

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (6): 1212.doi: 10.7503/cjcu20180160
收稿日期:2018-03-01
出版日期:2018-06-10
发布日期:2018-05-22
基金资助:
LI Yingli, WANG Jingbo*( ), LI Xiangyuan
), LI Xiangyuan
Received:2018-03-01
Online:2018-06-10
Published:2018-05-22
Contact:
WANG Jingbo
E-mail:wangjingbo@scu.edu.cn
Supported by:摘要:
采用量子化学方法研究了十氢化萘低温燃烧的动力学机理, 获得了脱氢反应、 自由基加氧反应及1,5氢迁移反应等反应的动力学参数, 并在CBS-QB3水平下获得了相关物种的热力学参数, 通过过渡态理论计算获得了具有紧致过渡态反应的高压极限速率常数, 而无能垒反应的速率常数则由变分过渡态理论得到. 基于此机理分析了十氢化萘低温反应的动力学规律和热力学机制. 相比于链烷烃和单环烷烃, 十氢化萘自由基加氧反应的速率常数随温度变化较快, 1,5-氢迁移反应的能垒较高, 揭示了物质结构对反应动力学的影响. 热力学平衡常数分析结果表明, 在低温下十氢化萘自由基加氧反应起主导作用. 通过拟合获得了所有反应Arrhenius形式的速率常数, 这些参数可用于双环烷烃低温燃烧机理的构建和优化.
中图分类号:
TrendMD:
李颖丽, 王静波, 李象远. 十氢化萘低温燃烧反应的动力学机理. 高等学校化学学报, 2018, 39(6): 1212.
LI Yingli, WANG Jingbo, LI Xiangyuan. Kinetic Mechanism Study on Low Temperature for Decalin Combustion†. Chem. J. Chinese Universities, 2018, 39(6): 1212.
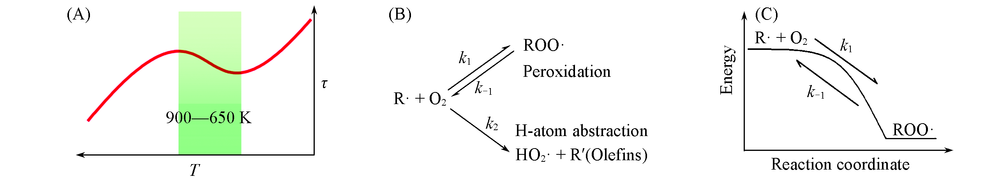
Fig.1 Negative temperature coefficient effect of low temperature ignition delay(A), the disproportionation reaction of radical oxidation(B) and the barrierless reaction for O2 addition(C)
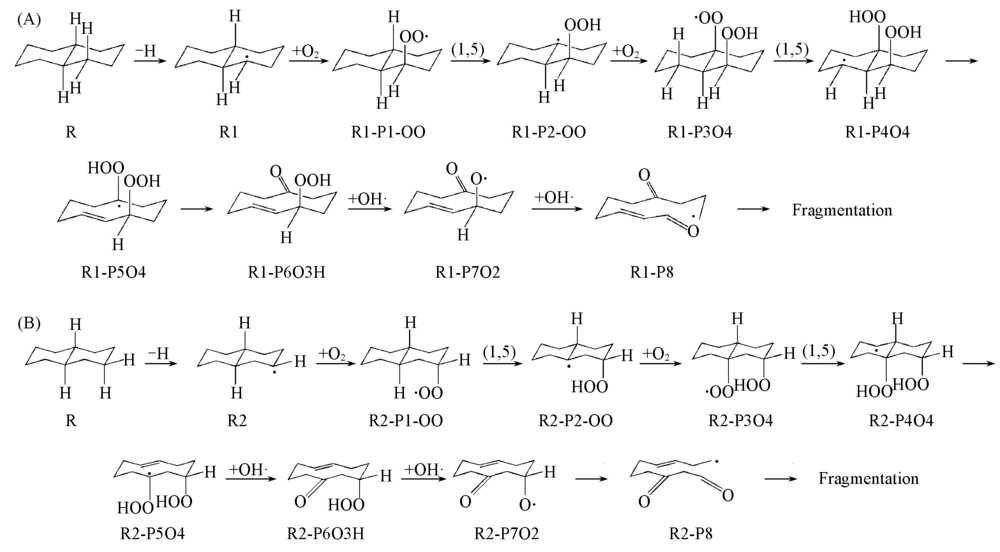
Fig.3 Low temperature oxidation reactions of decalin starting from C—H bond breaking at the α position(A) and β position(B)Species designation is also given.
