

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (7): 1125.doi: 10.7503/cjcu20160958
收稿日期:2016-12-29
出版日期:2017-07-10
发布日期:2017-05-02
作者简介:联系人简介: 李 夏, 女, 博士, 教授, 博士生导师, 主要从事无机配位化学研究. E-mail:基金资助:
WANG Qiushuang, ZHENG Xiaoli, QU Xianglong, LI Rui, LI Xia*( )
)
Received:2016-12-29
Online:2017-07-10
Published:2017-05-02
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:摘要:
以1,3-二(4-吡啶基)-丙烷(bpp)和邻苯二甲酸(1,2-H2bdc)为配体, 通过水热法合成了过渡金属配合物M2(1,2-bdc)2(bpp)2·2H2O[M=Co(1), Ni(2)]和Cd(1,2-bdc)(bpp)·H2O(3). 配合物1和2属单斜晶系P21空间群, 具有相似的三维骨架结构. 配合物中存在2种配位环境相似的金属中心, 每个金属中心采取六配位的畸变八面体构型, 与来自2个1,2-bdc配体的3个氧原子和2个bpp配体的2个氮原子以及1个水分子配位. 1,2-bdc配体采取单齿/双齿螯合的配位模式将金属离子连接成M1-(1,2-bdc)-M2右手螺旋链. bpp配体采取Trans-Gauche(TG)构型, 连接相邻的金属离子形成M1-(bpp)-M1链和M2-(bpp)-M2链. 这3种链交织在一起构筑成具有{65. 8}拓扑的三维结构. 配合物3属单斜晶系P21/c空间群, 具有单节点的双层二维结构. Cd(Ⅱ)离子采取七配位的畸变五角双锥体构型, 与来自2个1,2-bdc配体的4个氧原子, 2个bpp配体的2个氮原子和1个水分子配位. 1,2-bdc配体采取双齿螯合/双齿螯合的配位模式将Cd(Ⅱ)离子连接成Cd-(1,2-bdc)-Cd链. bpp配体采取TG构型, 连接相邻的Cd(Ⅱ)离子, 形成Cd-(bpp)-Cd链. 这2种链通过共享Cd(Ⅱ)离子交错排列构筑成二维结构. 配合物3显示出强的荧光, 最大发射位于408 nm处, 对应于配体的π*-π跃迁. 不同有机小分子对配合物3的荧光强度有不同程度的影响, 苯胺对其有显著的猝灭作用, 基于荧光猝灭机理, 配合物3可用于选择性检测苯胺分子.
TrendMD:
王秋爽, 郑晓丽, 屈相龙, 李睿, 李夏. 1,3-二(4-吡啶基)-丙烷与邻苯二甲酸构筑的过渡金属配合物的合成、结构和荧光性质. 高等学校化学学报, 2017, 38(7): 1125.
WANG Qiushuang, ZHENG Xiaoli, QU Xianglong, LI Rui, LI Xia. Synthesis, Structure and Luminescence Property of Transition Metal Complexes with 1,3-Di(4-pyridyl)-propane and 1,2-Benzenedicarboxylic Acid†. Chem. J. Chinese Universities, 2017, 38(7): 1125.
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C42H40N4O10Co2 | C42H40N4O10Ni2 | C21H20N2O5Cd |
| Formula weight | 878.64 | 878.20 | 492.79 |
| Temperature/K | 120(2) | 120(2) | 120(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21 | P21 | P21/c |
| a/nm | 1.16755(2) | 1.1609(3) | 1.04635(8) |
| b/nm | 1.4003(2) | 1.3986(3) | 1.01448(8) |
| c/nm | 1.18421(2) | 1.1847(3) | 1.87662(1) |
| β/(°) | 96.298(2) | 96.632(3) | 109.661(3) |
| V/nm3 | 1.9244(5) | 1.9107(7) | 1.8759(2) |
| Z | 2 | 2 | 4 |
| Dc/(g·cm-3) | 1.516 | 1.526 | 1.745 |
| Absorption coefficient/mm-1 | 0.928 | 1.052 | 1.202 |
| F(000) | 908 | 912 | 992 |
