

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (3): 20240458.doi: 10.7503/cjcu20240458
收稿日期:2024-10-09
出版日期:2025-03-10
发布日期:2024-11-19
通讯作者:
田泽民
E-mail:tzm@nuaa.edu.cn
基金资助:
SHEN Yuhao, TIAN Zemin( ), LI Wei, JI Yixuan, YAN Yingwen
), LI Wei, JI Yixuan, YAN Yingwen
Received:2024-10-09
Online:2025-03-10
Published:2024-11-19
Contact:
TIAN Zemin
E-mail:tzm@nuaa.edu.cn
Supported by:摘要:
采用DLPNO-CCSD(T)/CBS//B3LYP/6-311++G(d,p)量子化学方法计算了顺式-1,3-双甲基环己烷自由基低温二级加氧反应的反应物、 生成物和过渡态的分子结构、 振动频率和单点能, 构建了详细的反应势能面. 基于过渡态理论, 获得了主要基元反应的高压极限速率常数. 结果表明, 支链结构有利于过氧化氢过氧自由基(OOQOOH)的氢转移反应, 其中, 1,5-氢转移反应最占优势, 与其直接裂解生成酮基化合物(KHP)+OH路径形成竞争关系. 双过氧化氢自由基P(OOH)2由OOQOOH经氢转移反应生成, 主要裂解路径为环醚反应, 其能垒因支链有增加趋势. 基于RRKM/主方程方法获得的依压力变化的速率常数结果表明, 压力对上述反应的速率常数影响较小.
中图分类号:
TrendMD:
沈宇豪, 田泽民, 李伟, 纪亦轩, 颜应文. 构象结构对于顺式-1,3-双甲基环己烷二级加氧反应影响的理论研究. 高等学校化学学报, 2025, 46(3): 20240458.
SHEN Yuhao, TIAN Zemin, LI Wei, JI Yixuan, YAN Yingwen. Theoretical Study of the Effect of Conformational Structures on the Secondary Oxidation Reactions of cis-1,3-Dimethylcyclohexane. Chem. J. Chinese Universities, 2025, 46(3): 20240458.
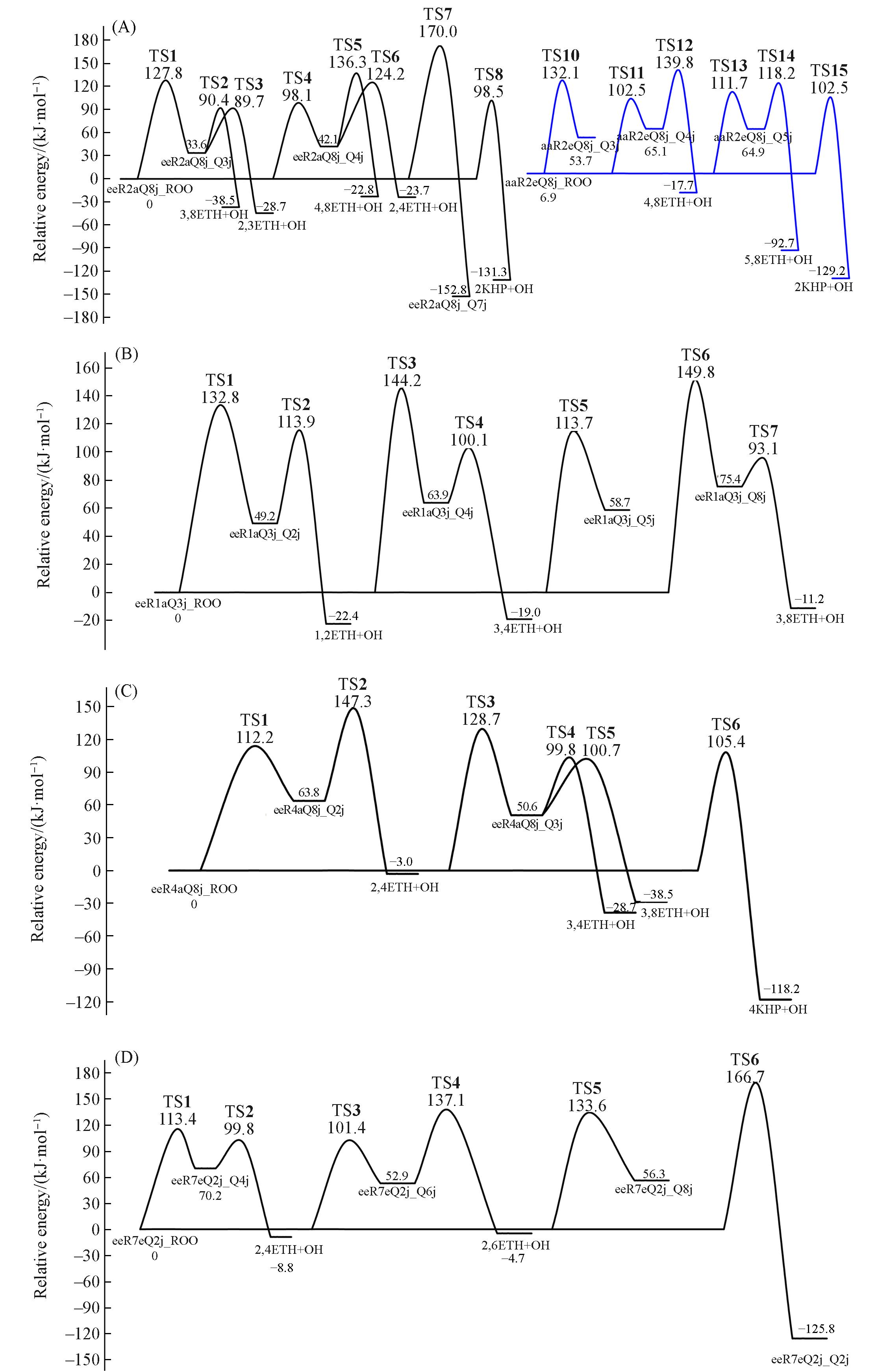
Fig.4 Five conformational structures reaction channel complete potential energy surface[DLNPO⁃CCSD(T) level energy](A) eeR2aQ8j_ROO and aaR2eQ8j_ROO; (B) eeR1aQ3j_ROO; (C) eeR4aQ8j_ROO; (D) eeR7eQ2j_ROO.
