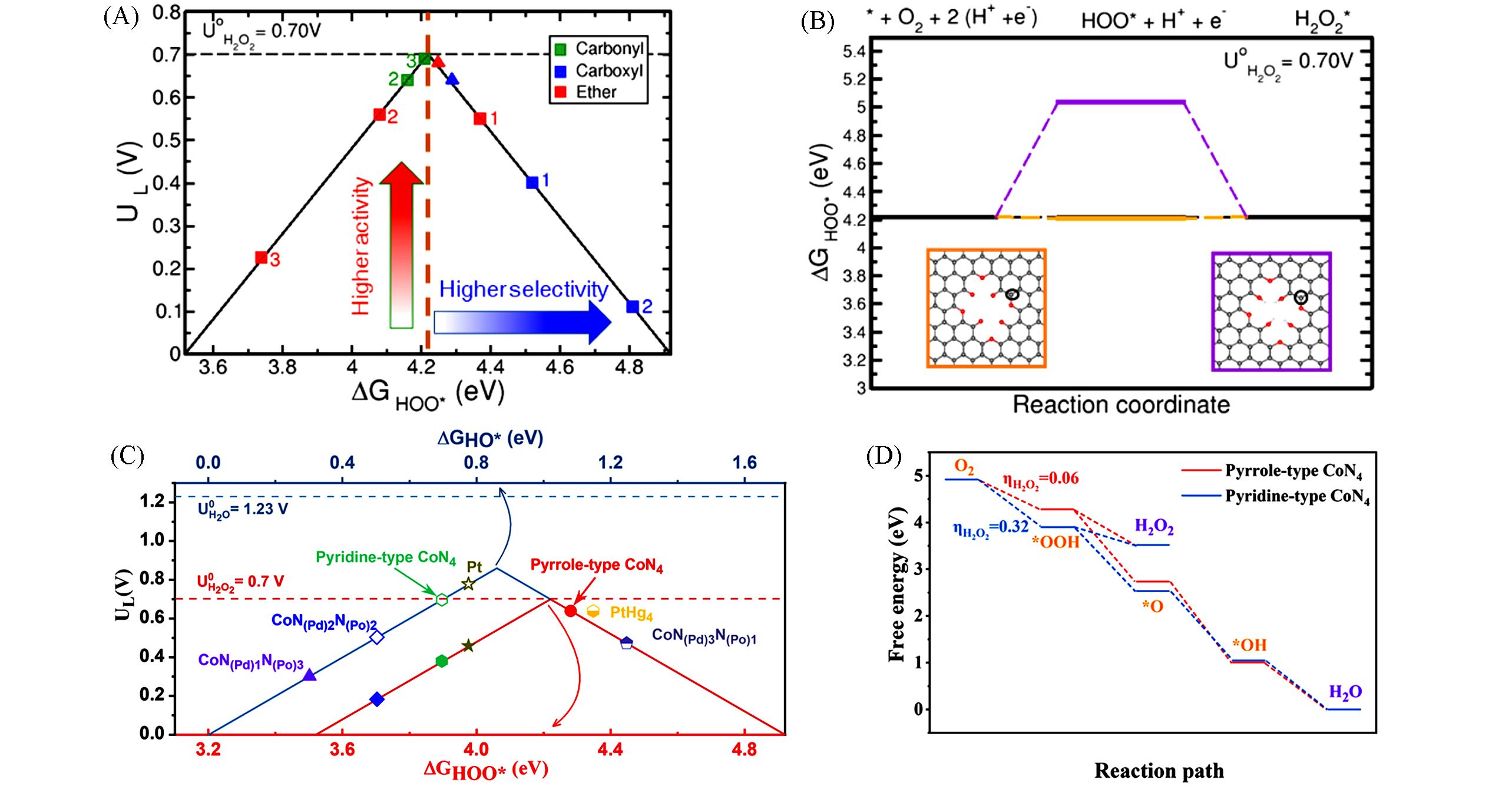
Fig.3 Activity volcano plots of 2e ‒ ORR pathway for different structures(A), free energy diagram for 2e ‒ ORR on different structuresl(B)[50], the activity volcano plots of 2e ‒ and 4e ‒ ORR pathways on different Co⁃N coordination structures(C), free energy diagram of ORR on CoN4 with different nitrogen coordination(D)[51]
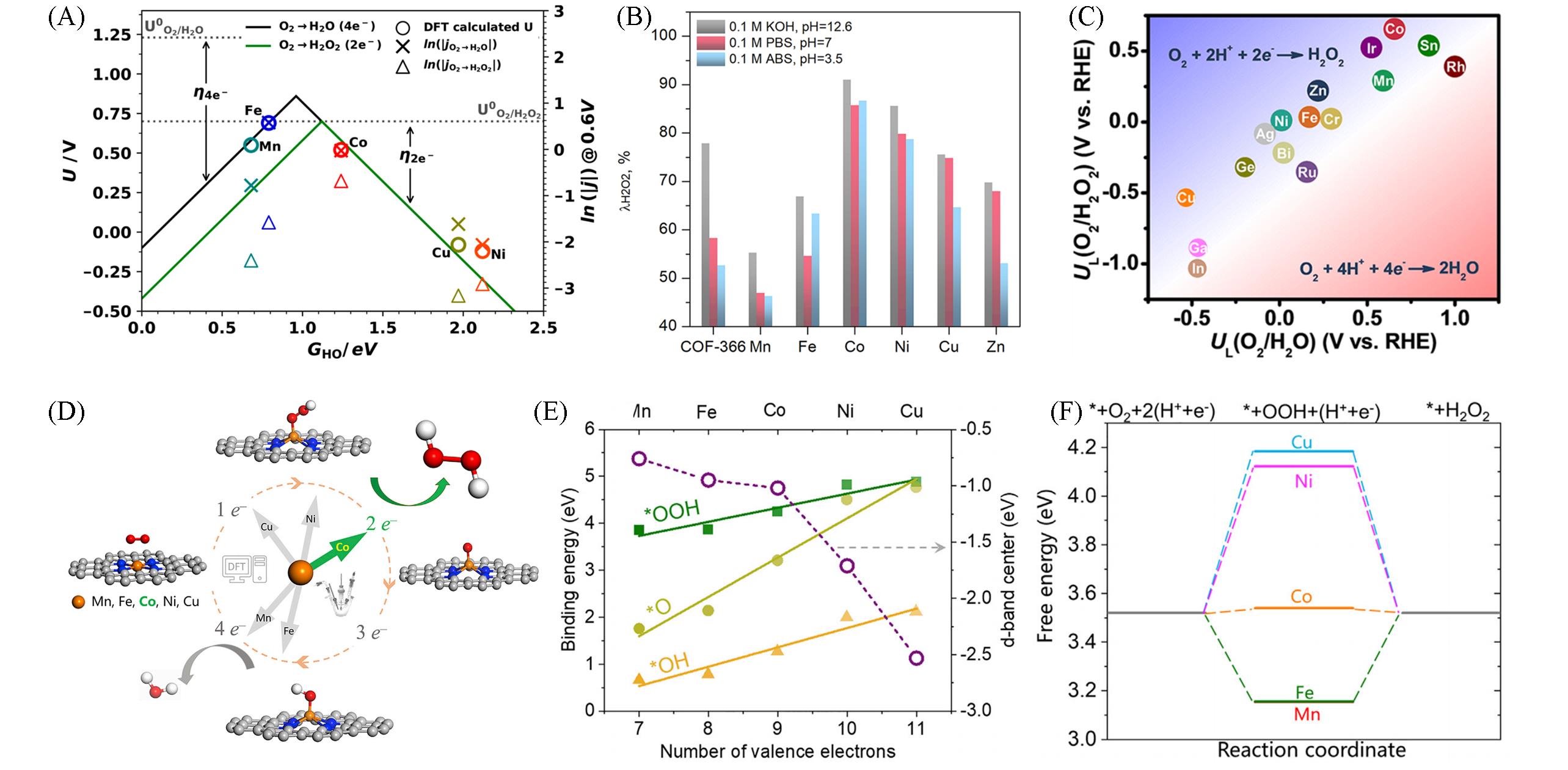
Fig.4 Activity volcano plots for the 2e‒ and 4e‒ ORR(A)[68], H2O2 selectivity in three types of electrolytes(B)[67], activity volcano plots of different metalloporphyrins for 2e‒ ORR and 4e‒ ORR(C)[69], schematic of ORR along the 2e‒ or 4e‒ pathway on transition metal SACs anchored in N⁃doped graphene(D), binding energy of *OOH, *O, and *OH on M⁃SAC(E) and free energy diagrams of 2e‒ ORR on M⁃SAC at U=0.7 V(vs. RHE)(F)[45](A) Copyright 2019, American Chemical Society; (B) Copyright 2020, American Chemical Society; (C) Copyright 2022, Springer Nature; (D—F) Copyright 2020, Elsevier.