| Crystal size/mm | 0.38×0.34×0.30 | 0.42×0.15×0.10 | 0.15×0.10×0.07 |
| θ range for data collection/(°) | 1.730—25.249 | 2.26—25.25 | 2.07—27.63 |
| Limiting indices | -14≤h≤10, -16≤k≤16, | -13≤h≤13, -16≤k≤8, | -13≤h≤12, -13≤k≤13, |
| -12≤l≤14 | -14≤l≤13 | -24≤l≤20 | |
| Reflections collected | 9762 | 9035 | 10882 |
| Rint | 0.0220 | 0.0322 | 0.0311 |
| Data/restraints/parameters | 6637/7/535 | 4556/31/554 | 4307/39/280 |
| Goodness-of-fit on F2 | 1.028 | 1.062 | 1.025 |
| Final R indices[I>2σ(I)] | R1=0.0373, wR2=0.0887 | R1=0.0501, wR2= 0.1463 | R1=0.0284, wR2= 0.0604 |
| R indices(all data) | R1=0.0405, wR2=0.0907 | R1=0.0525, wR2=0.1478 | R1=0.0358, wR2= 0.0636 |
| CCDC No. | 1522654 | 1522656 | 1522655 |
Table 1 Crystallographic data of complexes 1—3
| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C42H40N4O10Co2 | C42H40N4O10Ni2 | C21H20N2O5Cd |
| Formula weight | 878.64 | 878.20 | 492.79 |
| Temperature/K | 120(2) | 120(2) | 120(2) |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21 | P21 | P21/c |
| a/nm | 1.16755(2) | 1.1609(3) | 1.04635(8) |
| b/nm | 1.4003(2) | 1.3986(3) | 1.01448(8) |
| c/nm | 1.18421(2) | 1.1847(3) | 1.87662(1) |
| β/(°) | 96.298(2) | 96.632(3) | 109.661(3) |
| V/nm3 | 1.9244(5) | 1.9107(7) | 1.8759(2) |
| Z | 2 | 2 | 4 |
| Dc/(g·cm-3) | 1.516 | 1.526 | 1.745 |
| Absorption coefficient/mm-1 | 0.928 | 1.052 | 1.202 |
| F(000) | 908 | 912 | 992 |
| Crystal size/mm | 0.38×0.34×0.30 | 0.42×0.15×0.10 | 0.15×0.10×0.07 |
| θ range for data collection/(°) | 1.730—25.249 | 2.26—25.25 | 2.07—27.63 |
| Limiting indices | -14≤h≤10, -16≤k≤16, | -13≤h≤13, -16≤k≤8, | -13≤h≤12, -13≤k≤13, |
| -12≤l≤14 | -14≤l≤13 | -24≤l≤20 | |
| Reflections collected | 9762 | 9035 | 10882 |
| Rint | 0.0220 | 0.0322 | 0.0311 |
| Data/restraints/parameters | 6637/7/535 | 4556/31/554 | 4307/39/280 |
| Goodness-of-fit on F2 | 1.028 | 1.062 | 1.025 |
| Final R indices[I>2σ(I)] | R1=0.0373, wR2=0.0887 | R1=0.0501, wR2= 0.1463 | R1=0.0284, wR2= 0.0604 |
| R indices(all data) | R1=0.0405, wR2=0.0907 | R1=0.0525, wR2=0.1478 | R1=0.0358, wR2= 0.0636 |
| CCDC No. | 1522654 | 1522656 | 1522655 |