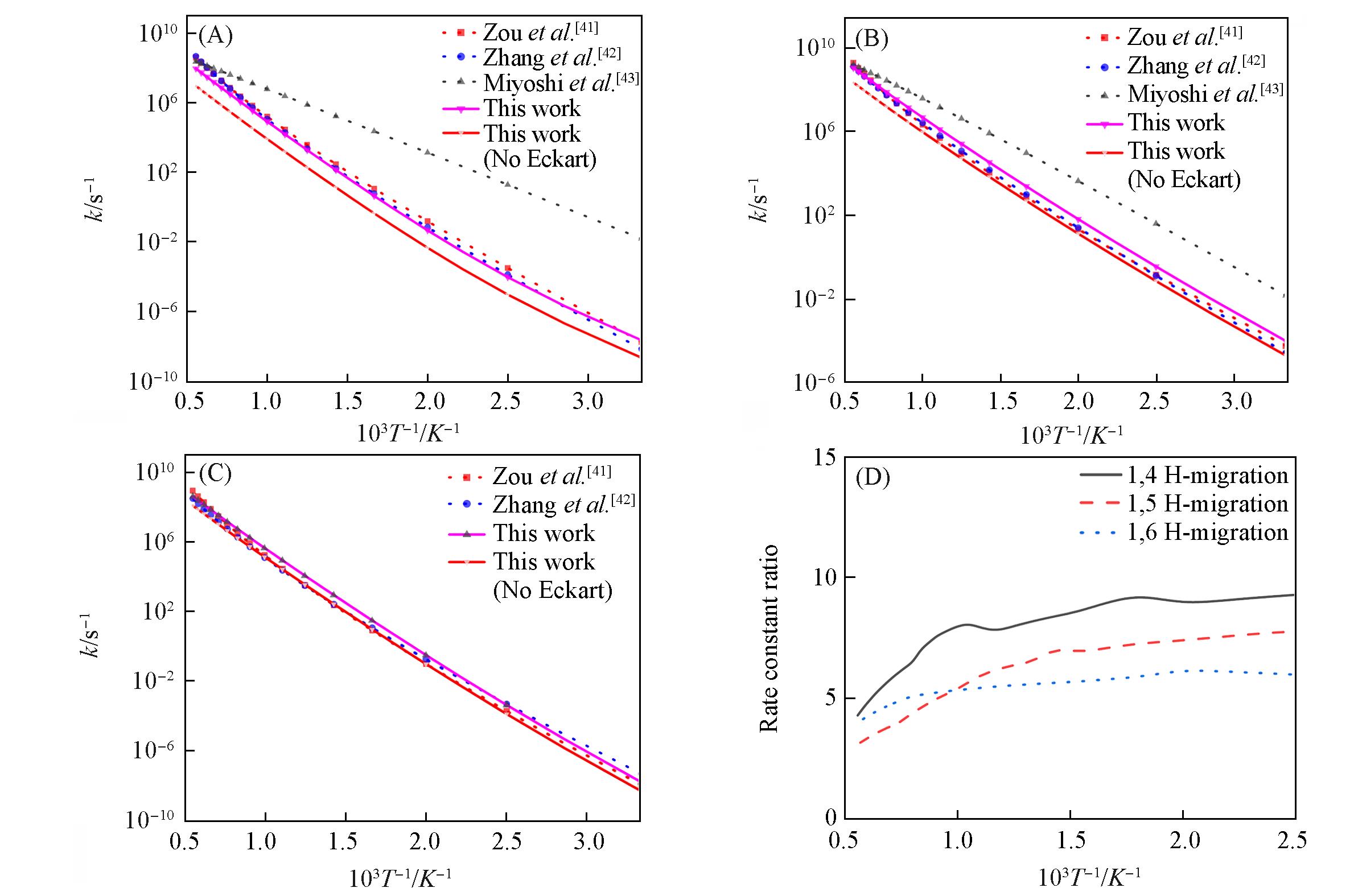
Fig.7 Comparison of hydrogen migration reaction rate constants calculated for cyclohexyl, ethylcyclohexane and N⁃C6H13, respectively(A) 1,4 H-migration; (B) 1,5 H-migration; (C) 1,6 H-migration; (D) comparison of the impact on tunnelling effects.
