

高等学校化学学报 ›› 2015, Vol. 36 ›› Issue (10): 1954.doi: 10.7503/cjcu20150326
收稿日期:2015-04-22
出版日期:2015-10-10
发布日期:2015-09-14
作者简介:联系人简介: 方德彩, 男, 教授, 博士生导师, 主要从事计算化学研究. E-mail:基金资助:Received:2015-04-22
Online:2015-10-10
Published:2015-09-14
Contact:
FANG Decai
E-mail:dcfang@bnu.edu.cn
Supported by:摘要:
采用多种密度泛函理论方法(如CAM-B3LYP, M062x和wB97x方法), 并辅以极化连续介质模型对叔丁氧基自由基(tBuO·)与一系列胺类、 烷烃、 醇类和醚类反应物之间氢迁移反应的反应机理进行研究. 计算结果表明, 这类氢迁移反应主要受熵的控制. 通过对液相平动熵和气相平动熵得到的活化自由能数据进行对比, 可以看出, 使用气相平动熵得出的活化自由能明显偏高于实验测量值, 而以液相平动熵计算的反应活化自由能垒与实际结果相近, 3种方法对胺类和烷烃类反应物体系得出的结果更可靠, 对醇类和醚类反应物体系自由能垒则略低.
中图分类号:
TrendMD:
李悦, 方德彩. 叔丁氧基自由基引发氢迁移过程的理论研究. 高等学校化学学报, 2015, 36(10): 1954.
LI Yue, FANG Decai. Density Functional Theory Studies on the t-Butoxyl Radical Mediated Hydrogen Atom Transfer Reactions†. Chem. J. Chinese Universities, 2015, 36(10): 1954.
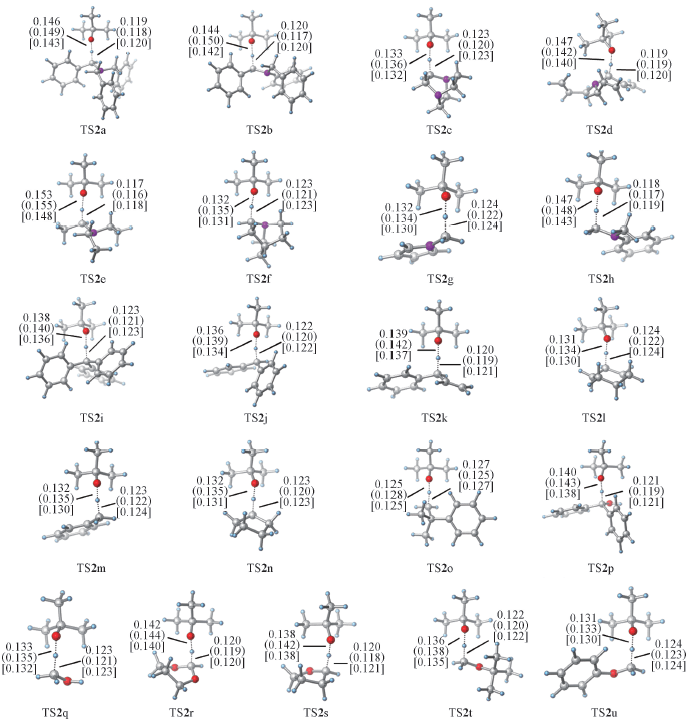
Fig.1 Main geometric parameters(nm) of 21 transition states in benzene solution, obtained with CAM-B3LYP, M062x and wB97x from top to bottom, respectively2a—2h: Amines; 2i—2o: hydrocarbons; 2p—2q: alcohols; 2r—2u: ethers.