| Co1—O6 | 0.2020(4) | Co1—O1 | 0.2153(4) | Co2—O8 | 0.2102(4) |
|---|---|---|---|---|---|
| Co1—N2A | 0.2105(5) | Co1—O2 | 0.2212(4) | Co2—N3 | 0.2112(5) |
| Co1—N1 | 0.2110(5) | Co2—O4B | 0.1987(4) | Co2—O10 | 0.2185(4) |
| Co1—O5 | 0.2152(4) | Co2—N4C | 0.2095(5) | Co2—O9 | 0.2237(4) |
| O6—Co1—N2A | 100.21(18) | O6—Co1—O2 | 94.71(16) | O8—Co2—N3 | 94.64(16) |
| O6—Co1—N1 | 92.04(17) | N2A—Co1—O2 | 164.30(16) | O4B—Co2—O10 | 89.72(16) |
| N2A—Co1—N1 | 92.14(18) | N1—Co1—O2 | 92.18(17) | N4C—Co2—O10 | 84.56(17) |
| O6—Co1—O5 | 90.58(16) | O5—Co1—O2 | 89.73(16) | O8—Co2—O10 | 85.50(15) |
| N2A—Co1—O5 | 85.30(17) | O1—Co1—O2 | 60.59(14) | N3—Co2—O10 | 179.62(18) |
| N1—Co1—O5 | 176.63(18) | O4B—Co2—N4C | 100.76(18) | O4B—Co2—O9 | 97.62(15) |
| O6—Co1—O1 | 154.37(16) | O4B—Co2—O8 | 158.35(16) | N4C—Co2—O9 | 159.14(16) |
| N2A—Co1—O1 | 103.95(17) | N4C—Co2—O8 | 99.79(17) | O8—Co2—O9 | 61.00(14) |
| N1—Co1—O1 | 95.45(17) | O4B—Co2—N3 | 90.28(17) | N3—Co2—O9 | 94.61(16) |
| O5—Co1—O1 | 83.07(15) | N4C—Co2—N3 | 95.07(18) | O10—Co2—O9 | 85.76(15) |
Table 2 Selected bond lengths(nm) and bond angles(°) for complex 1*
| Co1—O6 | 0.2020(4) | Co1—O1 | 0.2153(4) | Co2—O8 | 0.2102(4) |
|---|---|---|---|---|---|
| Co1—N2A | 0.2105(5) | Co1—O2 | 0.2212(4) | Co2—N3 | 0.2112(5) |
| Co1—N1 | 0.2110(5) | Co2—O4B | 0.1987(4) | Co2—O10 | 0.2185(4) |
| Co1—O5 | 0.2152(4) | Co2—N4C | 0.2095(5) | Co2—O9 | 0.2237(4) |
| O6—Co1—N2A | 100.21(18) | O6—Co1—O2 | 94.71(16) | O8—Co2—N3 | 94.64(16) |
| O6—Co1—N1 | 92.04(17) | N2A—Co1—O2 | 164.30(16) | O4B—Co2—O10 | 89.72(16) |
| N2A—Co1—N1 | 92.14(18) | N1—Co1—O2 | 92.18(17) | N4C—Co2—O10 | 84.56(17) |
| O6—Co1—O5 | 90.58(16) | O5—Co1—O2 | 89.73(16) | O8—Co2—O10 | 85.50(15) |
| N2A—Co1—O5 | 85.30(17) | O1—Co1—O2 | 60.59(14) | N3—Co2—O10 | 179.62(18) |
| N1—Co1—O5 | 176.63(18) | O4B—Co2—N4C | 100.76(18) | O4B—Co2—O9 | 97.62(15) |
| O6—Co1—O1 | 154.37(16) | O4B—Co2—O8 | 158.35(16) | N4C—Co2—O9 | 159.14(16) |
| N2A—Co1—O1 | 103.95(17) | N4C—Co2—O8 | 99.79(17) | O8—Co2—O9 | 61.00(14) |
| N1—Co1—O1 | 95.45(17) | O4B—Co2—N3 | 90.28(17) | N3—Co2—O9 | 94.61(16) |
| O5—Co1—O1 | 83.07(15) | N4C—Co2—N3 | 95.07(18) | O10—Co2—O9 | 85.76(15) |