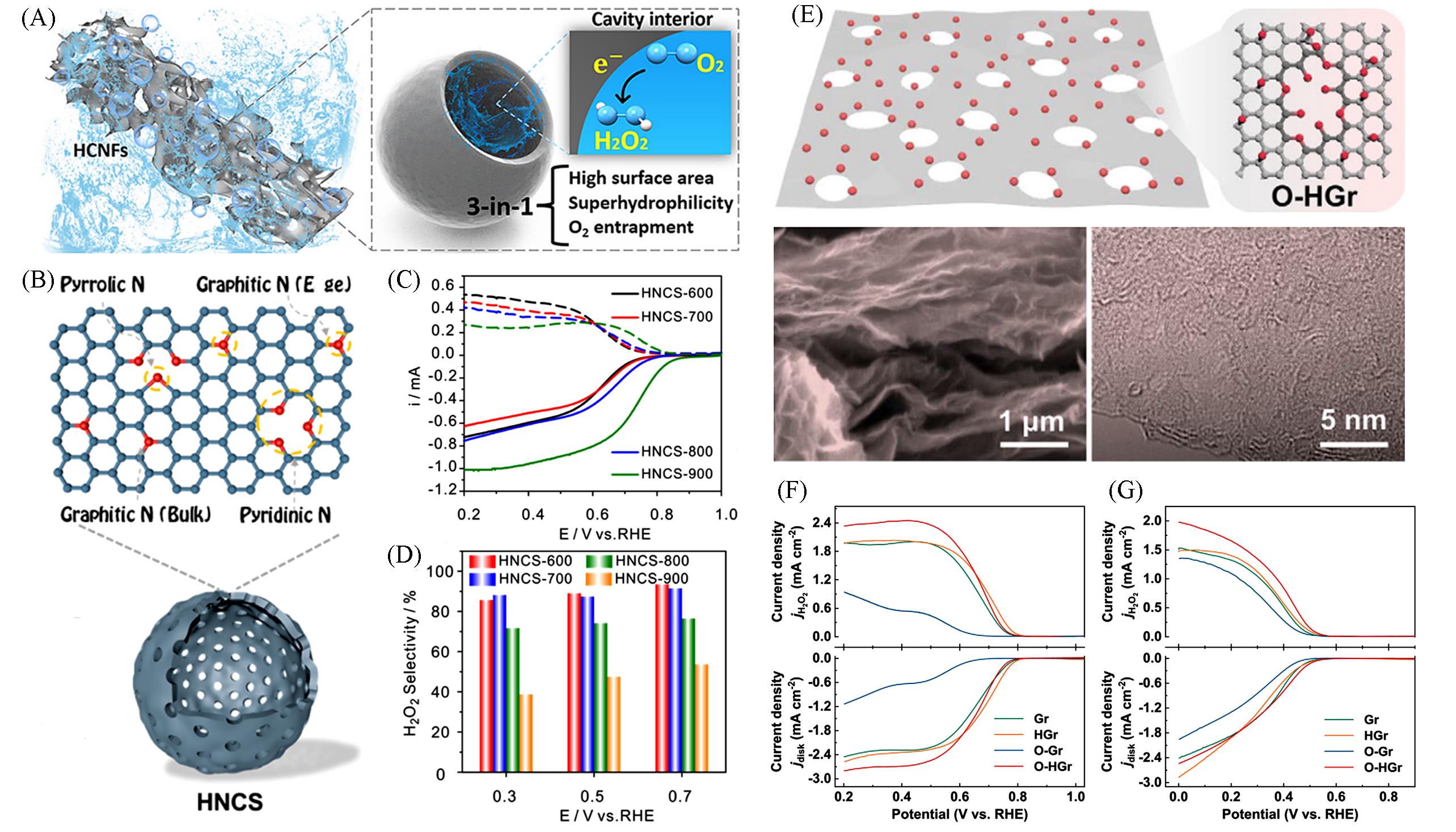
Fig.5 3⁃in⁃1 Effect of HCNFs promotes O2⁃to⁃H2O2 conversion(A)[88], schematic illustration of HNCS(B), LSV curves(solid lines) and H2O2 oxidation ring current adjusted by collection efficiency(C), H2O2 selectivity for different catalysts in O2⁃saturated 0.1 mol/L KOH(D)[89], schematic illustration of the microscopic characterization of O⁃HGr electrocatalysts(E), linear sweep voltammetry(LSV) of O⁃HGr at 1600 r/min in alkaline(F), LSV of O⁃HGr at 1600 r/min in neutral electrolytes(G)[50](A) Copyright 2021, Wiley-VCH; (B—D) Copyright 2021, American Chemical Society; (E—G) Copyright 2022, Wiley-VCH.
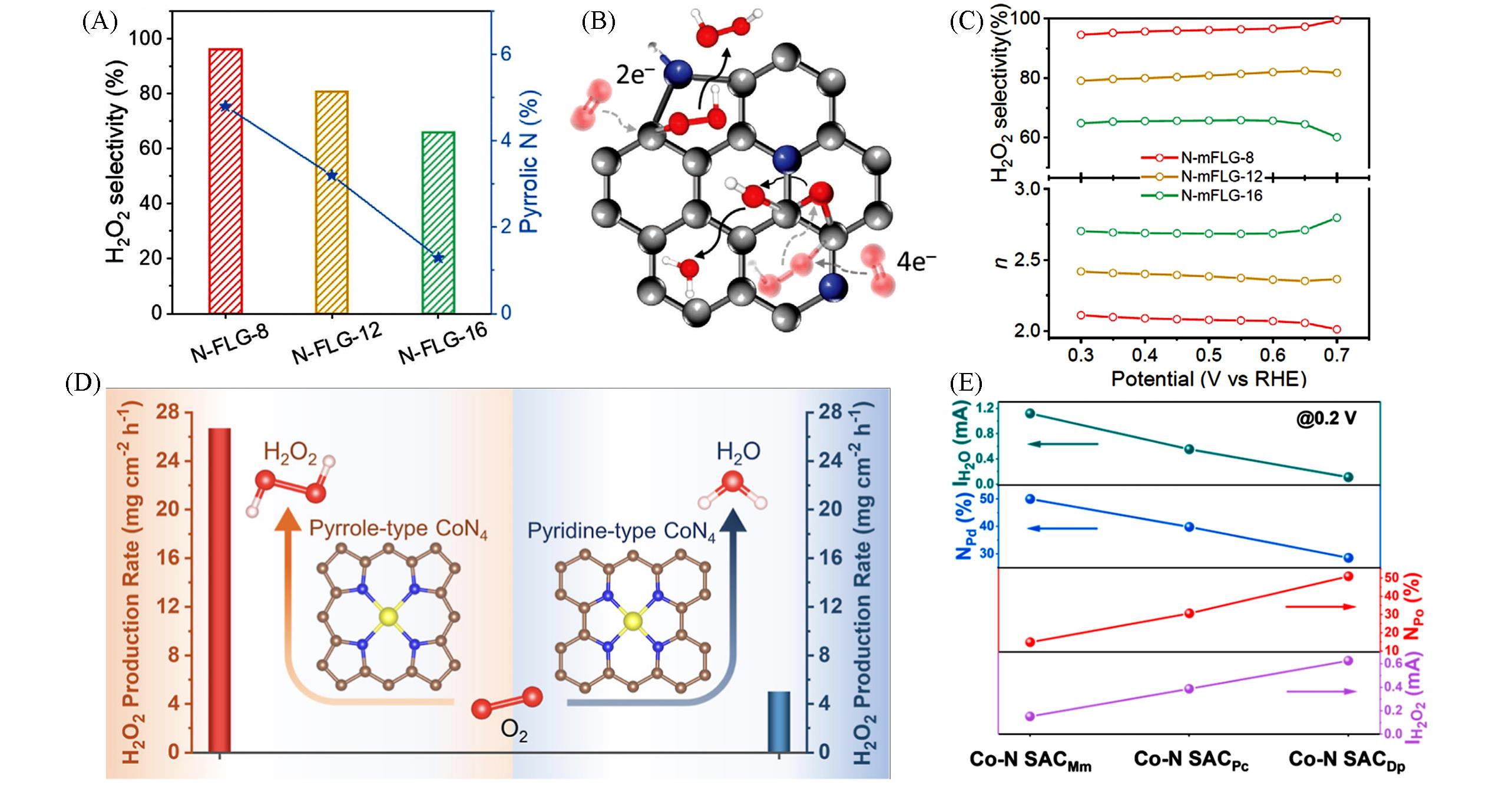
Fig.6 Relationship between H2O2 selectivity and atomic content of pyrrolic⁃N(A), schematic diagram of ORR pathways on N⁃FLG(B), H2O2 selectivity and electron transfer number(n) calculated from rotating ring disk electrode(RRDE) test(C)[94], simulated CoN4 coordination structures and H2O2 production rate for different catalysts(D), relationship between disk and ring current at 0.2 V(vs. RHE) and the content of N species for the three Co⁃N SACs(E)[51]
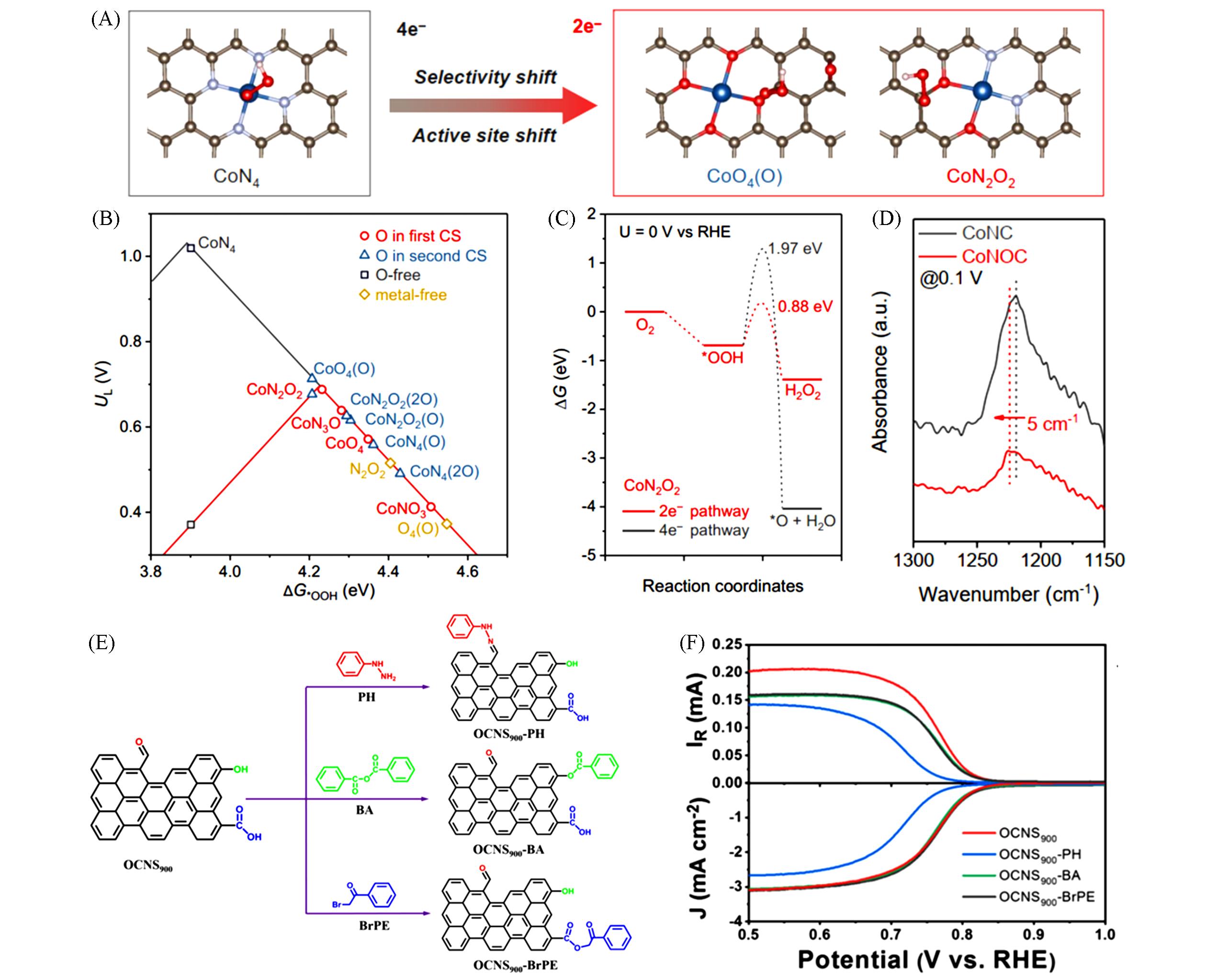
Fig.7 Optimized geometry structures of *OOH adsorption on Co SACs(A), computed activity volcano plots of ORR via the 2e ‒ or 4e ‒ pathway for varied Co SACs(B), free energy diagram for the 2e ‒ or 4e ‒ ORR pathway(C), in⁃situ ATR⁃SEIRAS spectra recorded on Co SACs(D)[74], schematic diagram of the chemical titration(E), LSV of different catalysts in 0.1 mol/L KOH(F)[103]
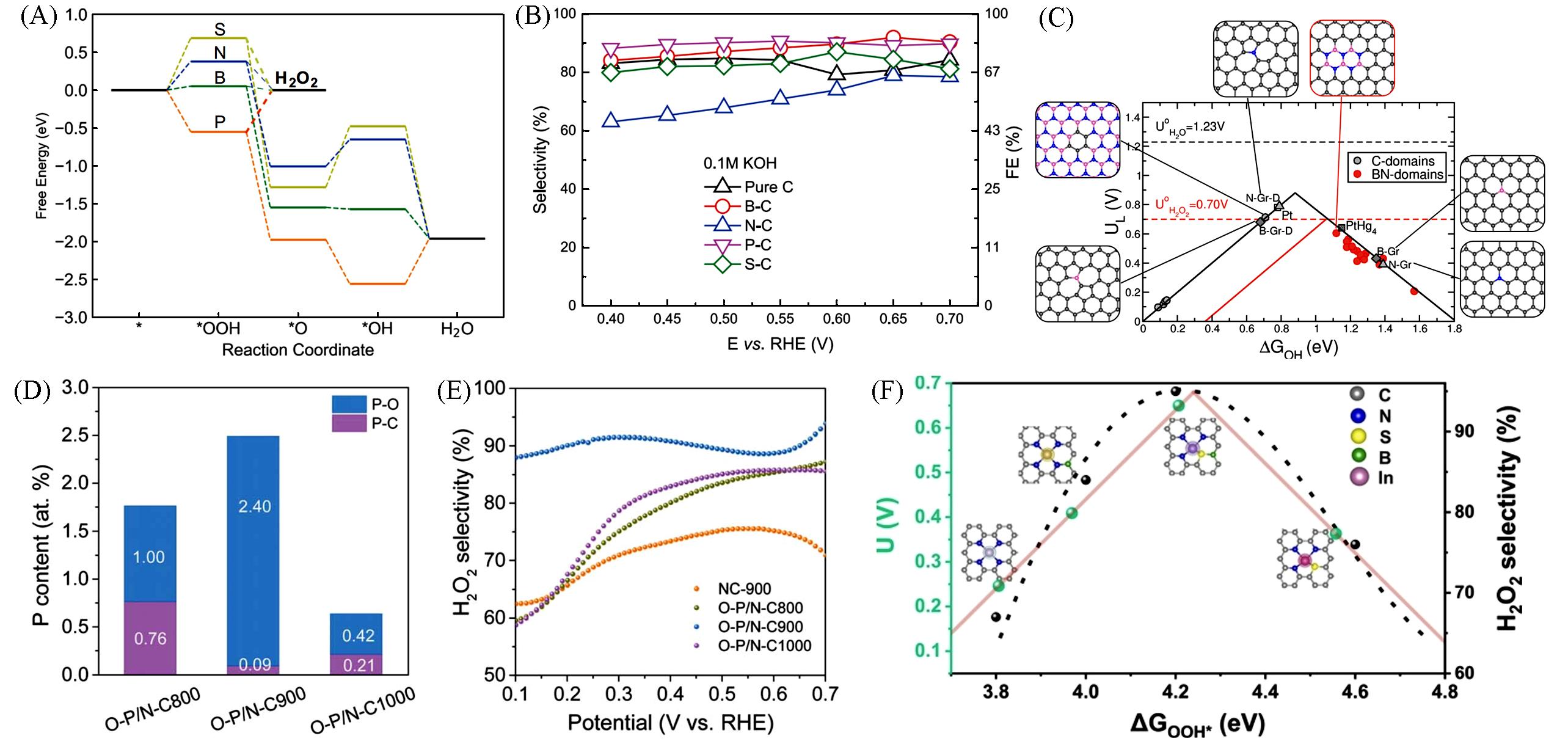
