

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (8): 20220122.doi: 10.7503/cjcu20220122
刘苏毓1, 丁飞2, 李茜2,3( ), 樊春海2, 冯景4(
), 樊春海2, 冯景4( )
)
收稿日期:2022-03-01
出版日期:2022-08-10
发布日期:2022-03-24
通讯作者:
李茜,冯景
E-mail:liqian2018@sjtu.edu.cn;fengjing71921@163.com
基金资助:
LIU Suyu1, DING Fei2, LI Qian2,3( ), FAN Chunhai2, FENG Jing4(
), FAN Chunhai2, FENG Jing4( )
)
Received:2022-03-01
Online:2022-08-10
Published:2022-03-24
Contact:
LI Qian,FENG Jing
E-mail:liqian2018@sjtu.edu.cn;fengjing71921@163.com
Supported by:摘要:
具备光致异构性及乏氧响应性的偶氮苯为构建DNA纳米机器提供了动态响应元件. 然而, 受限于偶氮苯类化合物有限的光异构化反应, 偶氮苯类DNA纳米机器的构建与应用仍然面临着巨大的挑战. 本文梳理了基于偶氮苯的DNA纳米机器的构建方式及相应优缺点, 总结了可见光响应的偶氮苯类DNA纳米机器的设计规则, 并进一步综合评述了偶氮苯类DNA纳米机器在调控酶活性、 物质运输和机械运动等方面的应用. 本文有望推动开发更灵活的偶氮苯与DNA的偶联方式, 并为偶氮苯类DNA纳米机器在生物医学上的应用带来一定启示.
中图分类号:
TrendMD:
刘苏毓, 丁飞, 李茜, 樊春海, 冯景. 偶氮苯类DNA纳米机器. 高等学校化学学报, 2022, 43(8): 20220122.
LIU Suyu, DING Fei, LI Qian, FAN Chunhai, FENG Jing. Azobenzene-integrated DNA Nanomachine. Chem. J. Chinese Universities, 2022, 43(8): 20220122.
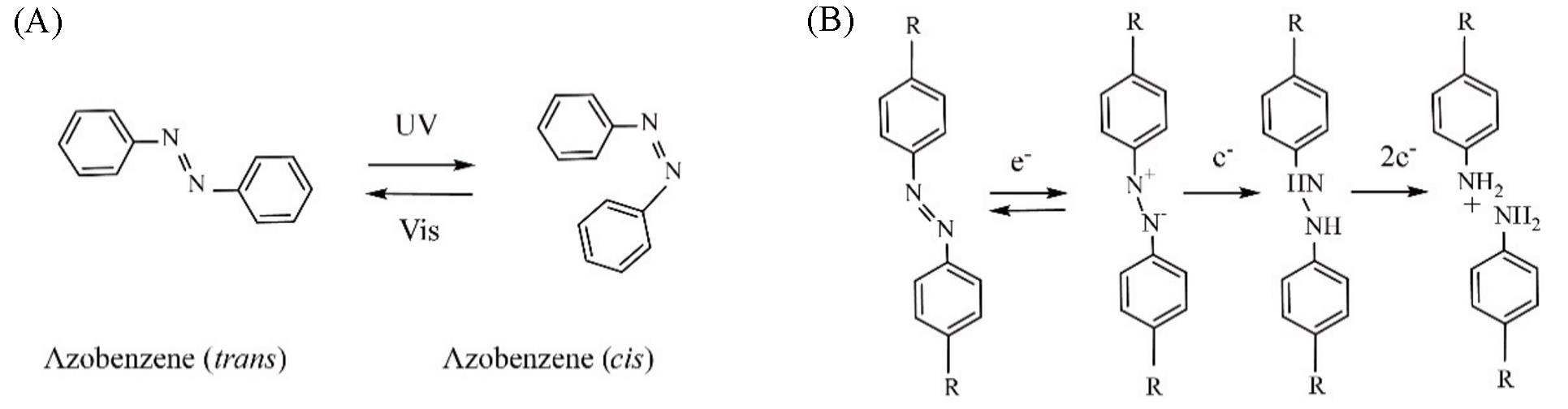
Fig.1 Photo?isomerized and hypoxia?responsive properties of azobenzene(A) Azobenzene reversibly isomerizes between cis? and trans?form under visible and UV light irradiation; (B) hypoxic reduction mechanism of azobenzene.