| Species | Qvibration | Qelectronic | Qtranslational | Qrotational |
|---|---|---|---|---|
| TS_aaR2eQ8j_Q3j | 0.620×1018 | 0.200×101 | 0.910×108 | 0.121×107 |
| TS_eeR2aQ8j_Q3j | 0.201×1019 | 0.200×101 | 0.910×108 | 0.120×107 |
| TS_aaR2eQ8j_Q4j | 0.426×1018 | 0.200×101 | 0.910×108 | 0.119×107 |
| TS_eeR2aQ8j_Q4j | 0.127×1018 | 0.200×101 | 0.910×108 | 0.106×107 |
Table 1 Isomerisable structural partition functions
| Species | Qvibration | Qelectronic | Qtranslational | Qrotational |
|---|---|---|---|---|
| TS_aaR2eQ8j_Q3j | 0.620×1018 | 0.200×101 | 0.910×108 | 0.121×107 |
| TS_eeR2aQ8j_Q3j | 0.201×1019 | 0.200×101 | 0.910×108 | 0.120×107 |
| TS_aaR2eQ8j_Q4j | 0.426×1018 | 0.200×101 | 0.910×108 | 0.119×107 |
| TS_eeR2aQ8j_Q4j | 0.127×1018 | 0.200×101 | 0.910×108 | 0.106×107 |
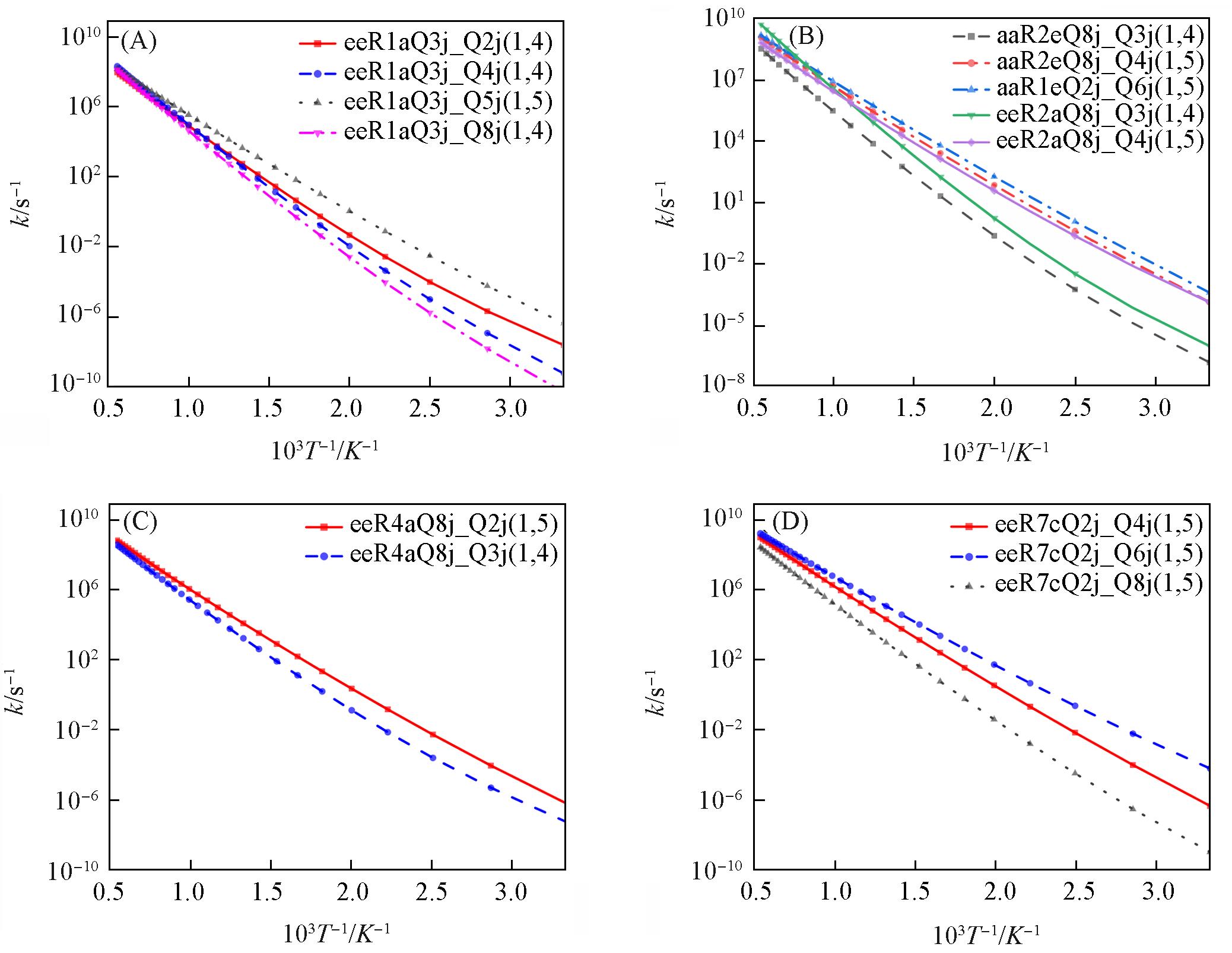
Fig.9 Comparison of rate constants for hydrogen transfer reactions in different conformations(A) eeR1aQ3j_ROO; (B) aaR2eQ8j_ROO; (C) eeR4aQ8j_ROO; (D) eeR7eQ2j_ROO.