Fig.8 Free⁃energy profile of O2 reduction paths(A), H2O2 selectivity and Faradaic efficiency during for different catalysts in 0.1 mol/L KOH(B)[105], theoretical activity volcano plots for 2e‒ and 4e‒ ORR pathway of the N⁃ or B⁃doped graphene(C)[106], atomic contents of different P species(D), H2O2 selectivity of different catalysts calculated from RRDE test(E)[107] and calculated catalytic activity volcano plots for the production of H2O2via the 2e‒ ORR process(F)[108]
| Catalyst | Strategy | Electrolyte | Selectivity [H2O2%] | Onset potential versus RHE | Ref. |
|---|---|---|---|---|---|
| Co⁃NC | Single atom | 0.1 mol/L HClO4 | 90 | 0.6 | [ |
| COF⁃366⁃Co | Single atom | 0.1 mol/L KOH | 91 | — | [ |
| Co⁃N⁃C | Single atom | 0.5 mol/L H2SO4 | 80 | 0.78 | [ |
| CoSA⁃N⁃CNTs | Single atom | 0.5 mol/L H2SO4 | 95 | 0.7 | [ |
| HCNFs | Defect engineering | 0.1 mol/L KOH | 97.3 | 0.75 | [ |
| HNCS | Defect engineering | 0.1 mol/L KOH | 90 | 0.7 | [ |
| MNC⁃50 | Defect engineering | 0.5 mol/L H2SO4 | 90 | — | [ |
| O⁃HGr | Defect engineering | 0.1 mol/L KOH | 95 | 0.79 | [ |
| N⁃FLG | Pyrrole nitrogen | 0.1 mol/L KOH | 100 | 0.8 | [ |
| Co⁃N SACDp | Pyrrole nitrogen | 0.1 mol/L HClO4 | 90 | — | [ |
| O⁃CNTs | Oxygen functionalization | 0.1 mol/L KOH | ca. 90 | 0.60 | [ |
| F⁃mrGO | Oxygen functionalization | 0.1 mol/L KOH | ca. 100 | 0.78 | [ |
| Co1⁃NG(O) | Oxygen functionalization | 0.1 mol/L KOH | 80 | 0.65 | [ |
| CQDs | Oxygen functionalization | 0.1 mol/L KOH | ca. 100 | 0.823 | [ |
| OCNs | Oxygen functionalization | 0.1 mol/L KOH | 94 | 0.825 | [ |
| O⁃GOMC | Oxygen functionalization | 0.1 mol/L KOH | 99 | — | [ |
| B⁃C | Other heteroatoms | 0.1 mol/L KOH | 90 | 0.773 | [ |
| O⁃P/N⁃C | Other heteroatoms | 0.1 mol/L KOH | 90 | 0.78 | [ |