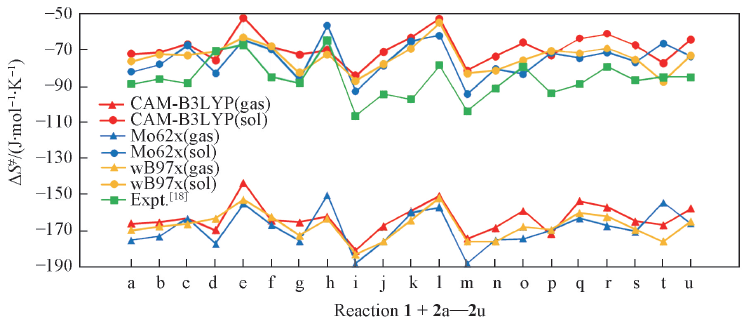
Fig.2 Calculated activation entropies(ΔS≠) for 21 hydrogen abstractions(1+2a—2u) obtained with gas-phase translational entropies ΔS≠(gas) for those methods and with solution translational entropies ΔS≠(sol) for those methods with PCM(in THF), along with experimental measurements[18]
| Species | Reaction | CAM-B3LYPa | CAM-B3LYPb | M062xa | wB97xa | Expt.c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠(g)d/ (kJ·mol-1) | ΔG≠(l)e/ (kJ·mol-1) | ΔG≠(g) / (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | |||
| Amines | 1+2a | 51.0 | 23.0 | 57.3 | 28.4 | 43.1 | 15.1 | 48.5 | 20.5 | 29.7 |
| 1+2b | 54.4 | 26.3 | 61.1 | 31.8 | 48.1 | 19.7 | 53.9 | 25.5 | 31.4 | |
| 1+2c | 64.0 | 35.1 | 63.6 | 35.1 | 56.5 | 28.0 | 65.7 | 37.2 | 32.6 | |
| 1+2d | 55.6 | 27.6 | 66.9 | 38.5 | 53.1 | 24.7 | 54.4 | 26.8 | 27.6 | |
| 1+2e | 45.6 | 18.4 | 51.4 | 23.8 | 43.5 | 16.3 | 47.3 | 20.5 | 27.6 | |
| 1+2f | 65.7 | 36.8 | 65.6 | 36.4 | 57.7 | 28.9 | 64.4 | 36.4 | 33.0 | |
| 1+2g | 70.7 | 43.1 | 68.6 | 40.6 | 63.6 | 36.8 | 72.8 | 45.6 | 33.9 | |
| 1+2h | 51.4 | 24.3 | 53.1 | 24.7 | 48.5 | 20.1 | 53.9 | 27.2 | 27.2 | |
| Hydrocarbons | 1+2i | 67.7 | 38.9 | 76.1 | 47.7 | 54.8 | 26.3 | 64.4 | 35.5 | 36.8 |
| 1+2j | 66.5 | 37.6 | 69.0 | 39.7 | 59.0 | 30.1 | 68.2 | 38.9 | 35.5 | |
| 1+2k | 63.6 | 34.7 | 63.1 | 33.9 | 57.7 | 29.3 | 65.2 | 36.8 | 36.0 | |
| 1+2l | 70.3 | 40.6 | 68.6 | 39.3 | 63.6 | 35.1 | 69.4 | 40.6 | 39.3 | |
| 1+2m | 77.8 | 49.8 | 74.9 | 46.4 | 74.0 | 46.0 | 78.2 | 50.2 | 43.1 | |
| 1+2n | 72.8 | 44.3 | 71.5 | 42.2 | 68.2 | 39.7 | 74.9 | 46.4 | 39.3 | |
| 1+2o | 83.2 | 55.6 | 82.8 | 55.2 | 75.7 | 48.5 | 85.3 | 57.7 | 46.8 | |
| Alcohols | 1+2p | 50.2 | 20.9 | 58.1 | 27.6 | 42.2 | 13.0 | 48.5 | 18.8 | 33.9 |
| 1+2q | 61.1 | 34.3 | 61.5 | 34.3 | 61.5 | 35.1 | 64.4 | 37.6 | 46.0 | |
| Ethers | 1+2r | 45.6 | 17.1 | 48.9 | 20.1 | 45.2 | 16.3 | 47.3 | 19.2 | 33.5 |
| 1+2s | 53.1 | 23.8 | 53.9 | 25.1 | 49.3 | 21.3 | 53.5 | 25.5 | 33.9 | |
| 1+2t | 63.6 | 36.8 | 62.7 | 35.5 | 56.4 | 30.1 | 65.7 | 39.3 | 44.3 | |
| 1+2u | 67.3 | 39.3 | 68.6 | 40.1 | 63.1 | 35.1 | 69.4 | 42.2 | 44.7 | |
Table 1 Calculated free-energy barriers obtained by different methods in benzene solution(298.15 K)