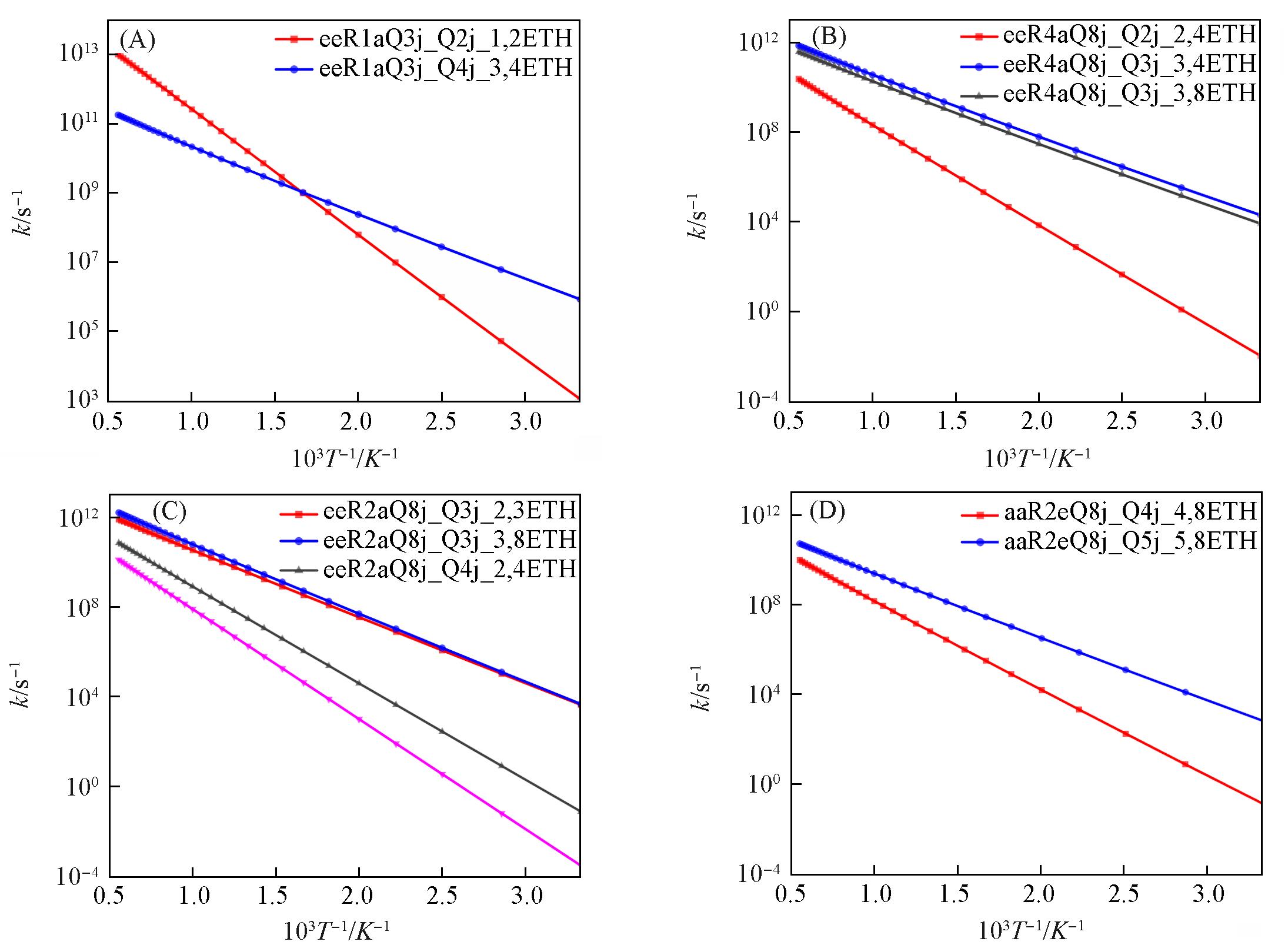
Fig.11 Comparison of high pressure limiting rate constants for cyclic ether reactions with different conformations(A) eeR1aQ3j_ROO; (B) eeR4aQ8j_ROO; (C) eeR2aQ8j_ROO; (D) aaR2eQ8j_ROO.
| Species | p | T/K | A/(cm3·mol-1·s-1) | n | Ea/(J·mol-1) |
|---|---|---|---|---|---|
| eeR1aQ3j_ROO=eeR1aQ3j_Q5j | High pressure limit | 300—1800 | 3810.91011 | 2.233127 | 91038.6100 |
| eeR1aQ3j_ROO=eeR1aQ3j_Q8j | High pressure limit | 300—1800 | 0.00603018 | 4.184921 | 110374.254 |
| aaR2eQ8j_ROO=aaR2eQ8j_Q3j | High pressure limit | 300—1800 | 0.00075039 | 4.380401 | 88013.0340 |
| eeR2aQ8j_ROO=eeR2aQ8j_Q4j | High pressure limit | 300—1800 | 511.615286 | 2.563875 | 76505.7788 |
| eeR4aQ8j_ROO=eeR4aQ8j_Q2j | High pressure limit | 300—1800 | 482.278753 | 2.694932 | 91171.6612 |
| eeR7eQ2j_ROO=eeR7eQ2j_Q6j | High pressure limit | 300—1800 | 106106.155 | 2.030745 | 83644.8125 |
eeR1aQ3j_Q4j=eeR1aQ3j_Q4j_ 3, 4ETH+OH | High pressure limit | 300—1800 | 28615758188 | 0.55146 | 34063.5255 |
aaR2eQ8j_Q5j=aaR2eQ8j_Q5j_ 5, 8ETH+OH | High pressure limit | 300—1800 | 43248373753 | 0.491631 | 52072.7251 |
eeR2aQ8j_Q3j=eeR2aQ8j_Q3j_ 2, 3ETH+OH | High pressure limit | 300—1800 | 2.017×1012 | 0.384468 | 55228.1305 |
eeR2aQ8j_Q3j=eeR2aQ8j_Q3j_ 3, 8ETH+OH | High pressure limit | 300—1800 | 4.089×1012 | 0.40081 | 57004.1967 |
| aaR2eQ8j=aaR2eQ8j_KHP+OH | High pressure limit | 300—1800 | 0.33388960 | 3.55955587 | 86321.7713 |