| Ni1—O6 | 0.2009(6) | Ni1—O1 | 0.2128(5) | Ni2—N3 | 0.2075(6) |
|---|---|---|---|---|---|
| Ni1—N2A | 0.2053(7) | Ni1—O2 | 0.2145(5) | Ni2—O8 | 0.2080(5) |
| Ni1—N1 | 0.2066(6) | Ni2—O4B | 0.1985(5) | Ni2—O10 | 0.2117(5) |
| Ni1—O5 | 0.2108(6) | Ni2—N4C | 0.2048(7) | Ni2—O9 | 0.2178(5) |
| O6—Ni1—N2A | 98.3(2) | O6—Ni1—O2 | 96.6(2) | N3—Ni2—O8 | 93.5(2) |
| O6—Ni1—N1 | 91.6(2) | N2A—Ni1—O2 | 164.2(2) | O4B—Ni2—O10 | 89.8(2) |
| N2A—Ni1—N1 | 91.5(3) | N1—Ni1—O2 | 93.2(2) | N4C—Ni2—O10 | 85.0(2) |
| O6—Ni1—O5 | 90.5(2) | O5—Ni1—O2 | 88.8(2) | N3—Ni2—O10 | 179.6(3) |
| N2A—Ni1—O5 | 85.9(2) | O1—Ni1—O2 | 62.2(2) | O8—Ni2—O10 | 86.6(2) |
| N1—Ni1—O5 | 176.9(3) | O4B—Ni2—N4C | 97.6(2) | O4B—Ni2—O9 | 100.1(2) |
| O6—Ni1—O1 | 158.1(2) | O4B—Ni2—N3 | 90.3(2) | N4C—Ni2—O9 | 160.3(2) |
| N2A—Ni1—O1 | 102.4(2) | N4C—Ni2—N3 | 94.6(3) | N3—Ni2—O9 | 94.0(2) |
| N1—Ni1—O1 | 94.8(2) | O4B—Ni2—O8 | 162.1(2) | O8—Ni2—O9 | 62.2(2) |
| O5—Ni1—O1 | 84.0(2) | N4C—Ni2—O8 | 99.5(2) | O10—Ni2—O9 | 86.4(2) |
Table 3 Selected bond lengths(nm) and bond angles(°) for complex 2*
| Ni1—O6 | 0.2009(6) | Ni1—O1 | 0.2128(5) | Ni2—N3 | 0.2075(6) |
|---|---|---|---|---|---|
| Ni1—N2A | 0.2053(7) | Ni1—O2 | 0.2145(5) | Ni2—O8 | 0.2080(5) |
| Ni1—N1 | 0.2066(6) | Ni2—O4B | 0.1985(5) | Ni2—O10 | 0.2117(5) |
| Ni1—O5 | 0.2108(6) | Ni2—N4C | 0.2048(7) | Ni2—O9 | 0.2178(5) |
| O6—Ni1—N2A | 98.3(2) | O6—Ni1—O2 | 96.6(2) | N3—Ni2—O8 | 93.5(2) |
| O6—Ni1—N1 | 91.6(2) | N2A—Ni1—O2 | 164.2(2) | O4B—Ni2—O10 | 89.8(2) |
| N2A—Ni1—N1 | 91.5(3) | N1—Ni1—O2 | 93.2(2) | N4C—Ni2—O10 | 85.0(2) |
| O6—Ni1—O5 | 90.5(2) | O5—Ni1—O2 | 88.8(2) | N3—Ni2—O10 | 179.6(3) |
| N2A—Ni1—O5 | 85.9(2) | O1—Ni1—O2 | 62.2(2) | O8—Ni2—O10 | 86.6(2) |
| N1—Ni1—O5 | 176.9(3) | O4B—Ni2—N4C | 97.6(2) | O4B—Ni2—O9 | 100.1(2) |
| O6—Ni1—O1 | 158.1(2) | O4B—Ni2—N3 | 90.3(2) | N4C—Ni2—O9 | 160.3(2) |
| N2A—Ni1—O1 | 102.4(2) | N4C—Ni2—N3 | 94.6(3) | N3—Ni2—O9 | 94.0(2) |
| N1—Ni1—O1 | 94.8(2) | O4B—Ni2—O8 | 162.1(2) | O8—Ni2—O9 | 62.2(2) |
| O5—Ni1—O1 | 84.0(2) | N4C—Ni2—O8 | 99.5(2) | O10—Ni2—O9 | 86.4(2) |
| Cd1—N1 | 0.2331(2) | Cd1—O3 | 0.2352(7) | Cd1—O5 | 0.2448(2) |