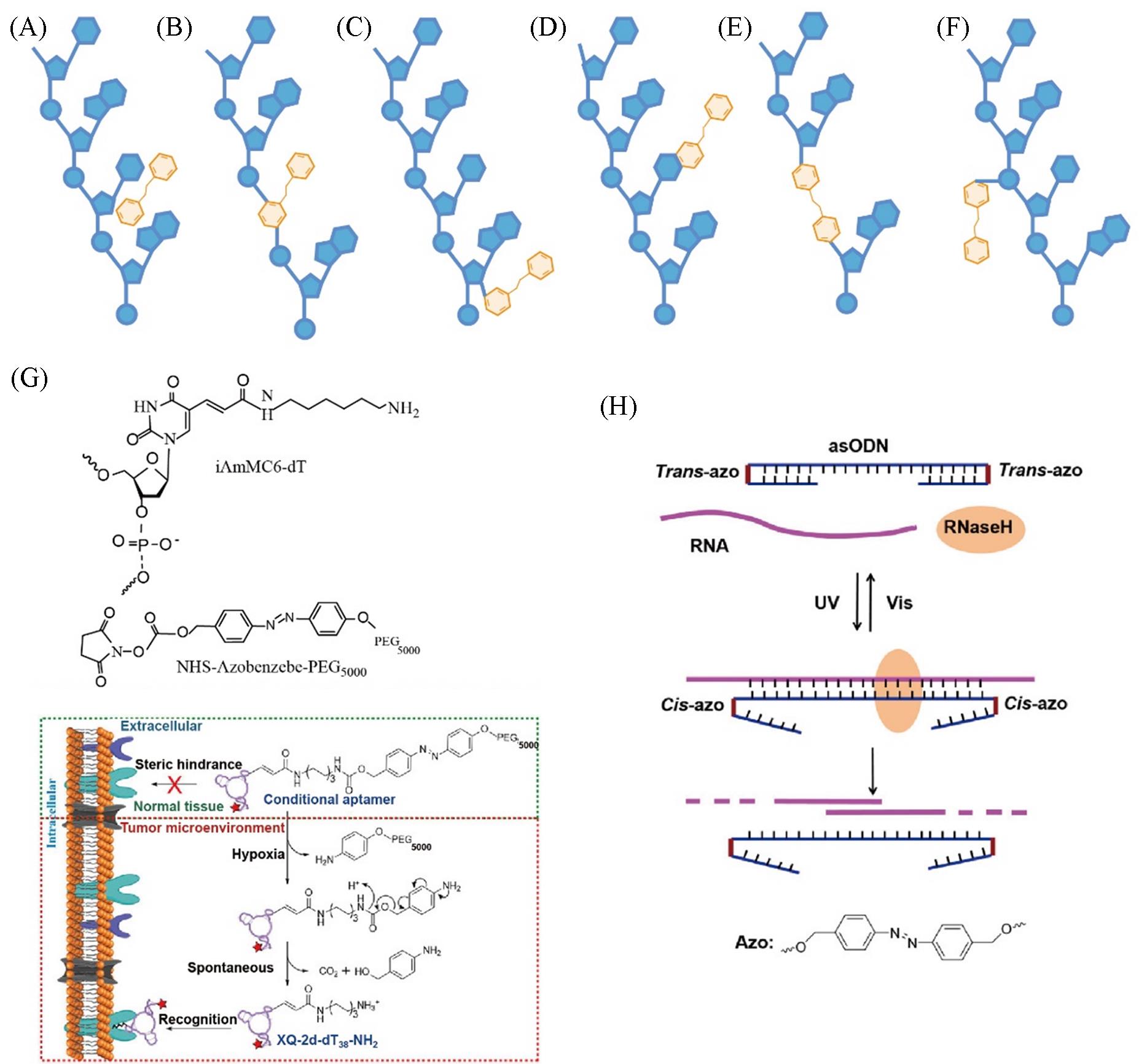
Fig.2 Interactions between azobenzene and nucleotides(A) Schematic illustration of noncovalent interaction; (B) schematic illustration of covalent interaction: azobenzene as nucleoside surrogates; (C) azobenzene attached to a nucleoside on the ribose unit; (D) azobenzene attached to a nucleoside on the nucleobase; (E) azobenzene used as a backbone linker between two nucleosides; (F) azobenzene attached on the phosphate backbone; (G) an example of azobenzene?based hypoxia?activated DNA nanodevice[32]; (H) light?responsive azobenzene?integrated asODNs for regula?ting RNA digestion[33].(G) Copyright 2019, American Chemical Society; (H) Copyright 2015, American Chemical Society.
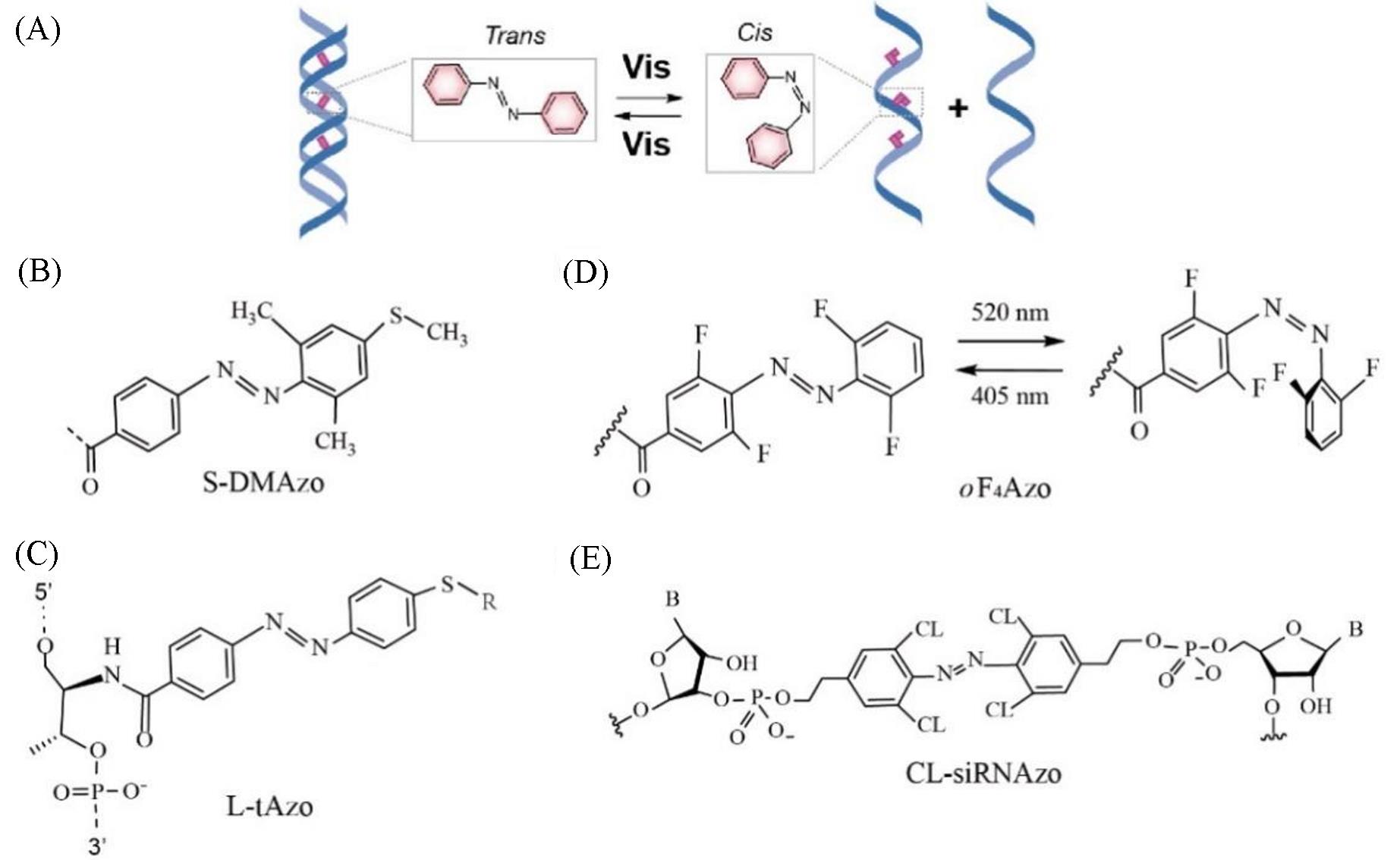
