

高等学校化学学报 ›› 2022, Vol. 43 ›› Issue (12): 20220375.doi: 10.7503/cjcu20220375
收稿日期:2022-05-25
出版日期:2022-12-10
发布日期:2022-07-21
基金资助:
ZHANG Qingpeng, GUAN Guoqiang, LIU Huiyi, LU Chang, ZHOU Ying( ), SONG Guosheng(
), SONG Guosheng( )
)
Received:2022-05-25
Online:2022-12-10
Published:2022-07-21
Contact:
ZHOU Ying, SONG Guosheng
E-mail:zhouying2021@hnu.edu.cn;songgs@hnu.edu.cn
Supported by:摘要:
磁粒子成像是基于功能和断层影像技术检测磁性纳米粒子空间分布的示踪方法, 具有正向的对比信号、 较低的组织背景、 无限的组织穿透深度、 非侵入性成像以及无电离辐射等优点, 是近年来一种很有前途的生物医学成像技术. 磁粒子成像信号是通过在无场点切换磁性纳米粒子的磁自旋矢量来产生的. 磁粒子成像的灵敏度和空间分辨率都高度依赖于作为磁粒子成像示踪剂的磁性纳米粒子本身的磁性能, 因此目前的研究主要集中在磁性纳米粒子的设计和合成上. 本文重点介绍了磁粒子成像示踪剂的最新研究进展, 总结了可作为磁粒子成像示踪剂的磁性纳米粒子的种类、 合成方法、 性能以及生物医学应用, 以期为磁粒子成像的未来研究提供参考.
中图分类号:
TrendMD:
张庆鹏, 关国强, 刘慧怡, 陆畅, 周颖, 宋国胜. 磁粒子成像示踪剂的研究进展. 高等学校化学学报, 2022, 43(12): 20220375.
ZHANG Qingpeng, GUAN Guoqiang, LIU Huiyi, LU Chang, ZHOU Ying, SONG Guosheng. Recent Development of Magnetic Particle Imaging Tracers. Chem. J. Chinese Universities, 2022, 43(12): 20220375.
| Imaging modality | Input | Penetration depth | Spatial resolution | Temporal resolution | Sensitivity/(mol·L-1) |
|---|---|---|---|---|---|
| MPI | Radio frequency | Unlimited | ~500 mm | ms | 10-6 |
| MRI | Radio frequency | Unlimited | 25—100 mm | min—h | 10-3—10-5 |
| Optical | Light | <2 cm | 2—3 mm | s—min | 10-9—10-12 |
| Photoacoustics | Light | <6 cm | 5 μm—1 mm | s—min | 10-9—10-11 |
| CT | X⁃Rays | Unlimited | 25—200 μm | s—min | 10-3 |