| Number of reaction | Reaction | lgA* | Ea/(kJ·mol-1) |
|---|---|---|---|
| r1 | R=R1+H | 14.91 | 300.33 |
| r2 | R1+O2=R1-P1-OO | -0.62 | 63.79 |
| r3 | R1-P1-OO→R1-P2-OO | 10.23 | 186.90 |
| r4 | R1-P2-OO+O2=R1-P3O4 | -8.17 | -114.99 |
| r5 | R1-P3O4→R1-P4O4 | 5.82 | 181.46 |
| r6 | R1-P4O4→R1-P5O4 | 10.51 | 72.12 |
| r7 | R1-P5O4→R1-P6O3H+OH | 12.39 | 65.93 |
| r8 | R1-P6O3H→R1-P7O2+OH | 11.22 | 40.56 |
| r9 | R1-P7O2→R1-P8 | 13.28 | 221.98 |
| r10 | R=R2+H | 12.39 | 376.02 |
| r11 | R2+O2=R2-P1-OO | -15.59 | -4.77 |
| r12 | R2-P1-OO→R2-P2-OO | 13.04 | 214.23 |
| r13 | R2-P2-OO+O2=R2-P3O4 | -13.58 | -126.50 |
| r14 | R2-P3O4→R2-P4O4 | 8.25 | 177.35 |
| r15 | R2-P4O4→R2-P5O4 | 8.48 | 63.67 |
| r16 | R2-P5O4→R2-P6O3H+OH | 13.84 | 46.17 |
| r17 | R2-P6O3H→R2-P7O2+OH | 22.58 | 28.05 |
| r18 | R2-P7O2→R2-P8 | 11.26 | 214.78 |
Table 1 High-pressure limit rate parameters for low temperature oxidation reactions of decalin in the temperature range of 500—1500 K*
| Number of reaction | Reaction | lgA* | Ea/(kJ·mol-1) |
|---|---|---|---|
| r1 | R=R1+H | 14.91 | 300.33 |
| r2 | R1+O2=R1-P1-OO | -0.62 | 63.79 |
| r3 | R1-P1-OO→R1-P2-OO | 10.23 | 186.90 |
| r4 | R1-P2-OO+O2=R1-P3O4 | -8.17 | -114.99 |
| r5 | R1-P3O4→R1-P4O4 | 5.82 | 181.46 |
| r6 | R1-P4O4→R1-P5O4 | 10.51 | 72.12 |
| r7 | R1-P5O4→R1-P6O3H+OH | 12.39 | 65.93 |
| r8 | R1-P6O3H→R1-P7O2+OH | 11.22 | 40.56 |
| r9 | R1-P7O2→R1-P8 | 13.28 | 221.98 |
| r10 | R=R2+H | 12.39 | 376.02 |
| r11 | R2+O2=R2-P1-OO | -15.59 | -4.77 |
| r12 | R2-P1-OO→R2-P2-OO | 13.04 | 214.23 |
| r13 | R2-P2-OO+O2=R2-P3O4 | -13.58 | -126.50 |
| r14 | R2-P3O4→R2-P4O4 | 8.25 | 177.35 |
| r15 | R2-P4O4→R2-P5O4 | 8.48 | 63.67 |
| r16 | R2-P5O4→R2-P6O3H+OH | 13.84 | 46.17 |
| r17 | R2-P6O3H→R2-P7O2+OH | 22.58 | 28.05 |
| r18 | R2-P7O2→R2-P8 | 11.26 | 214.78 |
| Name of reaction | Specified reaction | lg[A/(mol-1·cm3·s-1)] | Ea/(kJ·mol-1) |
|---|---|---|---|
| JetSurF 2.0[ |  | 12.06 | -6.36 |
| r1[ |  | 10.52 | -1.09 |
| r2[ |  | 10.36 | -11.09 |
| r3[ |  | 12.28 | -4.35 |
| r4[ |  | 11.28 | -17.62 |
Table 2 Results of one-step O2 addition to ethylcyclohexane radicals in the literature
| Name of reaction | Specified reaction | lg[A/(mol-1·cm3·s-1)] | Ea/(kJ·mol-1) |
|---|---|---|---|
| JetSurF 2.0[ |  | 12.06 | -6.36 |
| r1[ |  | 10.52 | -1.09 |
| r2[ |  | 10.36 | -11.09 |
| r3[ |  | 12.28 | -4.35 |
| r4[ |  | 11.28 | -17.62 |
| Name of reaction | Specified reaction | lg(A/s-1) | Ea/(kJ·mol-1) |
|---|---|---|---|
| r1[ |  | 11.51 | 100.75 |
| r2[ |  | 11.77 | 97.28 |
| r[ |  | 11.42 | 85.10 |
| r3[ |  | 11.78 | 85.35 |
Table 3 Kinetic parameters of 1,5 H-shift reactions from the literature
| Name of reaction | Specified reaction | lg(A/s-1) | Ea/(kJ·mol-1) |