Fig.4 Schematic illustration of azobenzene?modified DNA(A) The azobenzene-based DNA nanomachines responding to visible light; (B) S-DMAzo[18]; (C) L-tAzo[58]; (D) oF4Azo[59]; (E) CL-siRNAzo[60].(B) Copyright 2012, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (C) Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (D) Copyright 2019, Beilstein-Institut; (E) Copyright 2020, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
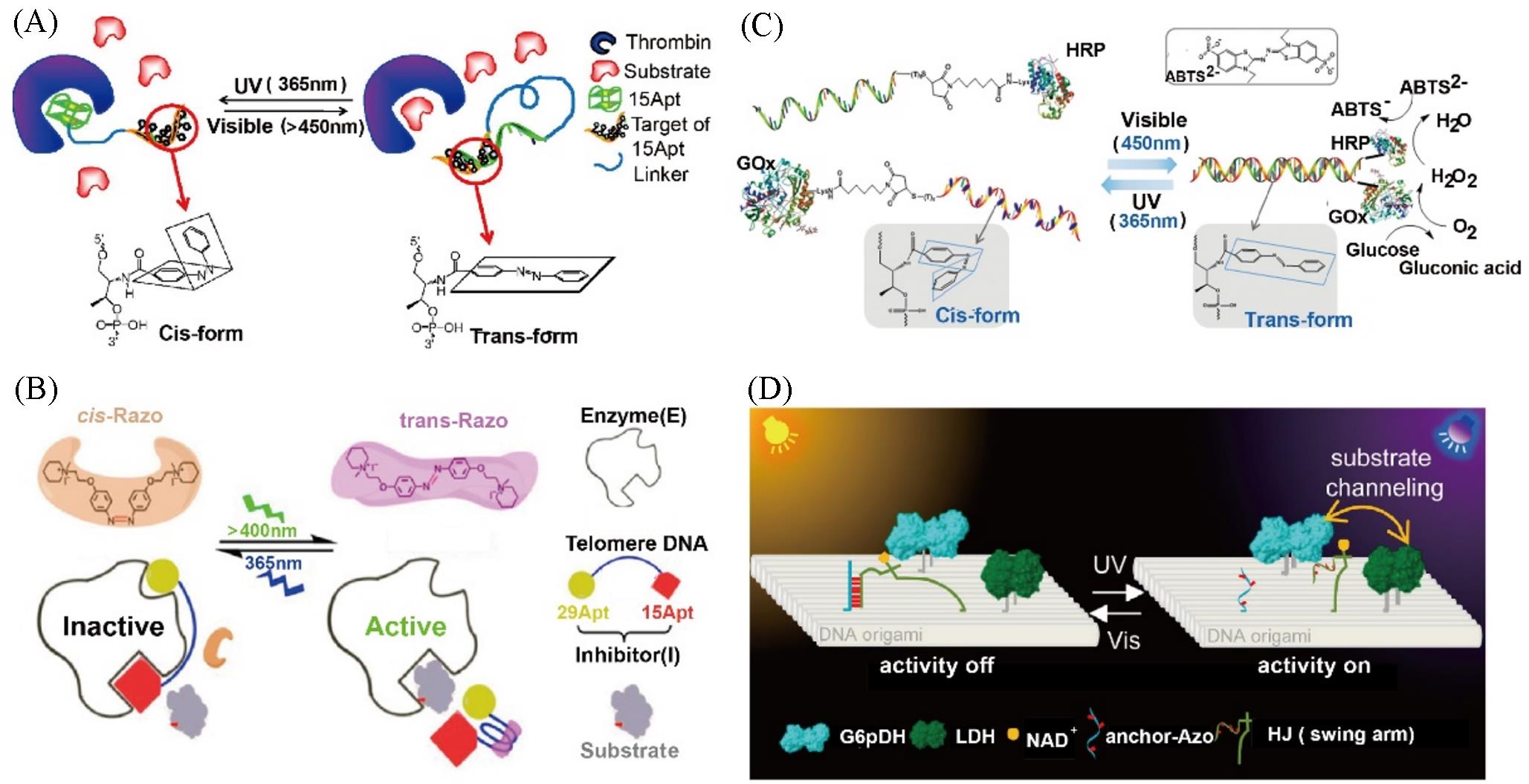
Fig.5 Azobenzene?based DNA nanomachines for regulation of enzymatic activity(A) The azobenzene-modified cDNA regulate thrombin activity[65]; (B) razo and telomere DNA regulate thrombin activity[68]; (C) light-responsive azobenzene-integrated DNA duplex for controlling GOx/HRP protein enzyme cascade activity[69]; (D) regulation of enzyme Cascade activity[70]. (A) Copyright 2009, National Academy of Sciences; (B) Copyright 2016, American Chemical Society; (C) Copyright 2011, American Chemical Society; (D) Copyright 2018, American Chemical Society.