|---|---|---|---|---|---|
| Cd1—N2A | 0.2351(2) | Cd1—O1B | 0.2421(2) | Cd1—O4 | 0.2467(1) |
| Cd1—O2B | 0.2352(4) | ||||
| N1—Cd1—N2A | 161.86(7) | N2A—Cd1—O1B | 85.12(7) | O1B—Cd1—O5 | 95.46(6) |
| N1—Cd1—O2B | 95.12(7) | O2B—Cd1—O1B | 54.97(6) | N1—Cd1—O4 | 87.61(7) |
| N2A—Cd1—O2B | 97.82(6) | O3—Cd1—O1B | 139.52(6) | N2A—Cd1—O4 | 90.69(7) |
| N1—Cd1—O3 | 101.52(7) | N1—Cd1—O5 | 82.41(7) | O2B—Cd1—O4 | 139.69(6) |
| N2A—Cd1—O3 | 92.11(7) | N2A—Cd1—O5 | 80.05(7) | O3—Cd1—O4 | 54.56(6) |
| O2B—Cd1—O3 | 85.67(6) | O2B—Cd1—O5 | 150.34(6) | O1B—Cd1—O4 | 165.32(6) |
| N1—Cd1—O1B | 92.00(7) | O3—Cd1—O5 | 123.87(6) | O5—Cd1—O4 | 69.93(6) |
Table 4 Selected bond lengths(nm) and bond angles(°) for complex 3*
| Cd1—N1 | 0.2331(2) | Cd1—O3 | 0.2352(7) | Cd1—O5 | 0.2448(2) |
|---|---|---|---|---|---|
| Cd1—N2A | 0.2351(2) | Cd1—O1B | 0.2421(2) | Cd1—O4 | 0.2467(1) |
| Cd1—O2B | 0.2352(4) | ||||
| N1—Cd1—N2A | 161.86(7) | N2A—Cd1—O1B | 85.12(7) | O1B—Cd1—O5 | 95.46(6) |
| N1—Cd1—O2B | 95.12(7) | O2B—Cd1—O1B | 54.97(6) | N1—Cd1—O4 | 87.61(7) |
| N2A—Cd1—O2B | 97.82(6) | O3—Cd1—O1B | 139.52(6) | N2A—Cd1—O4 | 90.69(7) |
| N1—Cd1—O3 | 101.52(7) | N1—Cd1—O5 | 82.41(7) | O2B—Cd1—O4 | 139.69(6) |
| N2A—Cd1—O3 | 92.11(7) | N2A—Cd1—O5 | 80.05(7) | O3—Cd1—O4 | 54.56(6) |
| O2B—Cd1—O3 | 85.67(6) | O2B—Cd1—O5 | 150.34(6) | O1B—Cd1—O4 | 165.32(6) |
| N1—Cd1—O1B | 92.00(7) | O3—Cd1—O5 | 123.87(6) | O5—Cd1—O4 | 69.93(6) |
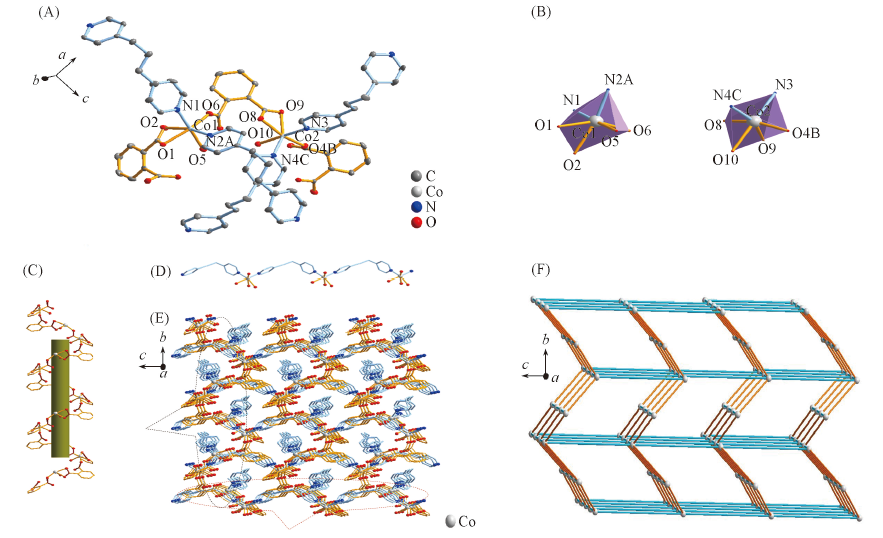
Fig.1 Asymmetric unit(A), coordination environment of Co(Ⅱ)(B), Co1-(1,2-bdc)-Co2 helix chain(C), Co2-(bpp)-Co2 chain(D), three-dimensional structure(E) and topological structure(F) of complex 1All hydrogen atoms in (A)—(E) are omitted; symmetry codes: A. x, y, z+1; B. -x, y-1/2, -z+2; C. x-1, y, z.
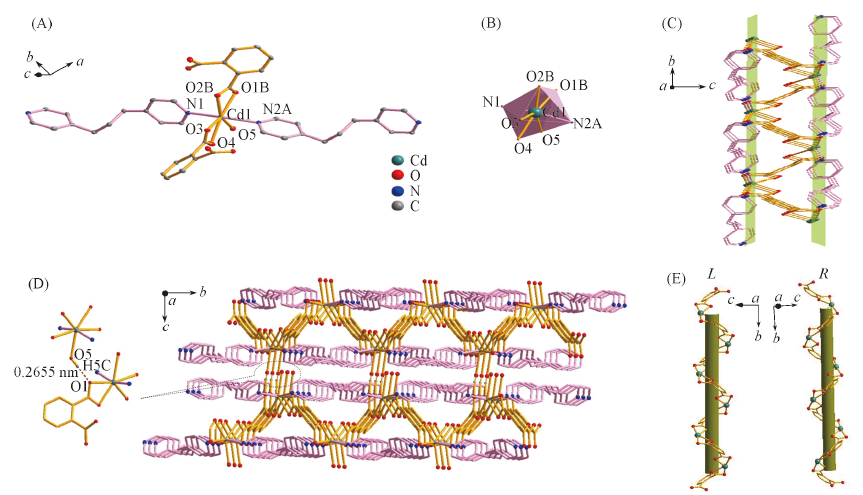
Fig.2 Asymmetric unit(A), coordination environment of Cd(Ⅱ)(B), two-dimensional network(C), three-dimensional structure by hydrogen bonds(D) and L-, R-helical chains(E) of complex 3All hydrogen atoms in (A)—(E) are omitted; symmetry codes: A. x+1, y-1, z; B. -x+2, y+1/2, -z+1/2; C. x, 1/2-y, -1/2+z.