| eeR2aQ8j=eeR2aQ8j_2KHP+OH | High pressure limit | 300—1800 | 8.628519146 | 3.276908 | 72540.9786 |
eeR4aQ8j_Q3j=eeR4aQ8j_Q3j_ 3, 4ETH+OH | High pressure limit | 300—1800 | 1.199×1011 | 0.68421 | 48920.7087 |
eeR4aQ8j_Q3j=eeR4aQ8j_Q3j_ 3, 8ETH+OH | High pressure limit | 300—1800 | 76225583785 | 0.665072 | 49742.3208 |
| eeR4aQ8j=eeR4aQ8j_4KHP+OH | High pressure limit | 300—1800 | 0.430188908 | 3.452753 | 76366.7445 |
eeR7eQ2j_Q6j=eeR7eQ2j_Q6j_ 2, 6ETH+OH | High pressure limit | 300—1800 | 1.804×1014 | -0.08062 | 87319.034 |
| eeR7eQ2j=eeR7eQ2j_Q2j+OH | High pressure limit | 300—1800 | 5.4001959 | 3.551797 | 137825.562 |
Table 2 Reaction rate constants for some important reactions
| Species | p | T/K | A/(cm3·mol-1·s-1) | n | Ea/(J·mol-1) |
|---|---|---|---|---|---|
| eeR1aQ3j_ROO=eeR1aQ3j_Q5j | High pressure limit | 300—1800 | 3810.91011 | 2.233127 | 91038.6100 |
| eeR1aQ3j_ROO=eeR1aQ3j_Q8j | High pressure limit | 300—1800 | 0.00603018 | 4.184921 | 110374.254 |
| aaR2eQ8j_ROO=aaR2eQ8j_Q3j | High pressure limit | 300—1800 | 0.00075039 | 4.380401 | 88013.0340 |
| eeR2aQ8j_ROO=eeR2aQ8j_Q4j | High pressure limit | 300—1800 | 511.615286 | 2.563875 | 76505.7788 |
| eeR4aQ8j_ROO=eeR4aQ8j_Q2j | High pressure limit | 300—1800 | 482.278753 | 2.694932 | 91171.6612 |
| eeR7eQ2j_ROO=eeR7eQ2j_Q6j | High pressure limit | 300—1800 | 106106.155 | 2.030745 | 83644.8125 |
eeR1aQ3j_Q4j=eeR1aQ3j_Q4j_ 3, 4ETH+OH | High pressure limit | 300—1800 | 28615758188 | 0.55146 | 34063.5255 |