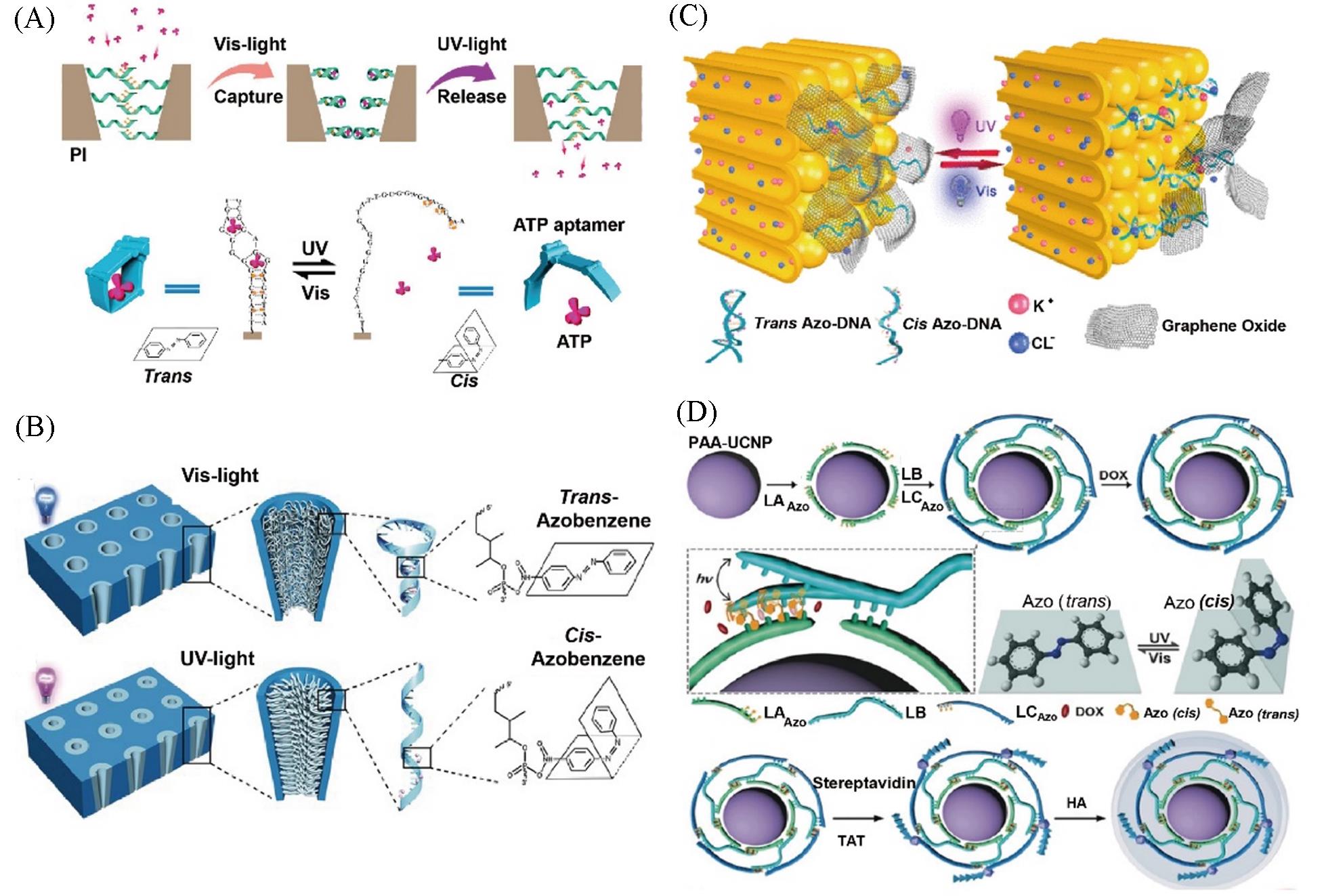
Fig.6 Azobenzene?based DNA nanomachines for photo?responsive regulation of material transport(A) Azobenzene-modified DNA nanochannel for ATP transmembrane transport[73]; (B) azobenzene-modified DNA nanochannel for ion transport[76]; (C) schematic illustration of the construction and working mechanism of the ionic gate based on the interaction between GO and azobenzene-DNA-modified PAA membrane[77]; (D) azobenzene-integrated DNA nanopump for photo-regulating Dox release[79].(A) Copyright 2018, American Chemical Society; (B) Copyright 2016, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (C) Copyright 2019, American Chemical Society; (D) Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
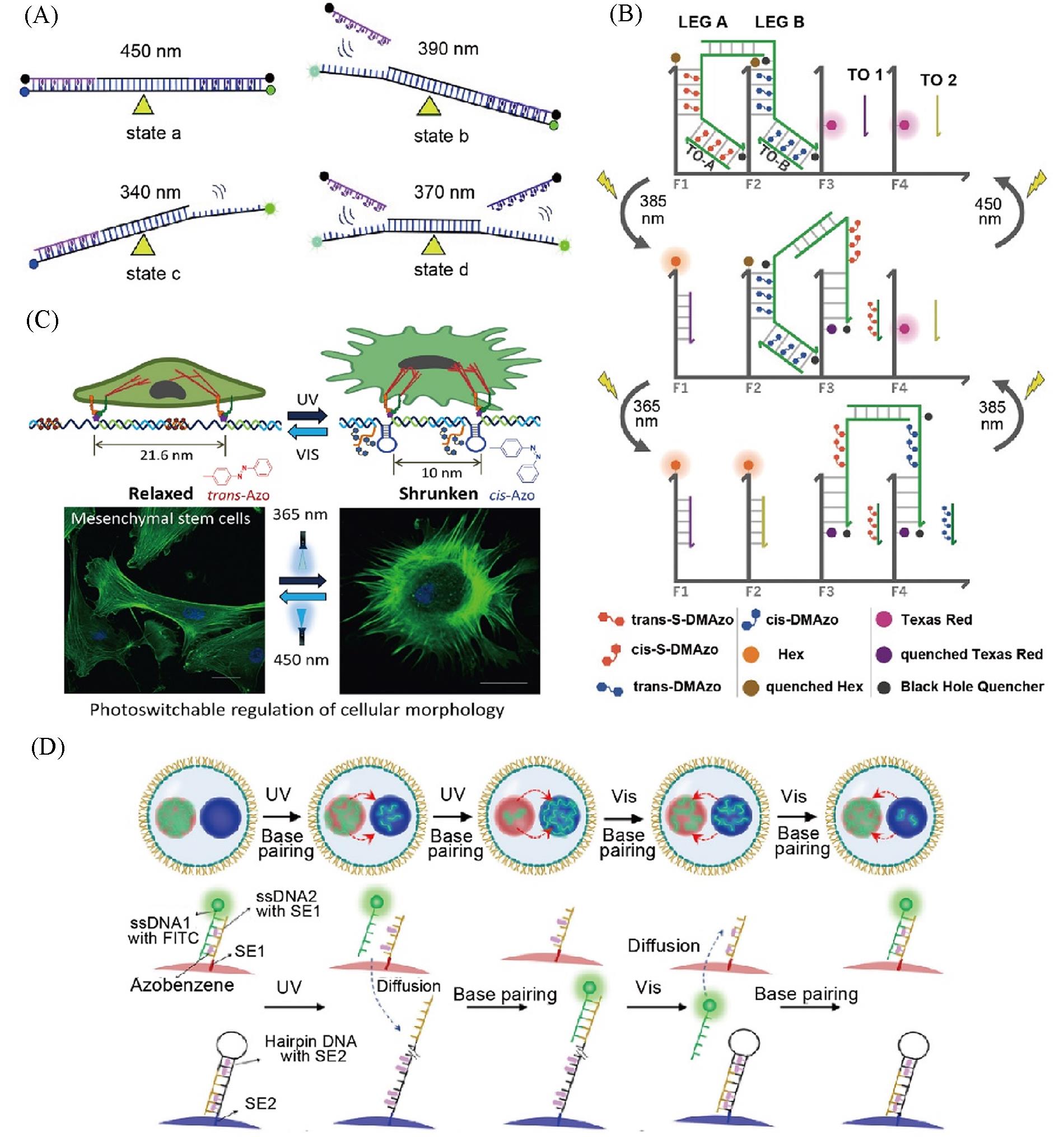
Fig.7 The azobenzene?based DNA nanomachines for photo?responsive regulation of mechanical motion, cellular morphology and molecular communication(A) Scheme illustration offour possible states(a, b, c, and d) of the DNA machines containing S-DMazo and azobenzene-modified DNAs are achieved byirradiation at four wavelengths(340, 370, 390 and 450 nm, respectively)[18]; (B) scheme illustration of DNA Walker[83]; (C) azobenzene-integrated DNA machine for photo-regulating cellular morphology[85]; (D) schematics of photo-actuated reversible transport of molecules between two DNA coacervates[86]. (A) Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (B) Copyright 2012, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (C) Copyright 2019, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim; (D) Copyright 2022, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
| 1 | Becker D., Konnertz N., Böhning M., Schmidt J., Thomas A., Chem. Mater., 2016, 28(23), 8523—8529 |
| 2 | Hartley G. S., Nature, 1937, 140(3537), 281 |
| 3 | Aggarwal K., Kuka T. P., Banik M., Medellin B. P., Ngo C. Q., Xie D., Fernandes Y., Dangerfield T. L., Ye E., Bouley B., Johnson K. A., Zhang Y. J., Eberhart J. K., Que E. L., J. Am. Chem. Soc., 2020, 142(34), 14522—14531 |
| 4 | Weis P., Wu S., Macromol. Rapid Commun., 2018, 39(1), 1700220 |
| 5 | Kou B., Tan L. H., Wang C. C., Xiao S. J., Prog. Chem., 2014, 26(10), 1720—1730 |
| 寇波, 谈玲华, 王倡春, 肖守军. 化学进展, 2014, 26(10), 1720—1730 | |
| 6 | Thambi T., Park J. H., Lee D. S., Chem. Commun., 2016, 52(55), 8492—8500 |
| 7 | Huang C., Tan W. L., Zheng J., Zhu C., Huo J., Yang R. H., ACS Appl. Mater. Interfaces, 2019, 11(29), 25740—25749 |
| 8 | Smith B. R., Gambhir S. S., Chem. Rev., 2017, 117(3), 901—986 |