Table 1 Various strategies, conditions and performances over different carbon-based catalysts for electrochemical H2O2 production
| Catalyst | Strategy | Electrolyte | Selectivity [H2O2%] | Onset potential versus RHE | Ref. |
|---|---|---|---|---|---|
| Co⁃NC | Single atom | 0.1 mol/L HClO4 | 90 | 0.6 | [ |
| COF⁃366⁃Co | Single atom | 0.1 mol/L KOH | 91 | — | [ |
| Co⁃N⁃C | Single atom | 0.5 mol/L H2SO4 | 80 | 0.78 | [ |
| CoSA⁃N⁃CNTs | Single atom | 0.5 mol/L H2SO4 | 95 | 0.7 | [ |
| HCNFs | Defect engineering | 0.1 mol/L KOH | 97.3 | 0.75 | [ |
| HNCS | Defect engineering | 0.1 mol/L KOH | 90 | 0.7 | [ |
| MNC⁃50 | Defect engineering | 0.5 mol/L H2SO4 | 90 | — | [ |
| O⁃HGr | Defect engineering | 0.1 mol/L KOH | 95 | 0.79 | [ |
| N⁃FLG | Pyrrole nitrogen | 0.1 mol/L KOH | 100 | 0.8 | [ |
| Co⁃N SACDp | Pyrrole nitrogen | 0.1 mol/L HClO4 | 90 | — | [ |
| O⁃CNTs | Oxygen functionalization | 0.1 mol/L KOH | ca. 90 | 0.60 | [ |
| F⁃mrGO | Oxygen functionalization | 0.1 mol/L KOH | ca. 100 | 0.78 | [ |
| Co1⁃NG(O) | Oxygen functionalization | 0.1 mol/L KOH | 80 | 0.65 | [ |
| CQDs | Oxygen functionalization | 0.1 mol/L KOH | ca. 100 | 0.823 | [ |
| OCNs | Oxygen functionalization | 0.1 mol/L KOH | 94 | 0.825 | [ |
| O⁃GOMC | Oxygen functionalization | 0.1 mol/L KOH | 99 | — | [ |
| B⁃C | Other heteroatoms | 0.1 mol/L KOH | 90 | 0.773 | [ |
| O⁃P/N⁃C | Other heteroatoms | 0.1 mol/L KOH | 90 | 0.78 | [ |
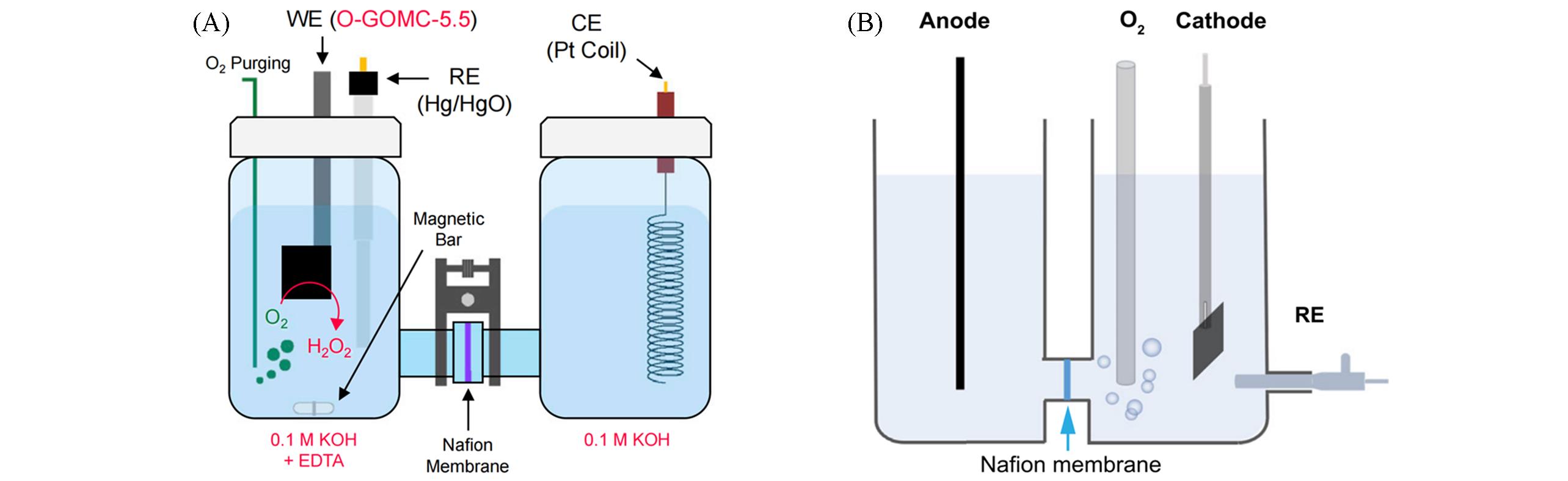
Fig.9 Schematic illustration of the H⁃type electrochemical cell(A)[104] and schematic of a home⁃made H⁃type electrolyzer(B)[111](A) Copyright 2021, Elsevier; (B) Copyright 2022, Springer Nature.