|---|---|---|---|
| r1[ |  | 11.51 | 100.75 |
| r2[ |  | 11.77 | 97.28 |
| r[ |  | 11.42 | 85.10 |
| r3[ |  | 11.78 | 85.35 |
| [1] | Hilpert M., Mora B. A., Ni J., Rule A. M., Nachman K. E., Curr. Environ. Health Rep., 2015, 2(4), 412—422 |
| [2] | Ogawa H., Ibuki T., Takayuki M. A., Miyamoto N., Energy Fuels, 2007, 21(3), 1517—1521 |
| [3] | Ranzi E., Energy & Fuels, 2006, 20(3), 1024—1032 |
| [4] | He J. N., Li Y. L., Zhang C. H., Li P., Li X. Y., Acta Phys. Chim. Sinica, 2015, 31(5), 836—842 |
| (何九宁, 李有亮, 张昌华, 李萍, 李象远. 物理化学学报, 2015, 31(5), 836—842) | |
| [5] | Battin-Leclerc F., Prog. Energ. Combust.Sci., 2008, 34(4), 440—498 |
| [6] | Kyungchan C., Violi A., J. Org. Chem., 2007, 72(9), 3179—85 |
| [7] | Dagaut P., Ristori A., Frassoldati A., Faravelli T., Dayma G., Ranzi E., Proc. Combust. Inst., 2013, 34(1), 289—296 |
| [8] | Zámostný P., Bělohlav Z., Starkbaumová L., Patera J., J. Anal. Appl. Pyrol., 2010, 87(2), 207—216 |
| [9] | Ondruschka B., Zimmermann G., Remmler M., Sedlackova M., Pola J., J. Anal. Appl. Pyrol., 1990, 18(1), 19—32 |
| [10] | Yang Y., Boehman A. L., Combust. Flame, 2010, 157(3), 495—505 |
| [11] | Zeng M., Li Y., Yuan W., Zhou Z., Wang Y., Zhang L., Qi F., Combust. Flame, 2016, 167(5), 228—237 |
| [12] | Zhu Y., Davidson D. F., Hanson R. K., Zhu Y., Davidson D. F., Hanson R. K., Combust. Flame, 2014, 161(2), 371—383 |
| [13] | Zhang W. F., Xian L. Y., Yong K. L., Acta Phys. Chim. Sin., 2016, 32(9), 2216—2222 |
| (张巍峰, 鲜雷勇, 雍康乐. 物理化学学报, 2016, 32(9), 2216—2222) | |
| [14] | Ranzi E., Frassoldati A., Grana R., Prog. Energ. Combust.Sci., 2012, 38(4), 468—501 |
| [15] | Tan N. X., Wang F., Liu A. K., Guo J. J., Xu J. Q., Li S. H., Li X. Y., The 29th Annual Meeting of the Chinese Chemical Societ., Beijing, 2014 |
| (谈宁馨, 王繁, 刘爱科, 郭俊江, 徐佳琪, 李树豪, 李象远. 中国化学会第29届学术年会摘要集, 北京, 2014) | |
| [16] | Zhang K., Banyon C., Bugler J., Curran H. J., Rodriguez A., Herbinet O., Battin-Leclerc F., B’Chirc C., Heuferc K. A., Combust. Flame, 2016, 172(10), 116—135 |
| [17] | Rashidi M. J. A., Mehl M., Pitz W. J., Mohamed S., Sarathy S. M., Combust. Flame, 2017, 183, 358—371 |
| [18] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.01, Gaussian Inc., Wallingford CT, 2009 |
| [19] | Becke A. D., J. Chem. Phys., 1998, 98(7), 5648—5652 |
| [20] | Lee C., Yang W., Parr R. G., Phys. Rev. B: Condens. Matter., 1988, 37(2), 785—789 |
| [21] | Gonzalez C., Schlegel H. B., J. Chem. Phys., 1989, 90(4), 2154—2161 |
| [22] | Montgomery J. A., Frisch M. J., Ochterski J. W., Petersson G. A., J. Chem. Phys., 1999, 110(6), 2822—2827 |
| [23] | Altarawneh M. K., Dlugogorski B. Z., Kennedy E. M., Mackie J. C., Combust. Flame, 2013, 160(1), 9—16 |