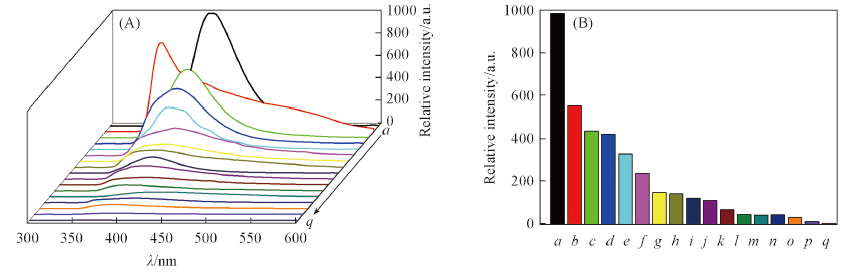
Fig.4 Emission spectra(A) and peak intensity at 408 nm(B) of complex 3 in different solvents Solvent: a. DMF; b. ethyl acetate; c. DMSO; d. dichloromethane; e. benzene; f. methanol; g. etanol; h. acetonitrile; i. hexane; j. formaldehyde; k. acetone; l. isopropanol; m. triethylamine; n. water; o. xylene; p. tetrahydrofuran; q. aniline.
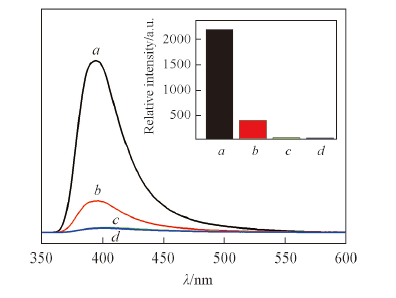
Fig.5 Emission spectra of complex 3 dispersed in ethanol solvent containing aniline with different concentrations(λex=314 nm)c(Aniline)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4. Inset shows peak intensity at 408 nm.
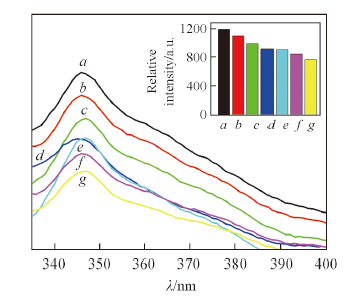
Fig.6 Emission spectra of complex 3 dispersed in ethanol solvent containing tetrahydrofuran with different concentrations(λex=314 nm)c(THF)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4; e. 10-3; f. 10-2; g. 10-1. Inset shows peak intensity at 345 nm.
| [1] | Furukawa H. K. N., Go Y. B., Aratani N., Choi S. B., Choi E., Yazaydin A. Ö., Snurr R. Q., O’Keeffe M., Kim J., Yaghi O. M., Science,2010, 329, 424—428 |
| [2] | Kreno L. E., Leong K., Farha O. K., Allendorf M., van Duyne R. P., Hupp J. T., Chem. Rev., 2012, 112, 1105—1125 |
| [3] | Nong R. Y., Kong J., Zhang J. H., Cheng L., Tang B., Xie S. M., Yuang L. M., Chem. J. Chinese Universities,2016, 37(1), 19—25 |
| (农蕊瑜, 孔娇, 章俊辉, 陈玲, 汤波, 谢生明, 袁黎明.高等学校化学学报, 2016, 37(1), 19—25) | |
| [4] | Brozek C. K., Dinca M., Chem. Soc. Rev., 2014, 43, 5456—5467 |
| [5] | Xu H., Chen R., Sun Q., La W., Su Q., Huang W., Liu X., Chem. Soc. Rev., 2014, 43, 3259—3302 |
| [6] | Hu Z. C., Deibert B. J., Li J., Chem. Soc. Rev., 2014, 43, 5815—5840 |
| [7] | Zhao L., Bai H. L., Sun E. J., Wang X. F., Wang Z. C., Chem. J. Chinese Universities,2016, 37(7), 1250—1256 |
| (赵仑, 白鹤龙, 孙二军, 王晓峰, 王子忱.高等学校化学学报, 2016, 37(7), 1250—1256) | |
| [8] | Pramanik S., Zheng C., Zhang X., Emge T. J., Li J., J. Am. Chem. Soc., 2011, 133, 4153—4155 |
| [9] | Vittal J. J., Coord. Chem. Rev., 2007, 251, 1781—1795 |
| [10] | Du M., Jiang X. J., Zhao X. J., Inorg. Chem., 2007, 46(10), 3984—3995 |
| [11] | Gang L. J., Wang L., Wang S. Y., Jing S. B., Chem. J. Chinese Universities,2016, 37(9), 1589—1595 |
| (高丽娟, 王莉,王圣燕,井淑波.高等学校化学学报, 2016, 37(9), 1589—1595) | |