| PET | Radionuclide | Unlimited | <1 mm | s—min | 10-11—10-12 |
| SPECT | Radionuclide | Unlimited | 0.5—2 mm | min | 10-10—10-11 |
| Ultrasound | Sound waves | Meter | 10—100 mm | s—min | 10-6—10-9 |
Table 1 Comparison of different imaging modalities
| Imaging modality | Input | Penetration depth | Spatial resolution | Temporal resolution | Sensitivity/(mol·L-1) |
|---|---|---|---|---|---|
| MPI | Radio frequency | Unlimited | ~500 mm | ms | 10-6 |
| MRI | Radio frequency | Unlimited | 25—100 mm | min—h | 10-3—10-5 |
| Optical | Light | <2 cm | 2—3 mm | s—min | 10-9—10-12 |
| Photoacoustics | Light | <6 cm | 5 μm—1 mm | s—min | 10-9—10-11 |
| CT | X⁃Rays | Unlimited | 25—200 μm | s—min | 10-3 |
| PET | Radionuclide | Unlimited | <1 mm | s—min | 10-11—10-12 |
| SPECT | Radionuclide | Unlimited | 0.5—2 mm | min | 10-10—10-11 |
| Ultrasound | Sound waves | Meter | 10—100 mm | s—min | 10-6—10-9 |
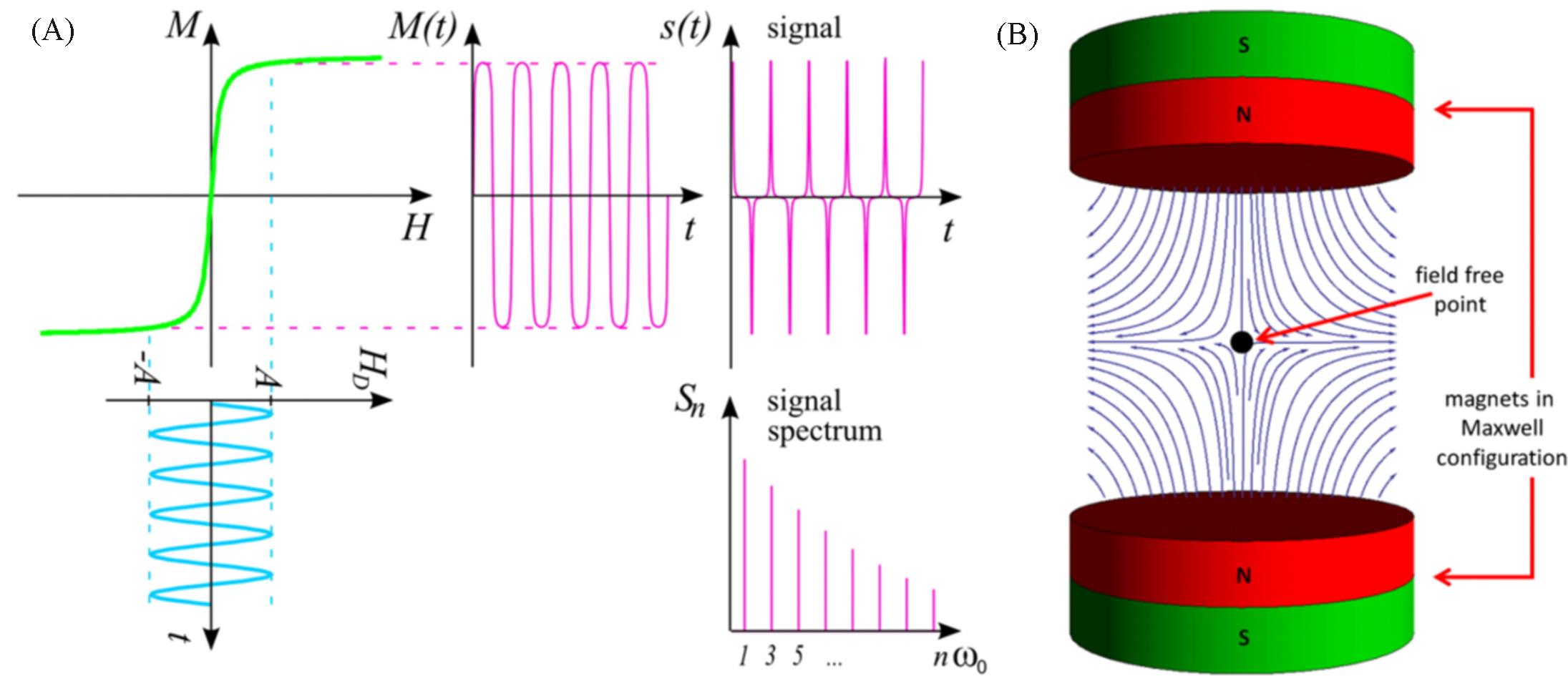
Fig.1 Schematic diagram of MPI signal generation[13](A) The upper left curve exemplifies the relation between the external magnetic drive field HD(usually measured in A/m or mT/μ0) and the magnetization of the particle M. The spectrum Sn of the signal s(t) generated by the changing magnetization then contains higher harmonics of the excitation frequency; (B) a selection field generated by 2 magnets in Maxwell configuration, that is, with opposing poles. Copyright 2012, Society of Cardiovascular Computed Tomography.