| [24] | NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database Number 101, Release 19, April 2018, Editor, Russell D. Johnson III, |
| [25] | Mokrushin V. T. W., Zachariah M., Knyazev V., Chem.Rate, Version 1.5.., National Institute of Standards and Technolog., Gaithersburg, MD, 2009 |
| [26] | Zádor J., Taatjes C. A., Fernandes R. X., Prog. Energ. Combust. Sci., 2011, 37(4), 371—421 |
| [27] | Li S. J., Tan N. X., Yao Q., Li Z. R., Li X. Y., Acta Phys. Chim. Sinica, 2015, 31(5), 859—865 |
| (李尚俊, 谈宁馨, 姚倩, 李泽荣, 李象远. 物理化学学报, 2015, 31(5), 859—865) | |
| [28] | Ning H. B., Gong C. M., Tan N. X., Li Z. R., Li X. Y., Combust. Flame, 2015, 162(11), 4167—4182 |
| [29] | Wang H., Dames E., Sirjean B., Sheen D.A., Tangko R., Violi A., Lai J. Y. W., Egolfopoulos F. N., Davidson D. F., Hanson R. K., Bowman C. T., Law C. K., Tsang W., Cernansky N. P., Miller D. L., Lindstedt R. P.,A High-temperature Chemical Kinetic Model of n-alkane(up to n-dodecane), Cyclohexane, and Methyl-, Ethyl-, n-Propyl and n-Butyl-cyclohexane Oxidation at High Temperatures, JetSurF Version 2.0, September 19, 2010() |
| [30] | Xing L., Zhang L., Zhang F., Jiang J., Combust. Flame, 2017, 182(8), 216—224 |
| [31] | Miyoshi A., J. Phys. Chem. A, 2011, 115(15), 3301—3325 |
| [1] | 任娜娜, 薛洁, 王治钒, 姚晓霞, 王繁. 热力学数据对1, 3-丁二烯燃烧特性的影响[J]. 高等学校化学学报, 2022, 43(6): 20220151. |
| [2] | 李宜蔚, 申屠江涛, 王静波, 李象远. 燃烧反应机理构建的极小反应网络方法: C1燃料燃烧[J]. 高等学校化学学报, 2021, 42(6): 1871. |
| [3] | 李象远, 申屠江涛, 李宜蔚, 李娟琴, 王静波. 燃烧反应机理构建的极小反应网络方法 |
| [4] | 李象远,姚晓霞,申屠江涛,孙晓慧,李娟琴,刘明夏,许诗敏. 燃烧反应机理构建的双参数速率常数方法[J]. 高等学校化学学报, 2020, 41(3): 512. |
| [5] | 王宁, 朱惠芳, 王璐, 张田田, 顾佳丽, 舒婕. 埃索美拉唑镁在特定溶液中的分子互变异构体结构表征及互变动力学的核磁共振波谱研究[J]. 高等学校化学学报, 2018, 39(9): 1919. |
| [6] | 方升, 刘静静, 段雪梅, 陶福明, 刘靖尧. 大气中一元酸催化亚硫酸分解反应的从头算及动力学研究[J]. 高等学校化学学报, 2017, 38(8): 1390. |
| [7] | 马倩, 王渭娜, 赵强莉, 刘峰毅, 王文亮. Criegee中间体RCHOO(R=H,CH3)与NCO的反应机理[J]. 高等学校化学学报, 2017, 38(4): 613. |
| [8] | 王睿, 李一粒, 凤旭凯, 宋亮, 张田雷, 王竹青, 靳玲侠, 张强, 许琼, 王志银. n(H2O)(n=1,2)在HO2+NO |
| [9] | 高志芳, 王渭娜, 马倩, 刘峰毅, wlwang@snnu.edu.cn. Criegee中间体CH3CHOO与OH自由基反应机理的理论研究[J]. 高等学校化学学报, 2016, 37(3): 513. |
| [10] | 朱鹏, 段雪梅, 刘靖尧. CF2ClC(O)OCH2CH3+OH的反应机理及动力学性质的理论研究[J]. 高等学校化学学报, 2016, 37(1): 79. |
| [11] | 王宽, 陈建刚, 王伯周, 吕剑, 王文亮, 刘峰毅, 周诚, 廉鹏, 刘忠文, 刘昭铁. FOX-12制备过程的反应机理及动力学[J]. 高等学校化学学报, 2015, 36(3): 531. |
| [12] | 胡茜茜, 杨俊英, 谢代前. 反应N+NH→N2+H的态-态量子动力学研究[J]. 高等学校化学学报, 2015, 36(11): 2198. |
| [13] | 李悦, 方德彩. 叔丁氧基自由基引发氢迁移过程的理论研究[J]. 高等学校化学学报, 2015, 36(10): 1954. |
| [14] | 马鹏, 宋金瓯, 宋崇林, 吕刚, 陈朝旭, 杨传旺. C2H3与C2H5OH和CH3HCO间的脱氢反应对乙醇-碳氢混合燃料燃烧过程的影响[J]. 高等学校化学学报, 2015, 36(1): 149. |
| [15] | 郭莎, 王渭娜, 靳玲侠, 王帅, 王文亮. CH3CH2O+HCHO反应机理及主通道速率常数[J]. 高等学校化学学报, 2014, 35(6): 1300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||