aaR2eQ8j_Q5j=aaR2eQ8j_Q5j_ 5, 8ETH+OH | High pressure limit | 300—1800 | 43248373753 | 0.491631 | 52072.7251 |
eeR2aQ8j_Q3j=eeR2aQ8j_Q3j_ 2, 3ETH+OH | High pressure limit | 300—1800 | 2.017×1012 | 0.384468 | 55228.1305 |
eeR2aQ8j_Q3j=eeR2aQ8j_Q3j_ 3, 8ETH+OH | High pressure limit | 300—1800 | 4.089×1012 | 0.40081 | 57004.1967 |
| aaR2eQ8j=aaR2eQ8j_KHP+OH | High pressure limit | 300—1800 | 0.33388960 | 3.55955587 | 86321.7713 |
| eeR2aQ8j=eeR2aQ8j_2KHP+OH | High pressure limit | 300—1800 | 8.628519146 | 3.276908 | 72540.9786 |
eeR4aQ8j_Q3j=eeR4aQ8j_Q3j_ 3, 4ETH+OH | High pressure limit | 300—1800 | 1.199×1011 | 0.68421 | 48920.7087 |
eeR4aQ8j_Q3j=eeR4aQ8j_Q3j_ 3, 8ETH+OH | High pressure limit | 300—1800 | 76225583785 | 0.665072 | 49742.3208 |
| eeR4aQ8j=eeR4aQ8j_4KHP+OH | High pressure limit | 300—1800 | 0.430188908 | 3.452753 | 76366.7445 |
eeR7eQ2j_Q6j=eeR7eQ2j_Q6j_ 2, 6ETH+OH | High pressure limit | 300—1800 | 1.804×1014 | -0.08062 | 87319.034 |
| eeR7eQ2j=eeR7eQ2j_Q2j+OH | High pressure limit | 300—1800 | 5.4001959 | 3.551797 | 137825.562 |
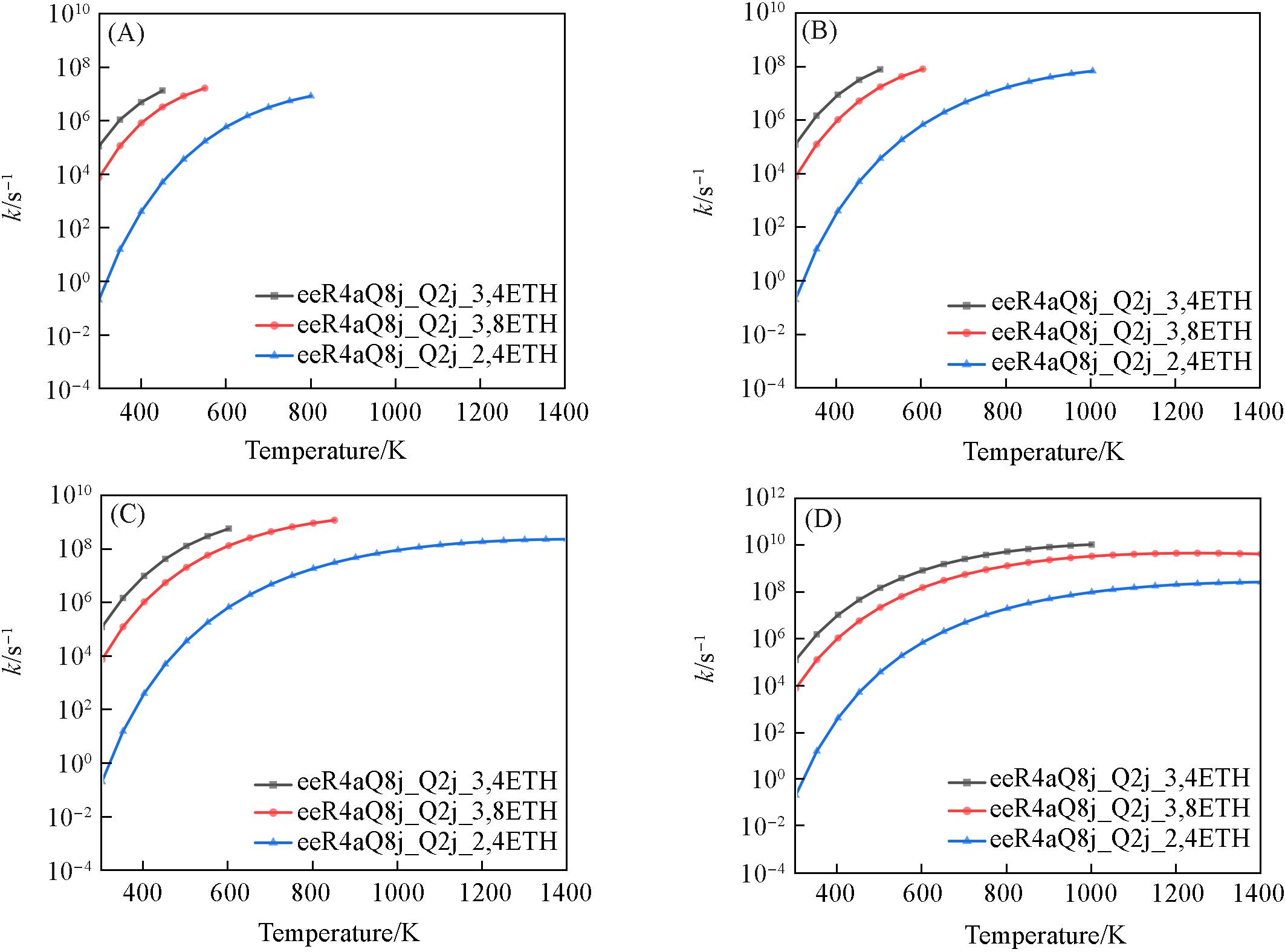
Fig.14 eeR4aQ8j_Q2j comparison of pressure dependent rate constants for cyclic ether reactions(A) 0.1×105 Pa; (B) 1×105 Pa; (C) 10×105 Pa; (D) 100×105 Pa.
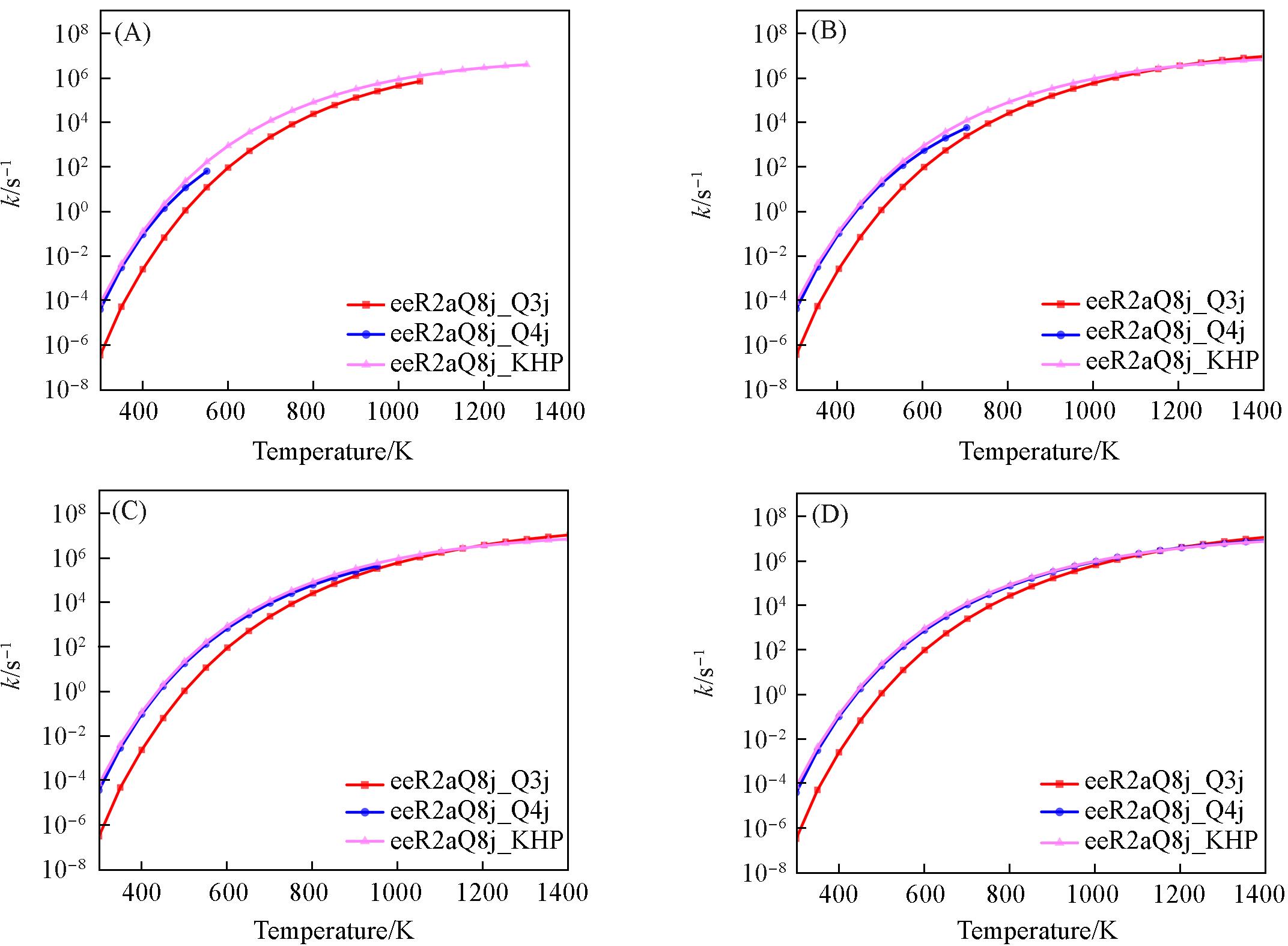
Fig.15 Comparison of pressure⁃dependent rate constants for eeR2aQ8j hydrogen transfer and KHP reactions(A) 0.1×105 Pa; (B) 1×105 Pa; (C) 10×105 Pa; (D) 100×105 Pa.