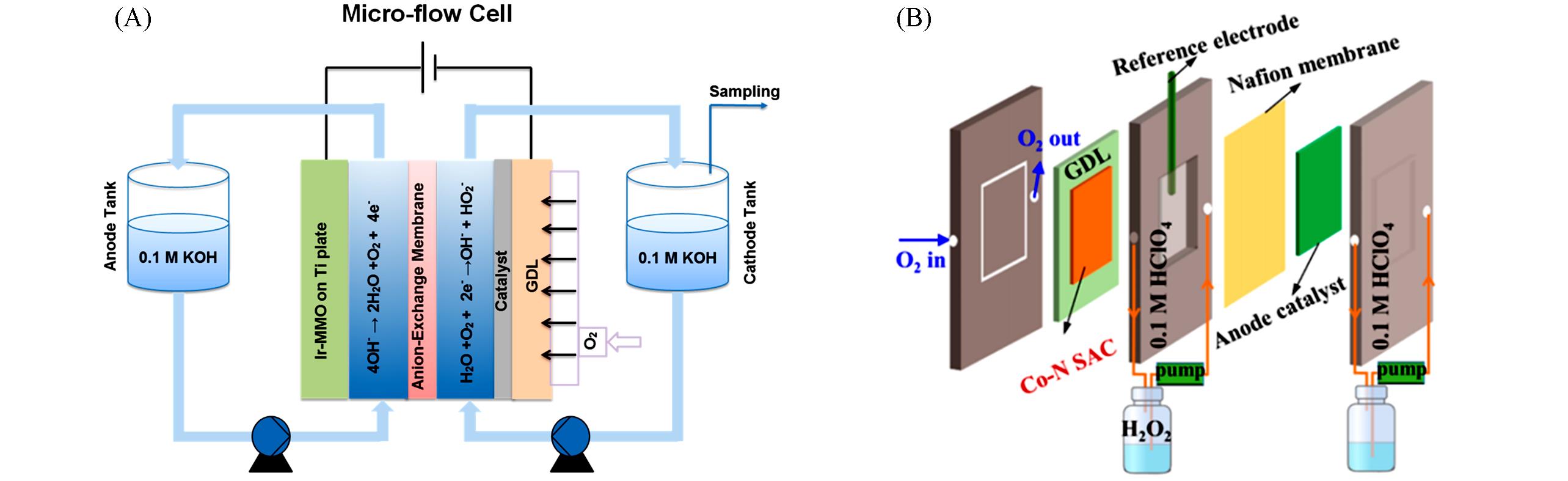
Fig.10 Scheme of the MFC setup(A)[68] and schematic diagram of the flow cell for H2O2 production(B)[51](A) Copyright 2019, American Chemical Society;(B) Copyright 2022, American Chemical Society.
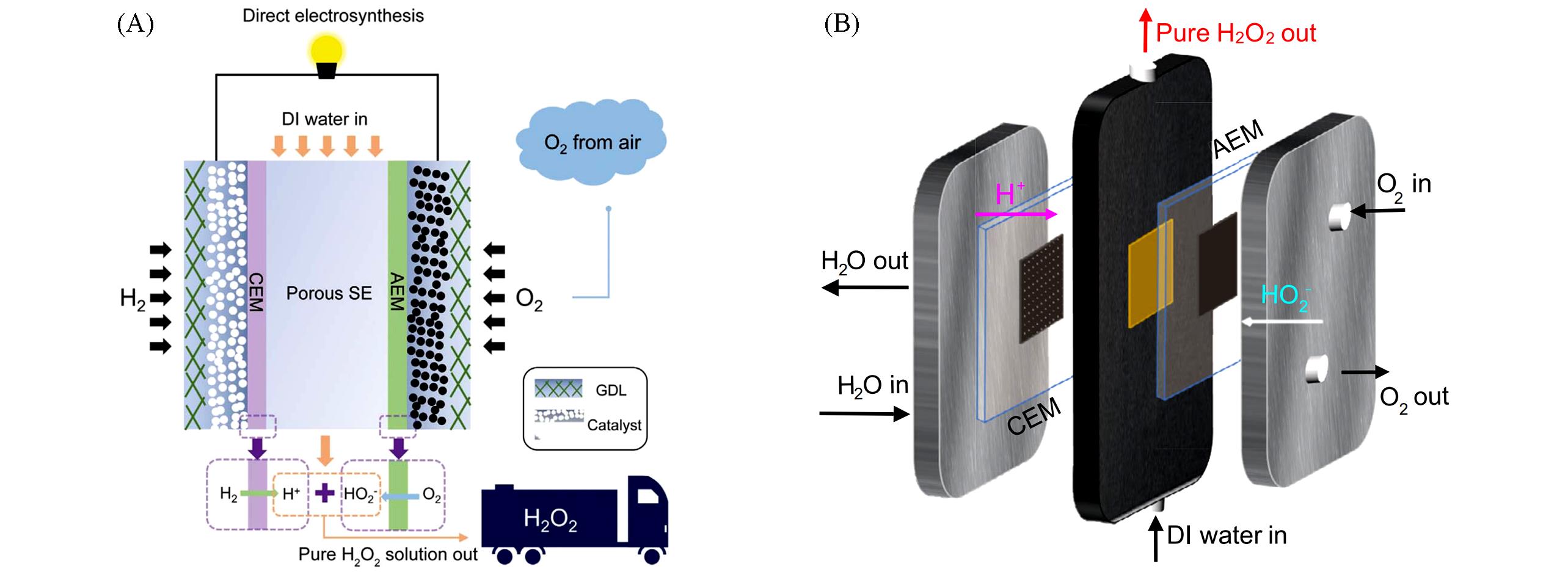
Fig.11 Electrosynthesis of H2O2 using pure H2 and O2 by solid⁃electrolyte cell[3](A) and schematic illustration of the solid⁃electrolyte cell configuration(B)[105](A) Copyright 2019, American Association for the Advancement of Science; (B) Copyright 2021, Springer Nature.