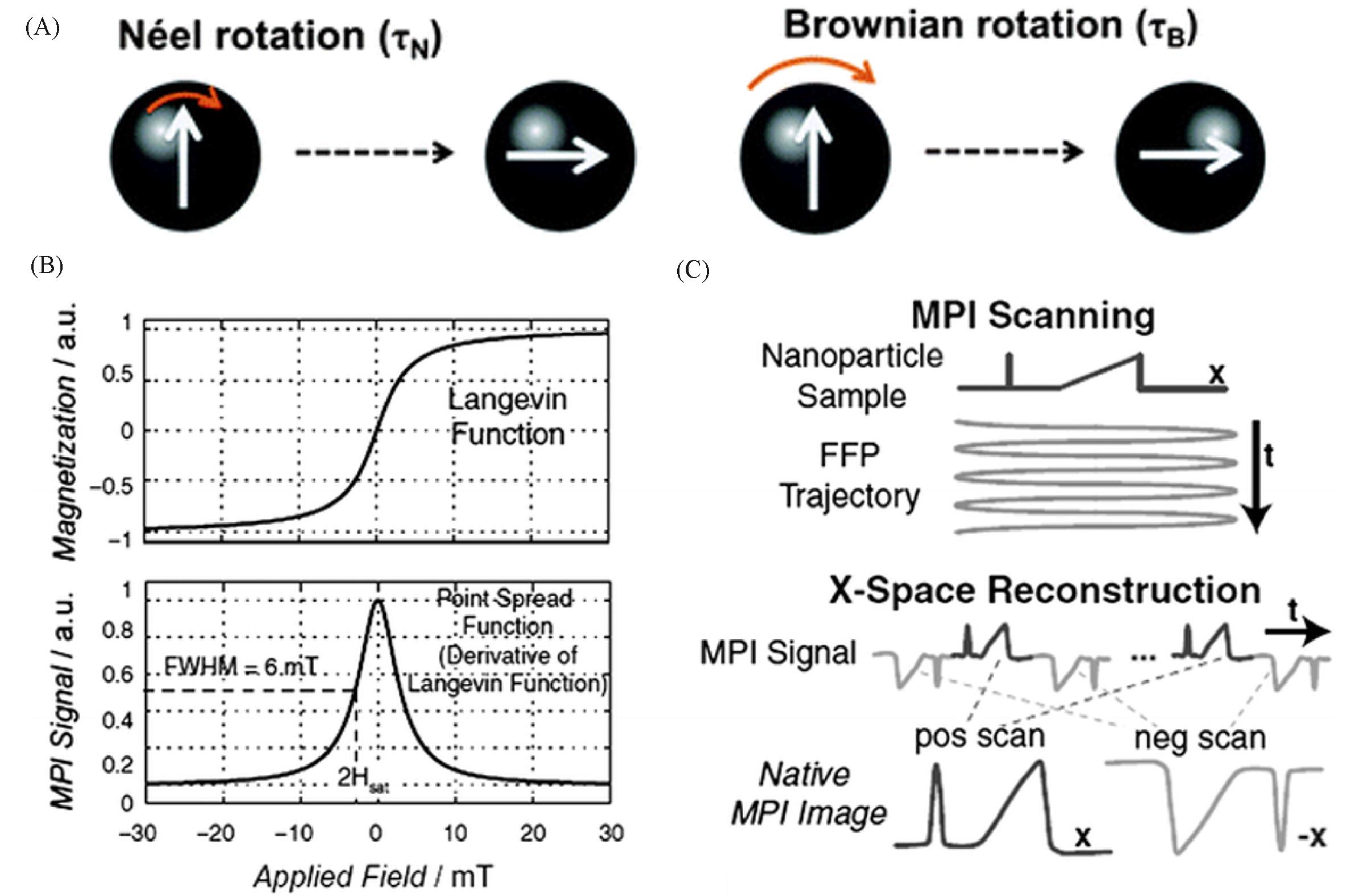
Fig.2 Schematic diagram of magnetic relaxation mechanism and X⁃space image reconstruction technique[16](A) Schematic representation of the magnetic relaxation mechanisms; (B) top figure shows magnetization of SPIONs modelled using a Langevin function; bottom figure shows the point spread function(PSF) as the derivative of the Langevin function; (C) X-space reconstruction involving a two-step process of velocity compensation and gridding of the instantaneous signal to the position of the FFP to form a native MPI image. Copyright 2012, John Wiley and Sons, Inc.