| 9 | Kumari R., Sunil D., Ningthoujam R. S., J. Control. Release, 2020, 319, 135—156 |
| 10 | Tian Y., Li Y. F., Jiang W. L., Zhou D. Y., Fei J. J., Li C. Y., Anal. Chem., 2019, 91(16), 10901—10907 |
| 11 | Wang M. F., Zhao Y. J., Chang M. Y., Ding B. B., Deng X. R., Cui S. Z., Hou Z. Y., Lin J., ACS Appl. Mater. Interfaces, 2019, 11(51), 47730—47738 |
| 12 | Wegener M., Hansen M. J., Driessen A. J. M., Szymanski W., Feringa B. L., J. Am. Chem. Soc., 2017, 139(49), 17979—17986 |
| 13 | Goldman N., Bertone P., Chen S. Y., Dessimoz C., LeProust E. M., Sipos B., Birney E., Nature, 2013, 494(7435), 77—80 |
| 14 | Ge Z. L., Li Q., Fan C. H., Chem. Res. Chinese Universities, 2020, 36(1), 1—9 |
| 15 | Rothemund P. W. K., Nature, 2006, 440(7082), 297—302 |
| 16 | Liu M. H., Fu J. L., Hejesen C., Yang Y. H., Woodbury N. W., Gothelf K., Liu Y., Yan H., Nat. Commun., 2013, 4, 2127 |
| 17 | Feng X. P., Zhang H., New Chem. Mater., 2016, 44(4), 29—31 |
| 冯西平, 张宏. 化工新型材料, 2016, 44(4), 29—31 | |
| 18 | Nishioka H., Liang X. G., Kato T., Asanuma H., Angew. Chem. Int. Ed., 2012, 51(5), 1165—1168 |
| 19 | Basu S., Johl R., Pacelli S., Gehrke S., Paul A., ACS Macro. Letters, 2020, 9(9), 1230—1236 |
| 20 | Zhao Y., Cao W. Q., Liu Y., Chem. J. Chinese Universities, 2020, 41(5), 909—923 |
| 赵宇, 曹琬晴, 刘阳. 高等学校化学学报, 2020, 41(5), 909—923 | |
| 21 | Hu Y. Q., Wang Y., Yan J. H., Wen N. C., Xiong H. J., Cai S. D., He Q. Y., Peng D. M., Liu Z. B., Liu Y. F., Adv. Sci., 2020, 7(14), 2000557 |
| 22 | Fan C. H., Li J., Science & Technology Vision, 2021, (36), 6—11 |
| 樊春海, 李江. 科技视界, 2021, (36), 6—11 | |
| 23 | Fan C. H., Chin. Sci. Bull., 2019, 64(10), 987—988 |
| 樊春海. 科学通报, 2019, 64(10), 987—988 | |
| 24 | Deiana M., Pokladek Z., Olesiak⁃Banska J., Młynarz P., Samoc M., Matczyszyn K., Sci. Rep., 2016, 6, 28605 |
| 25 | Dohno C., Uno S. N., Nakatani K., J. Am. Chem. Soc., 2007, 129(39), 11898—11899 |
| 26 | Liu K., Jin Y., Liang J. G., Wu Y., Chem. J. Chinese Universities, 2021, 42(11), 3477—3492 |
| 刘珂, 靳宇, 梁建功, 吴园. 高等学校化学学报, 2021, 42(11), 3477—3492 | |
| 27 | Zinchenko A. A., Tanahashi M., Murata S., ChemBioChem, 2012, 13(1), 105—111 |
| 28 | Le Ny A. L. M., Lee C. T., J. Am. Chem. Soc., 2006, 128(19), 6400—6408 |
| 29 | Schimka S., Santer S., Mujkić⁃Ninnemann N. M., Bléger D., Hartmann L., Wehle M., Lipowsky R., Santer M., Biomacromolecules, 2016, 17(6), 1959—1968 |
| 30 | Bergen A., Rudiuk S., Morel M., Le Saux T., Ihmels H., Baigl D., Nano Lett., 2016, 16(1), 773—780 |