| 1 | Singh D. K., Ganesan V., Yadav D. K., Yadav M., Sonkar P. K., Gupta R., Catal. Sci. Technol., 2021, 11, 1014—1026 |
| 2 | Lu Z., Chen G., Siahrostami S., Chen Z., Liu K., Xie J., Liao L., Wu T., Lin D., Liu Y., Jaramillo T. F., Nørskov J. K., Cui Y., Nat. Catal., 2018, 1, 156—162 |
| 3 | Xia C., Xia Y., Zhu P., Fan L., Wang H., Science, 2019, 366, 226—231 |
| 4 | Sun Y., Han L., Strasser P., Chem. Soc. Rev., 2020, 49, 6605—6631 |
| 5 | Zhang X. L., Su X., Zheng Y. R., Hu S. J., Shi L., Gao F. Y., Yang P. P., Niu Z. Z., Wu Z. Z., Qin S., Wu R., Duan Y., Gu C., Zheng X. S., Zhu J. F., Gao M. R., Angew. Chem. Int. Ed., 2021, 60, 26922—26931 |
| 6 | Dan M., Zhong R., Hu S., Wu H., Zhou Y., Liu Z. Q., Chem. Catal., 2022, 2, 1919—1960 |
| 7 | Yamanaka I., Murayama T., Angew. Chem. Int. Ed., 2008, 47, 1900—1902 |
| 8 | Edwards J. K., Freakley S. J., Lewis R. J., Pritchard J. C., Hutchings G. J., Catal. Today, 2015, 248, 3—9 |
| 9 | Yang S., Verdaguer⁃Casadevall A., Arnarson L., Silvioli L., Čolić V., Frydendal R., Rossmeisl J., Chorkendorff I., Stephens I. E. L., ACS Catal., 2018, 8, 4064—4081 |
| 10 | Dissanayake D. P., Lunsford J. H., J. Catal., 2002, 206, 173—176 |
| 11 | Lunsford J. H., J. Catal., 2003, 216, 455—460 |
| 12 | Edwards J. K., Ntainjua E., Carley A. F., Herzing A. A., Kiely C. J., Hutchings G. J., Angew. Chem. Int. Ed., 2009, 48, 8512—8515 |
| 13 | Liu Q., Bauer J. C., Schaak R. E., Lunsford J. H., Appl. Catal. A, 2008, 339, 130—136 |
| 14 | Abate S., Arrigo R., Schuster M. E., Perathoner S., Centi G., Villa A., Su D., Schlögl R., Catal. Today, 2010, 157, 280—285 |
| 15 | Liu Q., Bauer J. C., Schaak R. E., Lunsford J. H., Angew. Chem. Int. Ed., 2008, 47, 6221—6224 |
| 16 | Freakley S. J., He Q., Harrhy J. H., Lu L., Crole D. A., Morgan D. J., Ntainjua E. N., Edwards J. K., Carley A. F., Borisevich A. Y., Kiely C. J., Hutchings G. J., Science, 2016, 351, 965—968 |
| 17 | Cui L., Zhao X., Xie H., Zhang Z., ACS Catal., 2022, 12, 13334—13348 |
| 18 | Chu Y., Lan C., Luo E., Liu C., Ge J., Xing W., Chem. J. Chinese Universities, 2022, 43, 20220294 |
| 19 | Jung E., Shin H., Lee B. H., Efremov V., Lee S., Lee H. S., Kim J., Hooch Antink W., Park S., Lee K. S., Cho S. P., Yoo J. S., Sung Y. E., Hyeon T., Nat. Mater., 2020, 19, 436—442 |
| 20 | Wang Y., Shi R., Shang L., Waterhouse G. I. N., Zhao J., Zhang Q., Gu L., Zhang T., Angew. Chem. Int. Ed., 2020, 59, 13057—13062 |
| 21 | Wood K. N., O’Hayre R., Pylypenko S., Energy Environ. Sci., 2014, 7, 1212—1249 |
| 22 | Berl E., Trans. Electrochem. Soc., 1939, 76, 359—369 |
| 23 | Hage R.,Lienke A., Angew. Chem. Int. Ed., 2005, 45, 206—222 |
| 24 | Foller P. C., Bombard R. T., J. Appl. Electrochem., 1995, 25, 613—627 |
| 25 | Byers J. C., Guell A. G., Unwin P. R., J. Am. Chem. Soc., 2014, 136, 11252—11255 |
| 26 | Chen C. Y., Tang C., Wang H. F., Chen C. M., Zhang X., Huang X., Zhang Q., ChemSusChem, 2016, 9, 1194—1199 |
| 27 | Edwards J. K., Hutchings G. J., Angew. Chem. Int. Ed., 2008, 47, 9192—9198 |
| 28 | Siahrostami S., Verdaguer⁃Casadevall A., Karamad M., Deiana D., Malacrida P., Wickman B., Escudero⁃Escribano M., Paoli E. A., Frydendal R., Hansen T. W., Chorkendorff I., Stephens I. E., Rossmeisl J., Nat. Mater., 2013, 12, 1137—1143 |
| 29 | Shen Y., Zhu P., Liu B., Yang X., Dong S., Chem. J. Chinese Universities, 2003, 24, 2080—2082 |
| 30 | Huang J., Li Y., Yang S., Zhou Y., Cheng X., Zhu J., Yang J., Chem. J. Chinese Universities, 2018, 39, 1063—1071 |
| 31 | Strasser P., Gliech M., Kuehl S., Moeller T., Chem. Soc. Rev., 2018, 47, 715—735 |
| 32 | Beermann V., Gocyla M., Kuhl S., Padgett E., Schmies H., Goerlin M., Erini N., Shviro M., Heggen M., Dunin⁃Borkowski R. E., Muller D. A., Strasser P., J. Am. Chem. Soc., 2017, 139, 16536—16547 |
| 33 | Strasser P., Acc. Chem. Res., 2016, 49, 2658—2668 |
| 34 | Yang S., Tak Y. J., Kim J., Soon A., Lee H., ACS Catal., 2017, 7, 1301—1307 |
| 35 | Choi C. H., Kim M., Kwon H. C., Cho S. J., Yun S., Kim H. T., Mayrhofer K. J., Kim H., Choi M., Nat. Commun., 2016, 7, 10922 |
| 36 | Calle⁃Vallejo F., Tymoczko J., Colic V., Vu Q. H., Pohl M. D., Morgenstern K., Loffreda D., Sautet P., Schuhmann W., Bandarenka A. S., Science, 2015, 350, 185—189 |
| 37 | Song X., Li N., Zhang H., Wang H., Wang L., Bian Z., J. Power Sources, 2019, 435, 226771 |
| 38 | Zhang B., Xu W., Lu Z., Sun J., Trans. Tianjin Univ., 2020, 26, 188—196 |
| 39 | Gao J., Liu B., ACS Mater. Lett., 2020, 2, 1008—1024 |
| 40 | Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W., Fang L., Xiao S., Liang J., Small, 2020, 16, e1906206 |
| 41 | Melchionna M., Fornasiero P., Prato M., Adv. Mater., 2019, 31, e1802920 |
| 42 | Wang Y. H., Pegis M. L., Mayer J. M., Stahl S. S., J. Am. Chem. Soc., 2017, 139, 16458—16461 |
| 43 | Wang Z., Huang J., Wang L., Liu Y., Liu W., Zhao S., Liu Z. Q., Angew. Chem. Int. Ed., 2022, 61, e202114696 |
| 44 | Iglesias D., Giuliani A., Melchionna M., Marchesan S., Criado A., Nasi L., Bevilacqua M., Tavagnacco C., Vizza F., Prato M., Fornasiero P., Chem, 2018, 4, 106—123 |
| 45 | Gao J., Yang H. b., Huang X., Hung S. F., Cai W., Jia C., Miao S., Chen H. M., Yang X., Huang Y., Zhang T., Liu B., Chem, 2020, 6, 658—674 |
| 46 | Siahrostami S., Bjorketun M. E., Strasser P., Greeley J., Rossmeisl J., Phys. Chem. Chem. Phys., 2013, 15, 9326—9334 |