| [12] | He W. W., Li S. L., Zang H. Y., Yang G. S., Zhang S. R., Su Z. M., Lan Y. Q., Coord. Chem. Rev., 2014, 279, 141—160 |
| [13] | Matsuda R., Kitaura R., Kitagawa S., Kubota Y., Belosludov R. V., Kobayashi T. C., Sakamoto H., Chiba T., Takat M.,Kawazoe Y.,Mit Y., Nature,2005, 436(7048), 238—241 |
| [14] | Debal K. S., Prakash M., Sudip K. M., Partha M., RSC Adv., 2015, 5, 102076—102084 |
| [15] | Xu H., Hu H. C., Cao C. S., Inorg. Chem., 2015, 54, 4585—4587 |
| [16] | Zheng T. T., Zhao J., Fang Z. W., Li M. T., Sun C. Y., Li X., Wang X. L., Su Z. M., Dalton Trans., 2017, 46, 2456—2461 |
| [17] | Seong Y. L., Kwon H. B., RSC Adv., 2017, 7, 290—299 |
| [18] | Chen S. G., Shi Z. Z., Qin L., Jia H. L., Cryst. Growth Des., 2017, 17, 67—72 |
| [19] | Hou S., Liu Q. K., Ma J. P., Dong Y. B., Inorg. Chem., 2013, 52, 3225—3235 |
| [20] | Xu X. Y., Yan B., ACS Appl. Mater. Interfaces,2015, 7, 721—729 |
| [21] | Lu Y., Yan B., Liu K. L., Chem. Commun., 2014, 50, 9969—9972 |
| [22] | Huang X. L., Liu L., Gao M. L., Han Z. B., RSC Adv., 2016, 6, 87945—87949 |
| [23] | Zhang X. F., Liu X. L., Lu R., Zhang H. J., Gong P., J. Mater. Chem., 2012, 22, 1167—1172 |
| [24] | Håkansson K., Coorey R. V., Zubarev R. A., Talrose V. L., Hakansson P., J. Mass Spectrom., 2000, 35, 337—346 |
| [25] | del Nogal S. M., Sappo C. P., Perez P. J. L., Cordero B. M., Anal. Bioanal. Chem. , 2012, 404,2007—2015 |
| [26] | Imasaka T., Tashiro K., Ishibashi N., Anal. Chem., 1986, 58, 3242—3244 |
| [27] | Hou Y. L., Xu H., Cheng R. R., Zhao B., Chem. Commun., 2015, 51, 6769—6772 |
| [28] | Chen X. L., Li Z. B., Zhu Y. X., Xu J. G., Anal. Chimica Acta,2004, 505, 283—287 |
| [29] | Fan T. T., Li J. J., Qu X. L., Cryst. Eng. Comm., 2015, 17, 9443—9451 |
| [30] | Lu W. G., Wei Z. W., Gu Z. Y., Liu T. F., Park J. H., Tian J., Zhang M. W., Zhang Q., Gentle I., Thomas., Bosch M., Zhou H. C., Chem. Soc. Rev., 2014, 43, 5561—5593 |
| [31] | Sheldrick G.M., SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [32] | Sheldrick G M., SHELXL 97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [33] | Zhang L. P., Ma J. F., Yang J., Pang Y. Y., Ma J., Inorg. Chem., 2010, 49, 1535—1550 |
| [34] | Guo J., Ma J. F., Liu B., Kan W. Q., Yang J., Cryst. Growth Des., 2011, 11, 3609—3621 |
| [35] | Zhao J., Wang X. L., Shi X., Inorg. Chem., 2011, 50, 3198—3205 |