| Species | Reaction | CAM-B3LYPa | CAM-B3LYPb | M062xa | wB97xa | Expt.c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠(g)d/ (kJ·mol-1) | ΔG≠(l)e/ (kJ·mol-1) | ΔG≠(g) / (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | ΔG≠(g)/ (kJ·mol-1) | ΔG≠(l)/ (kJ·mol-1) | |||
| Amines | 1+2a | 51.0 | 23.0 | 57.3 | 28.4 | 43.1 | 15.1 | 48.5 | 20.5 | 29.7 |
| 1+2b | 54.4 | 26.3 | 61.1 | 31.8 | 48.1 | 19.7 | 53.9 | 25.5 | 31.4 | |
| 1+2c | 64.0 | 35.1 | 63.6 | 35.1 | 56.5 | 28.0 | 65.7 | 37.2 | 32.6 | |
| 1+2d | 55.6 | 27.6 | 66.9 | 38.5 | 53.1 | 24.7 | 54.4 | 26.8 | 27.6 | |
| 1+2e | 45.6 | 18.4 | 51.4 | 23.8 | 43.5 | 16.3 | 47.3 | 20.5 | 27.6 | |
| 1+2f | 65.7 | 36.8 | 65.6 | 36.4 | 57.7 | 28.9 | 64.4 | 36.4 | 33.0 | |
| 1+2g | 70.7 | 43.1 | 68.6 | 40.6 | 63.6 | 36.8 | 72.8 | 45.6 | 33.9 | |
| 1+2h | 51.4 | 24.3 | 53.1 | 24.7 | 48.5 | 20.1 | 53.9 | 27.2 | 27.2 | |
| Hydrocarbons | 1+2i | 67.7 | 38.9 | 76.1 | 47.7 | 54.8 | 26.3 | 64.4 | 35.5 | 36.8 |
| 1+2j | 66.5 | 37.6 | 69.0 | 39.7 | 59.0 | 30.1 | 68.2 | 38.9 | 35.5 | |
| 1+2k | 63.6 | 34.7 | 63.1 | 33.9 | 57.7 | 29.3 | 65.2 | 36.8 | 36.0 | |
| 1+2l | 70.3 | 40.6 | 68.6 | 39.3 | 63.6 | 35.1 | 69.4 | 40.6 | 39.3 | |
| 1+2m | 77.8 | 49.8 | 74.9 | 46.4 | 74.0 | 46.0 | 78.2 | 50.2 | 43.1 | |
| 1+2n | 72.8 | 44.3 | 71.5 | 42.2 | 68.2 | 39.7 | 74.9 | 46.4 | 39.3 | |
| 1+2o | 83.2 | 55.6 | 82.8 | 55.2 | 75.7 | 48.5 | 85.3 | 57.7 | 46.8 | |
| Alcohols | 1+2p | 50.2 | 20.9 | 58.1 | 27.6 | 42.2 | 13.0 | 48.5 | 18.8 | 33.9 |
| 1+2q | 61.1 | 34.3 | 61.5 | 34.3 | 61.5 | 35.1 | 64.4 | 37.6 | 46.0 | |
| Ethers | 1+2r | 45.6 | 17.1 | 48.9 | 20.1 | 45.2 | 16.3 | 47.3 | 19.2 | 33.5 |
| 1+2s | 53.1 | 23.8 | 53.9 | 25.1 | 49.3 | 21.3 | 53.5 | 25.5 | 33.9 | |
| 1+2t | 63.6 | 36.8 | 62.7 | 35.5 | 56.4 | 30.1 | 65.7 | 39.3 | 44.3 | |
| 1+2u | 67.3 | 39.3 | 68.6 | 40.1 | 63.1 | 35.1 | 69.4 | 42.2 | 44.7 | |
| Species | Reaction | k/(L·mol-1·s-1) | |||
|---|---|---|---|---|---|
| CAM-B3LYP | M062x | wB97x | Expt.[ | ||
| Amines | 1+2a | 6.1×108 | 1.4×1010 | 1.5×109 | 4.2×107 |
| 1+2b | 1.5×108 | 2.1×109 | 2.2×108 | 1.9×107 | |
| 1+2c | 4.2×106 | 7.5×107 | 1.8×106 | 1.2×107 | |
| 1+2d | 9.3×107 | 2.7×108 | 1.2×108 | 8.9×107 | |
| 1+2e | 3.9×109 | 8.9×109 | 1.6×109 | 9.8×107 | |
| 1+2f | 2.2×106 | 5.6×107 | 2.8×106 | 1.0×107 | |
| 1+2g | 1.8×105 | 2.2×106 | 6.2×104 | 7.3×106 | |