| 31 | Kong D. J., Mo M. W., Ji H. M., Lei H. J., Chen L., Li Y. H., He Y. J., Tang X. J., Zheng P. W., Wu L., Sci. Sin. Chim., 2018, 48(7), 698—711 |
| 孔德佳, 莫蒙武, 季禾茗, 雷华军, 陈露, 李元昊, 何裕建, 汤新景, 郑鹏武, 吴丽. 中国科学: 化学, 2018, 48(7), 698—711 | |
| 32 | Zhou F., Fu T., Huang Q., Kuai H. L., Mo L. T., Liu H. L., Wang Q. Q., Peng Y. B., Han D. M., Zhao Z. L., Fang X. H., Tan W. H., J. Am. Chem. Soc., 2019, 141(46), 18421—18427 |
| 33 | Wu L., He Y. J., Tang X. J., Bioconjugate Chem., 2015, 26(6), 1070—1079 |
| 34 | Asanuma H., Ito T., Komiyama M., Tetrahedron Lett., 1998, 39(49), 9015—9018 |
| 35 | Asanuma H., Ito T., Yoshida T., Liang X. G., Komiyama M., Angew. Chem. Int. Ed., 1999, 38(16), 2393—2395 |
| 36 | Nishioka H., Liang X. G., Asanuma H., Chem. Eur. J., 2010, 16(7), 2054—2062 |
| 37 | Ito H., Liang X. G., Nishioka H., Asanuma H., Org. Biomol. Chem., 2010, 8(24), 5519—5524 |
| 38 | Stafforst T., Hilvert D., Angew. Chem. Int. Ed., 2010, 49(51), 9998—10001 |
| 39 | Asanuma H., Liang X. G., Nishioka H., Matsunaga D., Liu M. Z., Komiyama M., Nat. Protoc., 2007, 2(1), 203—212 |
| 40 | Biswas M., Burghardt I., Biophys. J., 2014, 107(4), 932—940 |
| 41 | Li J., Wang X. Y., Liang X. G., Chem. Asian J., 2014, 9(12), 3344—3358 |
| 42 | Kou B., Guo X., Xiao S. J., Liang X. G., Small, 2013, 9(23), 3939—3943 |
| 43 | Goldau T., Murayama K., Brieke C., Steinwand S., Mondal P., Biswas M., Burghardt I., Wachtveitl J., Asanuma H., Heckel A., Chem. Eur. J., 2015, 21(7), 2845—2854 |
| 44 | Wang R. W., Jin C., Zhu X. Y., Zhou L. Y., Xuan W. J., Liu Y., Liu Q. L., Tan W. H., J. Am. Chem. Soc., 2017, 139(27), 9104—9107 |
| 45 | Keiper S., Vyle J. S., Angew. Chem. Int. Ed., 2006, 45(20), 3306—3309 |
| 46 | Freeman C., Vyle J. S., Heaney F., RSC Adv., 2013, 3(6), 1652—1655 |
| 47 | Lubbe A. S., Szymanski W., Feringa B. L., Chem. Soc. Rev., 2017, 46(4), 1052—1079 |
| 48 | Mori S., Morihiro K., Obika S., Molecules, 2014, 19(4), 5109—5118 |
| 49 | Mori S., Morihiro K., Kasahara Y., Tsunoda S. I., Obika S., Chemosensors, 2015, 3(2), 36—54 |
| 50 | Patnaik S., Kumar P., Garg B. S., Gandhi R. P., Gupta K. C., Bioorg. Med. Chem., 2007, 15(24), 7840—7849 |
| 51 | Wu L., Koumoto K., Sugimoto N., Chem. Commun., 2009,(14), 1915—1917 |
| 52 | Wu L., Wu Y., Jin H. W., Zhang L. R., He Y. J., Tang X. J., MedChemComm, 2015, 6(3), 461—468 |
| 53 | Kamiya Y., Asanuma H., Acc. Chem. Res., 2014, 47(6), 1663—1672 |
| 54 | Murayama K., Asanuma H., ChemBioChem, 2017, 18(1), 142—149 |
| 55 | Asano T., Okada T., J. Org. Chem., 1986, 51(23), 4454—4458 |