| 47 | Verdaguer⁃Casadevall A., Deiana D., Karamad M., Siahrostami S., Malacrida P., Hansen T. W., Rossmeisl J., Chorkendorff I., Stephens I. E., Nano Lett., 2014, 14, 1603—1608 |
| 48 | Siahrostami S., Villegas S. J., Bagherzadeh Mostaghimi A. H., Back S., Farimani A. B., Wang H., Persson K. A., Montoya J., ACS Catal., 2020, 10, 7495—7511 |
| 49 | Jiang K., Back S., Akey A. J., Xia C., Hu Y., Liang W., Schaak D., Stavitski E., Norskov J. K., Siahrostami S., Wang H., Nat. Commun., 2019, 10, 3997 |
| 50 | Koh K. H., Bagherzadeh Mostaghimi A. H., Chang Q., Kim Y. J., Siahrostami S., Han T. H., Chen Z., EcoMat, 2022, 5, e12266 |
| 51 | Chen S., Luo T., Li X., Chen K., Fu J., Liu K., Cai C., Wang Q., Li H., Chen Y., Ma C., Zhu L., Lu Y. R., Chan T. S., Zhu M., Cortes E., Liu M., J. Am. Chem. Soc., 2022, 144, 14505—14516 |
| 52 | Rodriguez P., Koper M. T., Phys. Chem. Chem. Phys., 2014, 16, 13583—13594 |
| 53 | Yang S., Kim J., Tak Y. J., Soon A., Lee H., Angew. Chem. Int. Ed., 2016, 55, 2058—2062 |
| 54 | Oh J. M., Venters C. C., Di C., Pinto A. M., Wan L., Younis I., Cai Z., Arai C., So B. R., Duan J., Dreyfuss G., Nat. Commun., 2020, 11, 1 |
| 55 | Jirkovsky J. S., Panas I., Ahlberg E., Halasa M., Romani S., Schiffrin D. J., J. Am. Chem. Soc., 2011, 133, 19432—19441 |
| 56 | Kunitski M., Eicke N., Huber P., Kohler J., Zeller S., Voigtsberger J., Schlott N., Henrichs K., Sann H., Trinter F., Schmidt L. P. H., Kalinin A., Schoffler M. S., Jahnke T., Lein M., Dorner R., Nat. Commun., 2019, 10, 1 |
| 57 | Shen R., Chen W., Peng Q., Lu S., Zheng L., Cao X., Wang Y., Zhu W., Zhang J., Zhuang Z., Chen C., Wang D., Li Y., Chem, 2019, 5, 2099—2110 |
| 58 | Wang Y., Su H., He Y., Li L., Zhu S., Shen H., Xie P., Fu X., Zhou G., Feng C., Zhao D., Xiao F., Zhu X., Zeng Y., Shao M., Chen S., Wu G., Zeng J., Wang C., Chem. Rev., 2020, 120, 12217—12314 |
| 59 | Fei H., Dong J., Chen D., Hu T., Duan X., Shakir I., Huang Y., Duan X., Chem. Soc. Rev., 2019, 48, 5207—5241 |
| 60 | Chen Y., Ji S., Chen C., Peng Q., Wang D., Li Y., Joule, 2018, 2, 1242—1264 |
| 61 | Zhang B. W., Wang Y. X., Chou S. L., Liu H. K., Dou S. X., Small Methods, 2019, 3, 1800497 |
| 62 | Zhao B., Xue D., Yuan P., Yan W., Zhang J., Mu S., Zhang J. N., Appl. Catal. B, 2023, 324, 122251 |
| 63 | Xue D., Yuan P., Jiang S., Wei Y., Zhou Y., Dong C. L., Yan W., Mu S., Zhang J. N., Nano Energy, 2023, 105, 108020 |
| 64 | Qu G., Guo K., Dong J., Huang H., Yuan P., Wang Y., Yuan H., Zheng L., Zhang J. N., Energy Storage Mater., 2023, 55, 490—497 |
| 65 | Gu Y., Xi B., Li J., Xiong S., Chem. J. Chinese Universities, 2022, 43, 20220036 |
| 66 | Xu S., Yin H., Xue D., Xia H., Zhao S., Yan W., Mu S. C., Zhang J., Chem. J. Chinese Universities, 2022, 43, 20220028 |
| 67 | Liu C., Li H., Liu F., Chen J., Yu Z., Yuan Z., Wang C., Zheng H., Henkelman G., Wei L., Chen Y., J. Am. Chem. Soc., 2020, 142, 21861—21871 |
| 68 | Sun Y., Silvioli L., Sahraie N. R., Ju W., Li J., Zitolo A., Li S., Bagger A., Arnarson L., Wang X., Moeller T., Bernsmeier D., Rossmeisl J., Jaouen F., Strasser P., J. Am. Chem. Soc., 2019, 141, 12372—12381 |
| 69 | Zhao X., Yin Q., Mao X., Cheng C., Zhang L., Wang L., Liu T. F., Li Y., Li Y., Nat. Commun., 2022, 13, 2721 |
| 70 | Zhao C. X., Li B. Q., Liu J. N., Zhang Q., Angew. Chem. Int. Ed., 2021, 60, 4448—4463 |
| 71 | Chen W., Xia H., Guo K., Jin W., Du Y., Yan W., Qu G., Zhang J., Chem. Res. Chin. Univ., 2022, 38, 1232—1238 |
| 72 | Lin L., Ni Y., Shang L., Sun H., Zhang Q., Zhang W., Yan Z., Zhao Q., Chen J., ACS Catal., 2022, 12, 7531—7540 |
| 73 | Gong H., Wei Z., Gong Z., Liu J., Ye G., Yan M., Dong J., Allen C., Liu J., Huang K., Liu R., He G., Zhao S., Fei H., Adv. Funct. Mater., 2021, 32, 2106886 |
| 74 | Tang C., Chen L., Li H., Li L., Jiao Y., Zheng Y., Xu H., Davey K., Qiao S. Z., J. Am. Chem. Soc., 2021, 143, 7819—7827 |
| 75 | Wu K. H., Liu Y., Tan X., Liu Y., Lin Y., Huang X., Ding Y., Su B. J., Zhang B., Chen J. M., Yan W., Smith S. C., Gentle I. R., Zhao S., Chem Catal., 2022, 2, 372—385 |
| 76 | Liu W., Zhang C., Zhang J., Huang X., Song M., Li J., He F., Yang H., Zhang J., Wang D., Appl. Catal. B, 2022, 310, 121312 |
| 77 | Xia F., Li B., Liu Y., Liu Y., Gao S., Lu K., Kaelin J., Wang R., Marks T. J., Cheng Y., Adv. Funct. Mater., 2021, 31, 2104716 |
| 78 | Ding Y., Zhou W., Gao J., Sun F., Zhao G., Adv. Mater. Interfaces, 2021, 8, 2002091 |
| 79 | Zhang J., Zhang J., He F., Chen Y., Zhu J., Wang D., Mu S., Yang H. Y., Nano⁃Micro Lett., 2021, 13, 65—94 |
| 80 | Zhao K., Su Y., Quan X., Liu Y., Chen S., Yu H., J. Catal., 2018, 357, 118—126 |
| 81 | Chen S., Chen Z., Siahrostami S., Kim T. R., Nordlund D., Sokaras D., Nowak S., To J. W. F., Higgins D., Sinclair R., Nørskov J. K., Jaramillo T. F., Bao Z., ACS Sustain. Chem. Eng., 2017, 6, 311—317 |
| 82 | Han L., Sun Y., Li S., Cheng C., Halbig C. E., Feicht P., Hübner J. L., Strasser P., Eigler S., ACS Catal., 2019, 9, 1283—1288 |
| 83 | Xu Z., Liang J., Wang Y., Dong K., Shi X., Liu Q., Luo Y., Li T., Jia Y., Asiri A. M., Feng Z., Wang Y., Ma D., Sun X., ACS Appl. Mater. Interfaces, 2021, 13, 33182—33187 |
| 84 | Jing L., Tang C., Tian Q., Liu T., Ye S., Su P., Zheng Y., Liu J., ACS Appl. Mater. Interfaces, 2021, 13, 39763—39771 |
| 85 | Zhao X., Yang H., Xu J., Cheng T., Li Y., ACS Mater. Lett., 2021, 3, 996—1002 |
| 86 | Jia Y., Chen J., Yao X., Mater. Chem. Front., 2018, 2, 1250—1268 |
| 87 | Zhu J., Mu S., Adv. Funct. Mater., 2020, 30, 2001097 |