| 1+2h | 3.8×108 | 1.8×109 | 1.1×108 | 1.0×108 | |
| Hydrocarbons | 1+2i | 9.2×105 | 1.5×108 | 3.6×106 | 2.1×106 |
| 1+2j | 1.5×106 | 3.3×107 | 1.0×106 | 3.4×106 | |
| 1+2k | 5.3×106 | 4.8×107 | 2.2×106 | 3.2×106 | |
| 1+2l | 4.6×105 | 4.3×106 | 4.8×105 | 8.1×105 | |
| 1+2m | 1.1×104 | 5.6×104 | 9.3×103 | 1.9×105 | |
| 1+2n | 1.1×105 | 6.8×105 | 4.5×104 | 8.6×105 | |
| 1+2o | 1.2×103 | 2.0×104 | 5.0×102 | 4.0×104 | |
| Alcohols | 1+2p | 1.4×109 | 3.2×1010 | 3.2×109 | 6.9×106 |
| 1+2q | 6.5×106 | 4.6×106 | 1.5×106 | 5.3×104 | |
| Ethers | 1+2r | 6.1×109 | 8.2×109 | 2.7×109 | 7.9×106 |
| 1+2s | 4.0×108 | 1.1×109 | 2.2×108 | 7.4×106 | |
| 1+2t | 2.2×106 | 3.3×107 | 8.7×105 | 1.1×105 | |
| 1+2u | 7.9×105 | 4.2×106 | 2.7×105 | 9.5×104 | |
Table 2 Comparison for reaction rate constants obtained by different DFT methods, along with the experimental rate constants at 298.15 K
| Species | Reaction | k/(L·mol-1·s-1) | |||
|---|---|---|---|---|---|
| CAM-B3LYP | M062x | wB97x | Expt.[ | ||
| Amines | 1+2a | 6.1×108 | 1.4×1010 | 1.5×109 | 4.2×107 |
| 1+2b | 1.5×108 | 2.1×109 | 2.2×108 | 1.9×107 | |
| 1+2c | 4.2×106 | 7.5×107 | 1.8×106 | 1.2×107 | |
| 1+2d | 9.3×107 | 2.7×108 | 1.2×108 | 8.9×107 | |
| 1+2e | 3.9×109 | 8.9×109 | 1.6×109 | 9.8×107 | |
| 1+2f | 2.2×106 | 5.6×107 | 2.8×106 | 1.0×107 | |
| 1+2g | 1.8×105 | 2.2×106 | 6.2×104 | 7.3×106 | |
| 1+2h | 3.8×108 | 1.8×109 | 1.1×108 | 1.0×108 | |
| Hydrocarbons | 1+2i | 9.2×105 | 1.5×108 | 3.6×106 | 2.1×106 |
| 1+2j | 1.5×106 | 3.3×107 | 1.0×106 | 3.4×106 | |
| 1+2k | 5.3×106 | 4.8×107 | 2.2×106 | 3.2×106 | |
| 1+2l | 4.6×105 | 4.3×106 | 4.8×105 | 8.1×105 | |
| 1+2m | 1.1×104 | 5.6×104 | 9.3×103 | 1.9×105 | |
| 1+2n | 1.1×105 | 6.8×105 | 4.5×104 | 8.6×105 | |
| 1+2o | 1.2×103 | 2.0×104 | 5.0×102 | 4.0×104 | |
| Alcohols | 1+2p | 1.4×109 | 3.2×1010 | 3.2×109 | 6.9×106 |
| 1+2q | 6.5×106 | 4.6×106 | 1.5×106 | 5.3×104 | |
| Ethers | 1+2r | 6.1×109 | 8.2×109 | 2.7×109 | 7.9×106 |
| 1+2s | 4.0×108 | 1.1×109 | 2.2×108 | 7.4×106 | |
| 1+2t | 2.2×106 | 3.3×107 | 8.7×105 | 1.1×105 | |
| 1+2u | 7.9×105 | 4.2×106 | 2.7×105 | 9.5×104 | |
| [1] | Walling C., Jacknow B. B., J. Am.Chem. Soc., 1960, 82, 6108—6112 |
| [2] | Walling C., McGuiness J. A., J. Am.Chem. Soc., 1969, 91(8), 2053—2058 |