| 56 | Fujii T., Kashida H., Asanuma H., Chem. Eur. J., 2009, 15(39), 10092—10102 |
| 57 | Liang X. G., Takenaka N., Nishioka H., Asanuma H., Chem. Asian J., 2008, 3(3), 553—560 |
| 58 | Asanuma H., Ishikawa T., Yamano Y., Murayama K., Liang X. G., ChemPhotoChem, 2019, 3(6), 418—424 |
| 59 | Zhang L., Linden G., Vazquez O., Beilstein. J. Org. Chem., 2019, 15, 2500—2508 |
| 60 | Hammill M. L., Islam G., Desaulniers J. P., ChemBioChem, 2020, 21(16), 2367—2372 |
| 61 | Ge Z. L., Fan C. H., Yan H., Chin. Sci. Bull., 2014, 59(2), 146—157 |
| 葛志磊, 樊春海, YAN Hao. 科学通报, 2014, 59(2), 146—157 | |
| 62 | Zhang C., Chen Y. G., Liu Y., Chem. J. Chinese Universities, 2008, 29(9), 1797—1800 |
| 张超, 陈银广, 刘燕. 高等学校化学学报, 2008, 29(9), 1797—1800 | |
| 63 | Rickles F. R., Patierno S., Fernandez P. M., Chest, 2003, 124(3, Supplement), 58S—68S |
| 64 | Dagliyan O., Shirvanyants D., Karginov A. V., Ding F., Fee L., Chandrasekaran S. N., Freisinger C. M., Smolen G. A., Huttenlocher A., Hahn K. M., Dokholyan N. V., Proc. Natl. Acad. Sci. USA, 2013, 110(17), 6800—6804 |
| 65 | Kim Y. M., Phillips J. A., Liu H. P., Kang H. Z., Tan W. H., Proc. Natl. Acad. Sci. USA, 2009, 106(16), 6489—6494 |
| 66 | Hu X. Y., Guo D. S., Chem. Res. Chinese Universities, 2021, 37(3), 619—620 |
| 67 | Wang X. L., Huang J., Zhou Y. Y., Yan S. Y., Weng X. C., Wu X. J., Deng M. G., Zhou X. A., Angew. Chem. Int. Ed., 2010, 49(31), 5305—5309 |
| 68 | Tian T., Song Y. Y., Wang J. Q., Fu B. S., He Z. Y., Xu X. Q., Li A. L., Zhou X., Wang S. R., Zhou X., J. Am. Chem. Soc., 2016, 138(3), 955—961 |
| 69 | You M. X., Wang R. W., Zhang X. B., Chen Y., Wang K. L., Peng L., Tan W. H., ACS Nano, 2011, 5(12), 10090—10095 |
| 70 | Chen Y. H., Ke G. L., Ma Y. L., Zhu Z., Liu M. H., Liu Y., Yan H., Yang C. J., J. Am. Chem. Soc., 2018, 140(28), 8990—8996 |
| 71 | Wheeldon I., Minteer S. D., Banta S., Barton S. C., Atanassov P., Sigman M., Nat. Chem., 2016, 8(4), 299—309 |
| 72 | Dahl G., Phil. Trans. R. Soc. B., 2015, 370(1672), 20140191 |
| 73 | Li P., Xie G. H., Liu P., Kong X. Y., Song Y. L., Wen L. P., Jiang L., J. Am. Chem. Soc., 2018, 140(47), 16048—16052 |
| 74 | Wang C. L., Di Z. H., Fan Z. T., Li L. L., Chem. Res. Chinese Universities, 2020, 36(2), 268—273 |
| 75 | Nagel G., Szellas T., Kateriya S., Adeishvili N., Hegemann P., Bamberg E., Biochem. Soc. Trans., 2005, 33(Part4), 863—866 |
| 76 | Li P., Xie G. H., Kong X. Y., Zhang Z., Xiao K., Wen L. P., Jiang L., Angew. Chem. Int. Ed., 2016, 55(50), 15637—15641 |