| 88 | Dong K., Liang J., Wang Y., Xu Z., Liu Q., Luo Y., Li T., Li L., Shi X., Asiri A. M., Li Q., Ma D., Sun X., Angew. Chem. Int. Ed., 2021, 60, 10583—10587 |
| 89 | Hu Y., Zhang J., Shen T., Li Z., Chen K., Lu Y., Zhang J., Wang D., ACS Appl. Mater. Interfaces, 2021, 13, 29551—29557 |
| 90 | Wang W., Zheng Y., Hu Y., Liu Y., Chen S., J. Phys. Chem. Lett., 2022, 13(38), 8914—8920 |
| 91 | Park J., Nabae Y., Hayakawa T., Kakimoto M. A., ACS Catal., 2014, 4, 3749—3754 |
| 92 | Wohlgemuth S. A., White R. J., Willinger M. G., Titirici M. M., Antonietti M., Green Chem., 2012, 14, 1515—1523 |
| 93 | Favaro M., Perini L., Agnoli S., Durante C., Granozzi G., Gennaro A., Electrochim. Acta, 2013, 88, 477—487 |
| 94 | Bu Y., Wang Y., Han G. F., Zhao Y., Ge X., Li F., Zhang Z., Zhong Q., Baek J. B., Adv. Mater., 2021, 33, e2103266 |
| 95 | Li L., Tang C., Zheng Y., Xia B., Zhou X., Xu H., Qiao S. Z., Adv. Energy Mater., 2020, 10, 2000789 |
| 96 | Zhang Y., Pang Y., Xia D., Chai G., New J. Chem., 2022, 46, 14510—14516 |
| 97 | Fellinger T. P., Hasche F., Strasser P., Antonietti M., J. Am. Chem. Soc., 2012, 134, 4072—4075 |
| 98 | Han G. F., Li F., Zou W., Karamad M., Jeon J. P., Kim S. W., Kim S. J., Bu Y., Fu Z., Lu Y., Siahrostami S., Baek J. B., Nat. Commun., 2020, 11, 2209 |
| 99 | Wu K. H., Wang D., Lu X., Zhang X., Xie Z., Liu Y., Su B. J., Chen J. M., Su D. S., Qi W., Guo S., Chem, 2020, 6, 1443—1458 |
| 100 | Kim H. W., Ross M. B., Kornienko N., Zhang L., Guo J., Yang P., McCloskey B. D., Nat. Catal., 2018, 1, 282—290 |
| 101 | Kim H. W., Park H., Roh J. S., Shin J. E., Lee T. H., Zhang L., Cho Y. H., Yoon H. W., Bukas V. J., Guo J., Park H. B., Han T. H., McCloskey B. D., Chem. Mater., 2019, 31, 3967—3973 |
| 102 | Guo Y., Zhang R., Zhang S., Hong H., Zhao Y., Huang Z., Han C., Li H., Zhi C., Energy Environ. Sci., 2022, 15, 4167—4174 |
| 103 | Chen S., Luo T., Chen K., Lin Y., Fu J., Liu K., Cai C., Wang Q., Li H., Li X., Hu J., Li H., Zhu M., Liu M., Angew. Chem. Int. Ed., 2021, 60, 16607—16614 |
| 104 | Lim J. S., Kim J. H., Woo J., Baek D. S., Ihm K., Shin T. J., Sa Y. J., Joo S. H., Chem, 2021, 7, 3114—3130 |
| 105 | Xia Y., Zhao X., Xia C., Wu Z. Y., Zhu P., Kim J. Y. T., Bai X., Gao G., Hu Y., Zhong J., Liu Y., Wang H., Nat. Commun., 2021, 12, 4225 |
| 106 | Chen S., Chen Z., Siahrostami S., Higgins D., Nordlund D., Sokaras D., Kim T. R., Liu Y., Yan X., Nilsson E., Sinclair R., Norskov J. K., Jaramillo T. F., Bao Z., J. Am. Chem. Soc., 2018, 140, 7851—7859 |
| 107 | Li Z., Kumar A., Liu N., Cheng M., Zhao C., Meng X., Li H., Zhang Y., Liu Z., Zhang G., Sun X., J. Mater. Chem. A, 2022, 10, 14355—14363 |
| 108 | Zhang E., Tao L., An J., Zhang J., Meng L., Zheng X., Wang Y., Li N., Du S., Zhang J., Wang D., Li Y., Angew. Chem. Int. Ed., 2022, 61, e202117347 |
| 109 | Weekes D. M., Salvatore D. A., Reyes A., Huang A., Berlinguette C. P., Acc. Chem. Res., 2018, 51, 910—918 |
| 110 | Burdyny T., Smith W. A., Energy Environ. Sci., 2019, 12, 1442—1453 |
| 111 | Zhao J., Fu C., Ye K., Liang Z., Jiang F., Shen S., Zhao X., Ma L., Shadike Z., Wang X., Zhang J., Jiang K., Nat. Commun., 2022, 13, 685 |
| 112 | Reis R. M., Beati A. A. G. F., Rocha R. S., Assumpção M. H. M. T., Santos M. C., Bertazzoli R., Lanza M. R. V., Ind. Eng. Chem. Res., 2011, 51, 649—654 |
| 113 | Nicoll W. D., Smith A. F., Ind. Eng. Chem., 1955, 47, 2548—2554 |
| 114 | Cota H. M., Katan T., Chin M., Schoenweis F. J., Nature, 1964, 203, 1281 |
| 115 | Zhou W., Jia J., Lu J., Yang L., Hou D., Li G., Chen S., Nano Energy, 2016, 28, 29—43 |
| 116 | Trzesniewski B. J., Diaz⁃Morales O., Vermaas D. A., Longo A., Bras W., Koper M. T., Smith W. A., J. Am. Chem. Soc., 2015, 137, 15112—15121 |
| 117 | Luo E., Chu Y., Liu J., Shi Z., Zhu S., Gong L., Ge J., Choi C. H., Liu C., Xing W., Energy Environ. Sci., 2021, 14, 2158—2185 |
| [1] | 刘晓磊, 陆永强, 游淇, 刘国辉, 姚伟, 胡日茗, 闫纪宪, 崔玉, 杨小凤, 孙国新, 蒋绪川. 基于3-羟基沙利度胺的比率型荧光探针对过氧化氢的检测[J]. 高等学校化学学报, 2022, 43(6): 20220070. |
| [2] | 朱浩天, 金美秀, 唐文思, 苏芳, 李阳光. 过渡金属-联咪唑-Dawson型钨磷酸盐杂化化合物的酶固定化性能[J]. 高等学校化学学报, 2022, 43(11): 20220328. |
| [3] | 高晓乐, 王家信, 李志芳, 李艳春, 杨冬花. 复合材料NiOx-ZSM-5的制备及微生物电解池催化析氢性能[J]. 高等学校化学学报, 2021, 42(9): 2886. |
| [4] | 徐梦祎, 黄雪雯, 李小杰, 魏玮, 刘晓亚. “串珠状”复合纳米组装体修饰丝网印刷电极构建的生物传感器[J]. 高等学校化学学报, 2021, 42(6): 1768. |
| [5] | 李健, 于明明, 孙源, 冯文华, 冯兆池, 吴剑峰. 水溶液pH对甲烷低温氧化制备甲醇的影响[J]. 高等学校化学学报, 2021, 42(3): 776. |
| [6] | 王瑞雪, 尹冬梅, 宋永新, 单桂晔. CuS/Ag2S纳米复合物的制备及类过氧化物酶性质[J]. 高等学校化学学报, 2020, 41(6): 1218. |
| [7] | 张晶晶, 金融, 方丹君, 江德臣. 电压调制型高灵敏电化学发光分析[J]. 高等学校化学学报, 2020, 41(11): 2421. |
| [8] | 滕渝,杨绍明,柏朝朋,张剑. 基于多壁碳纳米管增敏材料的辣根过氧化物酶分子印迹电化学传感器的制备及对H2O2的检测[J]. 高等学校化学学报, 2020, 41(1): 78. |
| [9] | 刘新超, 赵亚榕, 袁珍闫, 周丹, 鲁新环, 夏清华. Ti-Beta分子筛的控制合成及高效催化环己烯环氧化[J]. 高等学校化学学报, 2019, 40(6): 1222. |
| [10] | 陈艳, 董雪娇, 单桂晔. 脂质体@Ag/Au中空纳米壳层材料的制备及与H2O2的作用[J]. 高等学校化学学报, 2019, 40(4): 639. |
| [11] | 董德明, 张影, 花修艺, 姜旭, 梁大鹏, 郭志勇. 溶解有机质对光照自然水体生物膜体系中H2O2生成的影响[J]. 高等学校化学学报, 2019, 40(4): 800. |
| [12] | 方超, 朱焓毓, 刘晔, 赵外欧, 李亚鹏, 王静媛. 过氧化氢敏感的靶向荧光载药纳米粒子的制备及在动脉粥样硬化中的应用[J]. 高等学校化学学报, 2018, 39(9): 2071. |
| [13] | 王辉, 裴彦博, 胡绍争, 马文涛, 石朔宇. 能级可调的钾离子掺杂石墨相氮化碳的可控制备及“双渠道”光催化合成过氧化氢性能[J]. 高等学校化学学报, 2018, 39(7): 1503. |
| [14] | 蔡庄, 王贵领, 宋聪颖, 杨雪莹, 胡蓉, 叶克, 朱凯, 程魁, 闫俊, 曹殿学. A4纸-8B铅笔-NiAg电极的制备及对H2O2电还原反应的催化性能[J]. 高等学校化学学报, 2018, 39(5): 1041. |
| [15] | 代红艳, 杨慧敏, 刘宪, 简选, 郭敏敏, 曹乐乐, 梁镇海. MoS2/石墨烯复合阴极材料的制备及微生物电解池催化产氢性能[J]. 高等学校化学学报, 2018, 39(2): 351. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||