| [3] | Carter W. P. L., Darnall K. R., Lioyd A. C., Chem. Phys. Lett., 1976, 42(1), 22—27 |
| [4] | Adam W., Grimm G. N., Saha-Moeller C. R., Dall A. F., Miolo G., Daniela V., Chem. Res. Toxicology., 1998, 11, 1089—1097 |
| [5] | Adam W., Marquardt S., Kemmer D., Saha-Moeller C. R., Schreier P., Org. Lett., 2002, 4, 225—228 |
| [6] | Mahler H. C., Schulz I., Adam W., Grimm G. N., Saha-Moeller C. R., Epe B., Mutation Research, 2001, 46, 289—299 |
| [7] | Jones C. M., Burkitt M. J., J. Am. Chem. Soc., 2003, 125, 6946—6954 |
| [8] | Lindsay S. J. R., Nagatomi E., Stead A., Waddington D. J., Beviere S. D., J. Chem. Soc., Perkin Trans., 2000, 2, 1193—1198 |
| [9] | Karki S. B., Treemaneekam V., KaufmanM. J., J. Pharm. Sci., 2000, 89, 1518—1524 |
| [10] | Hartung J., Schneiders N., Gottwald T., Terahedron Lett., 2007, 48, 6027—6030 |
| [11] | Paul H., Small R. D., Scaiano J. C., J. Am. Chem. Soc., 1978, 100(14), 4520—4527 |
| [12] | Encinas M. V., Scaiano J. C., J. Am. Chem. Soc., 1981, 103(21), 6393—6397 |
| [13] | Russell G.A., Reactivity, Selectivity, and Polar Effects in Hydrogen Atom Transfer Reactions, Wiley,New York, 1973, 1—13 |
| [14] | Suleman N. K., Flores J., Tanko J. M., Isin E. M., Castaqnoli N. Jr., Bioorg. Med. Chem., 2008, 16(18), 8557—8562 |
| [15] | Tsentalovich Y. P., Kulik L. V., Gritsan N. P., Yurkovskaya A. V., J.Phys. Chem. A, 1998, 102, 7975—7980 |
| [16] | Baciocchi E., Bietti M., Salamone M., Steenken S., J. Org. Chem., 2002, 67, 2266—2270 |
| [17] | Roberts B. P., Chem. Soc. Rev., 1999, 28, 25—35 |
| [18] | Finn M., Friegline R., Suleman N. K., J. Am. Chem. Soc., 2004, 126(24), 7578—7584 |
| [19] | Wong S. K., J. Am. Chem. Soc., 1979, 101, 1235—1239 |
| [20] | Salamone M., Giammarioli I., Bietti M., J. Org. Chem., 2011, 76(11), 4645—4651 |
| [21] | Poleshchuk O. K., Yureva A. G., Filimonov V. D., Frenking G., Journal of Molecular Structure, 2009, 912, 67—72 |
| [22] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Gaussian Inc., Wallingford CT, 2009 |
| [23] | Lee C., Yang W., Parr R. G., Phys. Rev. B, 1988, 37, 785—789 |
| [24] | Becke A. D., J. Chem. Phys., 1993, 98, 5648—5652 |
| [25] | Yanai T., Tew D. P., Handy N. C., Chem. Phys. Lett., 2004, 393, 51—56 |
| [26] | Ditchfield R., Hehre W. J., Pople J. A., J. Chem. Phys., 1971, 54, 724—728 |