| 1 | Zhang T., Sun W., Wang L., Ju Y., Combust. Flame, 2019, 200, 342—353 |
| 2 | Okada Y., Miyashita S., Izumi Y., Hayakawa Y., SAE Int. J. Engines, 2014, 7, 584—594 |
| 3 | Dec J. E., Pro. Combust. Inst., 2009, 488, 294—303 |
| 4 | Splitter D., Reitz R., Hanson R., SAE Int. J. Fuels Lubr., 2010, 3, 742—756 |
| 5 | Ju Y., Combust. Flame, 2017, 178, 61—69 |
| 6 | Liu D., Santner J., Togbé C., Felsmann D., Koppmann J., Lackner A., Kohse⁃Höinghaus K., Combust. Flame, 2013, 160(12), 2654—2668 |
| 7 | Dong X., Duan H., Jia M., J. Fuel Sci., 2023, 127—531 |
| 8 | Bergthorson J. M., Thomson M. J., Renew Sustain Energy Rev., 2015, 42, 1393—1417 |
| 9 | Tan N. X., Wang J. B., Hua X. X., Li Z. R., Li X. Y., Chem. J. Chinese Universities, 2011, 32(8), 1832—1837 |
| 谈宁馨, 王静波, 华晓筱, 李泽荣, 李象远. 高等学校化学学报, 2011, 32(8), 1832—1837 | |
| 10 | Li Y. L., Wang J. B., Li X. Y., Chem. J. Chinese Universities, 2018, 39(6), 1212—1220 |
| 李颖丽, 王静波, 李象远. 高等学校化学学报, 2018, 39(6), 1212—1220 | |
| 11 | Shang Y., Li X., Zhang Z., Sun R., Luo S., Combust. Flame, 2024, 261, 113—320 |
| 12 | Oleinikov A. D., Azyazov V. N., Mebel A. M., Combust. Flame, 2018, 191, 209—319 |
| 13 | Ruan S., Yin J., Shi Y., Qin C., Xu K., He C., Hu X., Zhang L., Combust. Flame, 2023, 249, 112616 |
| 14 | Ye L., Wang D., Bian H., Li B., Gao W., Bi M., Combust. Flame, 2021, 227, 95—105 |
| 15 | Villano S. M., Huynh L. K., Carstensen H. H., Dean A. M., J. Phys. Chem. A, 2011, 115, 13425—13442 |
| 16 | Villano S. M., Huynh L. K., Carstensen H. H., Dean A. M., J. Phys. Chem. A, 2012, 116, 5068—5089 |
| 17 | Xing L., Bao J. L., Wang Z., Wang X., Truhlar D. G., Combust. Flame, 2018, 197, 88—101 |
| 18 | Xing L., Bao J. L., Wang Z., Wang X., Truhlar D. G., J. Am. Chem. Soc., 2018, 140, 17556—17570 |
| 19 | Yao Q., Sun X. H., Li Z. R., Chen F. F., Li X. Y., J. Phys. Chem. A, 2017, 121, 3001—3018 |
| 20 | Sarathy S. M., Farooq A., Kalghatgi G. T., Prog. Energy Combust. Sci., 2018, 65, 67—108 |
| 21 | Edwards T., Maurice L. Q., J. Propul. Power, 2001, 17, 461—466 |
| 22 | Pitz W. J., Mueller C. J., Prog. Energy Combust. Sci., 2011, 37(3), 330—350 |
| 23 | Liu G., Yan B., Chen G., Renew. Sustain. Energy Rev., 2013, 25, 59—70 |
| 24 | Balster L. M., Corporan E., DeWitt M. J., Edwards J. T., Ervin J. S., Graham J. L., Lee S. Y., Pal S., Phelps D. K., Rudnick L. R., Santoro R. J., Schobert H. H., Shafer L. M., Striebich R. C., West Z. J., Wilson G. R., Woodward R., Zabarnick S., Fuel Process. Technol., 2008, 89, 364—378 |
| 25 | Zhang X., Yan H., Zhu L. J., Li T., Wang S. R., Adv. Sustain. Syst., 2020, 4(10), 1900136 |
| 26 | Mao Y., Wang S., Wu Z., Qiu Y., Yu L., Ruan C., Chen F., Zhu L., Lu X., Combust. Flame, 2019, 206, 83—97 |
| 27 | Yang Y., Boehman A. L., Siimmie J. M., Combust. Flame, 2010, 157, 2357—2368 |
| 28 | Tian Z., Li J., Yan Y., Chem. Phys. Lett., 2020, 755, 137784 |
| 29 | Xing L., Lian L., Truhlar D. G., Combust. Flame, 2021, 231, 111503 |