| [36] | Majumder S., Mandal L., Mohanta S., Inorg. Chem., 2012, 51, 8739—8749 |
| [37] | Gu J. Z., Liang X. X., Cui Y. H., Wu J., Shi Z. F., Cryst. Eng. Comm., 2017, 19(18), 2570—2588 |
| [38] | Zhu X. D., Zhou W. X., Liu R. M., Ding Y. J., Lu J., Proserpio D. M., Cryst. Eng. Comm., 2016, 18(24), 4530—4537 |
| [39] | Yang L. L., Xiao Q. C., Shao K. Z., Su Z. M., Cryst. Eng. Comm., 2016, 18(25), 4765—4771 |
| [40] | Zhang X., Wang Z. J., Chen S. G., Shi Z. Z., Chen J. X., Zheng H. G., Dalton Trans., 2017, 46(7), 2332—2338 |
| [41] | de Silva A. P., Gunaratne H. Q. N., Gunnlaugsson T., Huxley A. J. M., McCoy C. P., Rademacher J. T., Rice T. E., Chem. Rev., 1997, 97, 1515—1566 |
| [1] | 葛怡聪, 聂万丽, 孙国峰, 陈稼轩, 田冲. 银催化2-烯基苯胺与苯并异噁唑的[5+1]环化反应[J]. 高等学校化学学报, 2022, 43(8): 20220142. |
| [2] | 王君旸, 刘争, 张茜, 孙春燕, 李红霞. DNA银纳米簇在功能核酸荧光生物传感器中的应用[J]. 高等学校化学学报, 2022, 43(6): 20220010. |
| [3] | 鲁聪, 李振华, 刘金露, 华佳, 李光华, 施展, 冯守华. 一种新的镧系金属有机骨架材料的合成、 结构及荧光检测性质[J]. 高等学校化学学报, 2022, 43(6): 20220037. |
| [4] | 刘晓磊, 陆永强, 游淇, 刘国辉, 姚伟, 胡日茗, 闫纪宪, 崔玉, 杨小凤, 孙国新, 蒋绪川. 基于3-羟基沙利度胺的比率型荧光探针对过氧化氢的检测[J]. 高等学校化学学报, 2022, 43(6): 20220070. |
| [5] | 蒋小康, 周琦, 周恒为. Gd2ZnTiO6∶Dy3+, Eu3+单基质白光荧光粉的制备与发光性能[J]. 高等学校化学学报, 2022, 43(6): 20220029. |
| [6] | 施耐克, 张娅, SANSON Andrea, 王蕾, 陈骏. Zn(NCN)单轴的负热膨胀性及机理研究[J]. 高等学校化学学报, 2022, 43(6): 20220124. |
| [7] | 王明智, 郑燕萍, 翁维正. CeO2负载的PdO与Ce1‒x Pd x O2‒δ 物种的甲烷催化燃烧性能[J]. 高等学校化学学报, 2022, 43(4): 20210816. |
| [8] | 赵永梅, 穆叶舒, 洪琛, 罗稳, 田智勇. 双萘酰亚胺衍生物用于检测水溶液中的苦味酸[J]. 高等学校化学学报, 2022, 43(3): 20210765. |
| [9] | 李巧, 赵洋, 王恩举. 基于芳叉丙二腈的高活性迈克尔系统的吸湿反应及荧光性质[J]. 高等学校化学学报, 2022, 43(3): 20210690. |
| [10] | 高京, 何文涛, 王欣欣, 向宇姝, 龙丽娟, 秦舒浩. DOPO衍生物改性碳纳米管的制备及对聚乳酸阻燃性能的影响[J]. 高等学校化学学报, 2022, 43(3): 20210670. |
| [11] | 田雪琴, 莫争, 丁鑫, 武鹏彦, 王雨, 王健. 方胺功能化荧光金属-有机框架材料的制备及对组氨酸的识别研究[J]. 高等学校化学学报, 2022, 43(2): 20210589. |
| [12] | 唐倩, 但飞君, 郭涛, 兰海闯. 喹啉酮-香豆素类Hg2+比色荧光探针的合成及应用[J]. 高等学校化学学报, 2022, 43(2): 20210660. |
| [13] | 章丽玲, 刘浏, 郑明秋, 方文凯, 刘达, 唐宏武. 基于上转换发光共振能量转移的CRISPR/Cas12a生物传感系统用于HPV16 DNA双信号检测[J]. 高等学校化学学报, 2022, 43(11): 20220412. |
| [14] | 伍泽鑫, 朱渊杰, 王泓中, 王均安, 贺英. 甲基修饰的咔唑/二苯砜基AIE-TADF蓝光材料及其OLED器件[J]. 高等学校化学学报, 2022, 43(11): 20220371. |
| [15] | 王迪, 钟克利, 汤立军, 侯淑华, 吕春欣. 席夫碱共价有机框架的合成及对I ‒ 的识别[J]. 高等学校化学学报, 2022, 43(10): 20220115. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||