| [27] | Francl M. M., Pietro W. J., Hehre W. J., Binkley J. S., DeFrees D. J., Pople J. A., Gordon M. S., J. Chem. Phys., 1982, 77, 3654—3665 |
| [28] | Miertus S., Scrocco E., Tomasi J., Chem. Phys., 1981, 55, 117—129 |
| [29] | Scalmani G., Frisch M. J., J. Chem. Phys., 2010, 132, 114—110 |
| [30] | Tao J. Y., Mu W. H., Chass G. A., Tang T. H., Fang D. C., Int. J. Quantum Chem., 2013, 113, 975—984 |
| [31] | Zhao Y., Truhlar D. G., J. Chem. Phys., 2006, 125, 194101-1—194101-5 |
| [32] | Chai J. D., Head-Gordon M., J. Chem. Phys., 2008, 128, 084106-1—084106-15 |
| [33] | McLean A. D., Chandler G. S., J. Chem. Phys., 1980, 72, 5639—5648 |
| [34] | Raghavachari K., Binkley J. S., Seeger R., Pople J. A., J. Chem. Phys., 1980, 72, 650—654 |
| [35] | Fang D.C., Thermo Program, Beijing Normal University,Beijing, 2013 |
| [36] | Trouton F., Philosophical Magazine, 1884, 18, 54—57 |
| [37] | Atkins P., Physical Chemistry, Oxford University Press, London, 1978 |
| [38] | Liang Y., Liu S., Xia Y., Li Y., Yu Z. X., Chem. Eur. J., 2008, 14, 4361—4373 |
| [39] | Lin S. H., Lau K. H., Volk L., Richardson W., Eyring H., Proc. Natl. Acad. Sci. USA, 1972, 69, 2778—2782 |
| [40] | Volk L., Richardson W., Lau K.H., Hall M., Lin S.H., J. Chem. Ed., 1977, 54, 95—97 |
| [1] | 王宁, 朱惠芳, 王璐, 张田田, 顾佳丽, 舒婕. 埃索美拉唑镁在特定溶液中的分子互变异构体结构表征及互变动力学的核磁共振波谱研究[J]. 高等学校化学学报, 2018, 39(9): 1919. |
| [2] | 韩蕾 朱彤 孙凤龙 尚静. 除草剂2-甲基-4-氯苯氧乙酸(MCPA)与OH自由基的气相化学反应[J]. 高等学校化学学报, 2006, 27(1): 112. |
| [3] | 何鼎胜, 马铭, 王艳. 三正辛胺-二甲苯液膜迁移Cd(Ⅱ)的研究[J]. 高等学校化学学报, 2000, 21(4): 605. |
| [4] | 叶建农, 金薇, 赵学伟, 方禹之. 蔗糖水解反应速率常数的毛细管电泳测定方法研究[J]. 高等学校化学学报, 1998, 19(1): 31. |
| [5] | 于浩, 刘若庄, 李宗和, 马思渝. C2H2++H2→C2H3++H反应振动选态速率常数的理论研究[J]. 高等学校化学学报, 1995, 16(3): 444. |
| [6] | 栗方星, 王红军, 马克勤. CH3COSbCl6催化四氢呋喃聚合的研究[J]. 高等学校化学学报, 1994, 15(5): 785. |
| [7] | 蒋亦芹, 刘宛乔, 唐开清, 廖展如, 阵沛玲, 石巨恩. 采样极谱法测定SOD模型化合物催化超氧离子歧化反应速率常数[J]. 高等学校化学学报, 1992, 13(11): 1444. |
| [8] | 张祖训. 单滴汞上可逆和不可逆过程电流~时间曲线方程式[J]. 高等学校化学学报, 1991, 12(3): 326. |
| [9] | 罗勤慧, 沈孟长, 高伟, 彭庆云. 光照法研究超氧化物歧化酶及其模型化合物与超氧离子的反应动力学[J]. 高等学校化学学报, 1990, 11(9): 928. |
| [10] | 沈家骢, 王国斌, 杨梅林, 郑莹光. 本体聚合中增长自由基的微环境效应[J]. 高等学校化学学报, 1990, 11(12): 1439. |
| [11] | 袁云程, 高大彬. 离子交换树脂催化合成丙烯酸-β-羟乙酯的动力学研究[J]. 高等学校化学学报, 1990, 11(11): 1259. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