| 30 | Yao X. X., Wang J. B., Yao Q., Li Y. Q., Li Z. R., Li X. Y., Combust. Flame, 2019, 204, 176—188 |
| 31 | Zou J., Jin H., Liu D., Zhang X., Combust. Flame, 2022, 235, 111550 |
| 32 | Bian H., Zhang Z., Kuang Y., Li N., Chem. Phys. Lett., 2024, 853, 141496 |
| 33 | Bian H., Wang Y., Li J., Zhao J., Int. J. Quantum Chem., 2022, 122, e26890 |
| 34 | Bian H., Zhang Y., Wang Y., Zhao J., Ruan X., Li J., Int. J. Quantum Chem., 2021, 121(11), e26636 |
| 35 | Ye L., Zhang L., Qi F., Combust. Flame, 2018, 190, 119—132 |
| 36 | Alecu I. M., Truhlar D. G., J. Phys. Chem. A, 2011, 115(13), 2811—2829 |
| 37 | Constantinou L., Gani R., Aiche J., 1994, 40(10), 1697—1710 |
| 38 | Georgievskii Y., Miller J. A., Burke M. P., Klippenstein S. J., J. Phys. Chem. A, 2013 117(46), 12146—12154 |
| 39 | Yang Y., Boehman A. L., Simmie J. M., Combust. Flame, 2010, 157(12), 2369—2379 |
| 40 | Bian H., Ye L., Li J., Sun J., Liang T., Zhong W., Zhao J., Combust. Flame, 2019, 205, 193—205 |
| 41 | Zou J., Li Y., Ye L., Jin H., Combust. Flame, 2022, 235, 111658 |
| 42 | Zhang H., Guo J., Xu P., Zhang C., Wang J., Combust. Flame, 2022, 245, 112307 |
| 43 | Miyoshi A., J. Phys. Chem. A, 2011, 115(15), 3301 |
| 44 | Serinyel Z., Herbinet O., Frottier O., Dirrenberger P., Warth V., Glaude P. A., Battin⁃Leclerc F., Combust. Flame, 2013, 160(11), 2319—2332 |
| 45 | Silke E. J., Pitz W. J., Westbrook C. K., Ribaucour M., J. Phys. Chem. A, 2007, 111(19), 3761—3775 |
| 46 | Xing L., Zhang F., Zhang L., Proc. Combust. Inst., 2017, 36(1), 179—186 |
| [1] | 秦海敬, 贺乾军, 徐敏敏, 袁亚仙, 姚建林. 离子液体中PMBA脱羧反应及界面水影响的电化学SERS研究[J]. 高等学校化学学报, 2024, 45(1): 20230349. |
| [2] | 徐德义, 丁超俊, 李芳, 刘月明, 何鸣元. 磷改性失活TS-1高效催化C5=裂解制备C2=/C3=反应的研究[J]. 高等学校化学学报, 2023, 44(8): 20230094. |
| [3] | 李象远,姚晓霞,申屠江涛,孙晓慧,李娟琴,刘明夏,许诗敏. 燃烧反应机理构建的双参数速率常数方法[J]. 高等学校化学学报, 2020, 41(3): 512. |
| [4] | 王宁, 朱惠芳, 王璐, 张田田, 顾佳丽, 舒婕. 埃索美拉唑镁在特定溶液中的分子互变异构体结构表征及互变动力学的核磁共振波谱研究[J]. 高等学校化学学报, 2018, 39(9): 1919. |
| [5] | 李颖丽, 王静波, 李象远. 十氢化萘低温燃烧反应的动力学机理[J]. 高等学校化学学报, 2018, 39(6): 1212. |
| [6] | 方升, 刘静静, 段雪梅, 陶福明, 刘靖尧. 大气中一元酸催化亚硫酸分解反应的从头算及动力学研究[J]. 高等学校化学学报, 2017, 38(8): 1390. |
| [7] | 马倩, 王渭娜, 赵强莉, 刘峰毅, 王文亮. Criegee中间体RCHOO(R=H,CH3)与NCO的反应机理[J]. 高等学校化学学报, 2017, 38(4): 613. |
| [8] | 杨明艳, 张莉, 王道全, 王明安. 反式-3-苯基-12-取代环十二酮的合成及晶体结构[J]. 高等学校化学学报, 2017, 38(3): 403. |
| [9] | 王睿, 李一粒, 凤旭凯, 宋亮, 张田雷, 王竹青, 靳玲侠, 张强, 许琼, 王志银. n(H2O)(n=1,2)在HO2+NO |
| [10] | 高志芳, 王渭娜, 马倩, 刘峰毅, wlwang@snnu.edu.cn. Criegee中间体CH3CHOO与OH自由基反应机理的理论研究[J]. 高等学校化学学报, 2016, 37(3): 513. |
| [11] | 朱鹏, 段雪梅, 刘靖尧. CF2ClC(O)OCH2CH3+OH的反应机理及动力学性质的理论研究[J]. 高等学校化学学报, 2016, 37(1): 79. |
| [12] | 王宽, 陈建刚, 王伯周, 吕剑, 王文亮, 刘峰毅, 周诚, 廉鹏, 刘忠文, 刘昭铁. FOX-12制备过程的反应机理及动力学[J]. 高等学校化学学报, 2015, 36(3): 531. |
| [13] | 杨明艳, 张莉, 王道全, 王明安. 2-苯基/环己基环十二酮的还原选择性及trans-1,2-二取代环十二烷的构象分析[J]. 高等学校化学学报, 2015, 36(3): 489. |
| [14] | 胡茜茜, 杨俊英, 谢代前. 反应N+NH→N2+H的态-态量子动力学研究[J]. 高等学校化学学报, 2015, 36(11): 2198. |
| [15] | 李悦, 方德彩. 叔丁氧基自由基引发氢迁移过程的理论研究[J]. 高等学校化学学报, 2015, 36(10): 1954. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||