| 77 | Shi L., Mu C., Gao T., Chai W., Sheng A., Chen T., Yang J., Zhu X., Li G., J. Am. Chem. Soc., 2019, 141(20), 8239—8243 |
| 78 | Yuan Q., Zhang Y. F., Chen T., Lu D. Q., Zhao Z. L., Zhang X. B., Li Z. X., Yan C. H., Tan W. H., ACS Nano, 2012, 6(7), 6337—6344 |
| 79 | Zhang Y., Song G. B., He Y. L., Zhang X. B., Liu Y., Ju H. X., Angew. Chem. Int. Ed., 2019, 58(50), 18207—18211 |
| 80 | Modi S., Swetha M. G., Goswami D., Gupta G. D., Mayor S., Krishnan Y., Nat. Nanotech., 2009, 4(5), 325—330 |
| 81 | Bandy T. J., Brewer A., Burns J. R., Marth G., Nguyen T., Stulz E., Chem. Soc. Rev., 2011, 40(1), 138—148 |
| 82 | Zhou M. G., Liang X. G., Mochizuki T., Asanuma H., Angew. Chem. Int. Ed., 2010, 49(12), 2167—2170 |
| 83 | Skugor M., Valero J., Murayama K., Centola M., Asanuma H., Famulok M., Angew. Chem. Int. Ed., 2019, 58(21), 6948—6951 |
| 84 | Frantz C., Stewart K. M., Weaver V. M., J. Cell Sci., 2010, 123(24), 4195—4200 |
| 85 | Sethi S., Hidaka K., Sugiyama H., Endo M., Angew. Chem. Int. Ed., 2021, 60(37), 20342—20349 |
| 86 | Zhao Q. H., Cao F. H., Luo Z. H., Huck W. T. S., Deng N. N., Angew. Chem. Int. Ed., 2022, 61(14), e202117500 |
| [1] | 高健, 冯奕钰, 方文宇, 王慧, 葛婧, 封伟. 基于低温热释放的烷基接枝相变偶氮苯材料[J]. 高等学校化学学报, 2022, 43(8): 20220146. |
| [2] | 桂晨, 王颢霖, 邵柏璇, 杨育景, 徐光青. 熔盐辅助法制备g-C3N4纳米结构及其光催化制氢性能[J]. 高等学校化学学报, 2021, 42(3): 827. |
| [3] | 李闪闪, 赵文娟, 李辉, 方千荣. 偶氮苯功能化的光响应共价有机框架材料[J]. 高等学校化学学报, 2020, 41(6): 1384. |
| [4] | 赵瑞阳, 于春燕, 韩吉姝, 傅云磊, 李明, 胡德华, 刘福胜. 电化学沉积制备光响应聚合物薄膜及其光信息存储应用[J]. 高等学校化学学报, 2019, 40(2): 358. |
| [5] | 李志明, 丁强, 谷晓俊, 辛红, 白炳莲, 李敏. 含偶氮苯基元联酰胺衍生物的有机凝胶和光响应性质[J]. 高等学校化学学报, 2017, 38(9): 1695. |
| [6] | 严超, 肖玉龙, 戴衡, 程晓红. 对称偶氮苯的合成及性质[J]. 高等学校化学学报, 2016, 37(3): 475. |
| [7] | 蒋红波, 龚成斌, 王强, 唐倩, 马学兵. 基于氨基偶氮苯的光和pH响应聚[(三甲基丙烯酸乙酯胺)8-(4-氨基-4'-甲基丙烯酰胺基偶氮苯)1](T8A1)的合成与表征[J]. 高等学校化学学报, 2014, 35(9): 2043. |
| [8] | 樊冬丽, 翟岩, 张妍, 涂伟, 黄耀东. 光响应偶氮苯类小分子凝胶因子的合成及性能[J]. 高等学校化学学报, 2014, 35(11): 2447. |
| [9] | 高超, 杨瑜珠, 张广播, 彭敬东, 唐倩, 龚成斌. 光响应性有机-无机杂化分子印迹聚合物的制备及表征[J]. 高等学校化学学报, 2012, 33(12): 2801. |
| [10] | 何立兵 边秀杰 晁单明 李智亮 李野 王策 刘新才. 主链含偶氮电活性聚酰胺材料的合成与性质[J]. 高等学校化学学报, 2011, 32(8): 1664. |
| [11] | 李向亮, 唐倩, 郭聪聪, 马荣, 常文斌, 龚成斌. 含氟偶氮苯光响应性聚合物的合成及性能[J]. 高等学校化学学报, 2011, 32(11): 2706. |
| [12] | 王洋 唐先忠 唐翔 汪云龙 赵帅. 两种含偶氮苯共轭桥新型生色团分子的合成及性能[J]. 高等学校化学学报, 2011, 32(10): 2327. |
| [13] | 刘宇芳 刘博 董振明 金硕 梁彩云 朱瑞涛 鲁云. 氧化偶氮苯类化合物的新法合成[J]. 高等学校化学学报, 2010, 31(12): 2396. |
| [14] | 张文泉, 施文芳 . 全偶氮苯官能化树枝状聚合物的合成[J]. 高等学校化学学报, 2007, 28(5): 1002. |
| [15] | 白炳莲, 李敏 . 含甲氧基偶氮苯基元的苯甲酸衍生物的合成与液晶性研究[J]. 高等学校化学学报, 2006, 27(9): 1767. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||