| MNPs | Composition | Size/nm | Sharp | Ms/(emu·g-1)* | MPI Signal(MNPs/Vivotrax) | Ref. |
|---|---|---|---|---|---|---|
| Vivotrax | Fe | ca. 10 | Amorphous | 54 | 1.0 | [ |
| SIONs⁃17 | Fe | 17 | Sphere | 32 | 0.5 | [ |
| CIONs⁃22 | Fe | 22 | Cube | 54 | 4.2 | [ |
| CIONs⁃26 | Fe | 26 | Cube | 72 | 3.5 | [ |
| CIONs⁃46 | Fe | 46 | Cube | 61 | 0.5 | [ |
| ZnFe2O4/C | Zn, Fe, C | 7—9 | Spindle | 32 | 4.7 | [ |
| CoFe2O4/C | Ni, Fe, C | 7—9 | Spindle | 36 | 1.8 | [ |
| MnFe2O4/C | Mn, Fe, C | 7—9 | Spindle | 50 | 2.1 | [ |
| ZnFe2O4 | Zn, Fe | 7—9 | Sphere | 48 | 0.6 | [ |
| γ⁃Fe2O3/C | Fe, C | 7—9 | Spindle | 48 | 1.9 | [ |
| FeCo@C | Fe, Co, C | 10 | Core⁃shell | 192 | 6.8 | [ |
| Fe@Fe3O4 | Fe | 14 | Core⁃shell | 176 | 0.8 | [ |
Table 2 Comparison of MPI performance of different MNPs
| MNPs | Composition | Size/nm | Sharp | Ms/(emu·g-1)* | MPI Signal(MNPs/Vivotrax) | Ref. |
|---|---|---|---|---|---|---|
| Vivotrax | Fe | ca. 10 | Amorphous | 54 | 1.0 | [ |
| SIONs⁃17 | Fe | 17 | Sphere | 32 | 0.5 | [ |
| CIONs⁃22 | Fe | 22 | Cube | 54 | 4.2 | [ |
| CIONs⁃26 | Fe | 26 | Cube | 72 | 3.5 | [ |
| CIONs⁃46 | Fe | 46 | Cube | 61 | 0.5 | [ |
| ZnFe2O4/C | Zn, Fe, C | 7—9 | Spindle | 32 | 4.7 | [ |
| CoFe2O4/C | Ni, Fe, C | 7—9 | Spindle | 36 | 1.8 | [ |
| MnFe2O4/C | Mn, Fe, C | 7—9 | Spindle | 50 | 2.1 | [ |
| ZnFe2O4 | Zn, Fe | 7—9 | Sphere | 48 | 0.6 | [ |
| γ⁃Fe2O3/C | Fe, C | 7—9 | Spindle | 48 | 1.9 | [ |
| FeCo@C | Fe, Co, C | 10 | Core⁃shell | 192 | 6.8 | [ |
| Fe@Fe3O4 | Fe | 14 | Core⁃shell | 176 | 0.8 | [ |
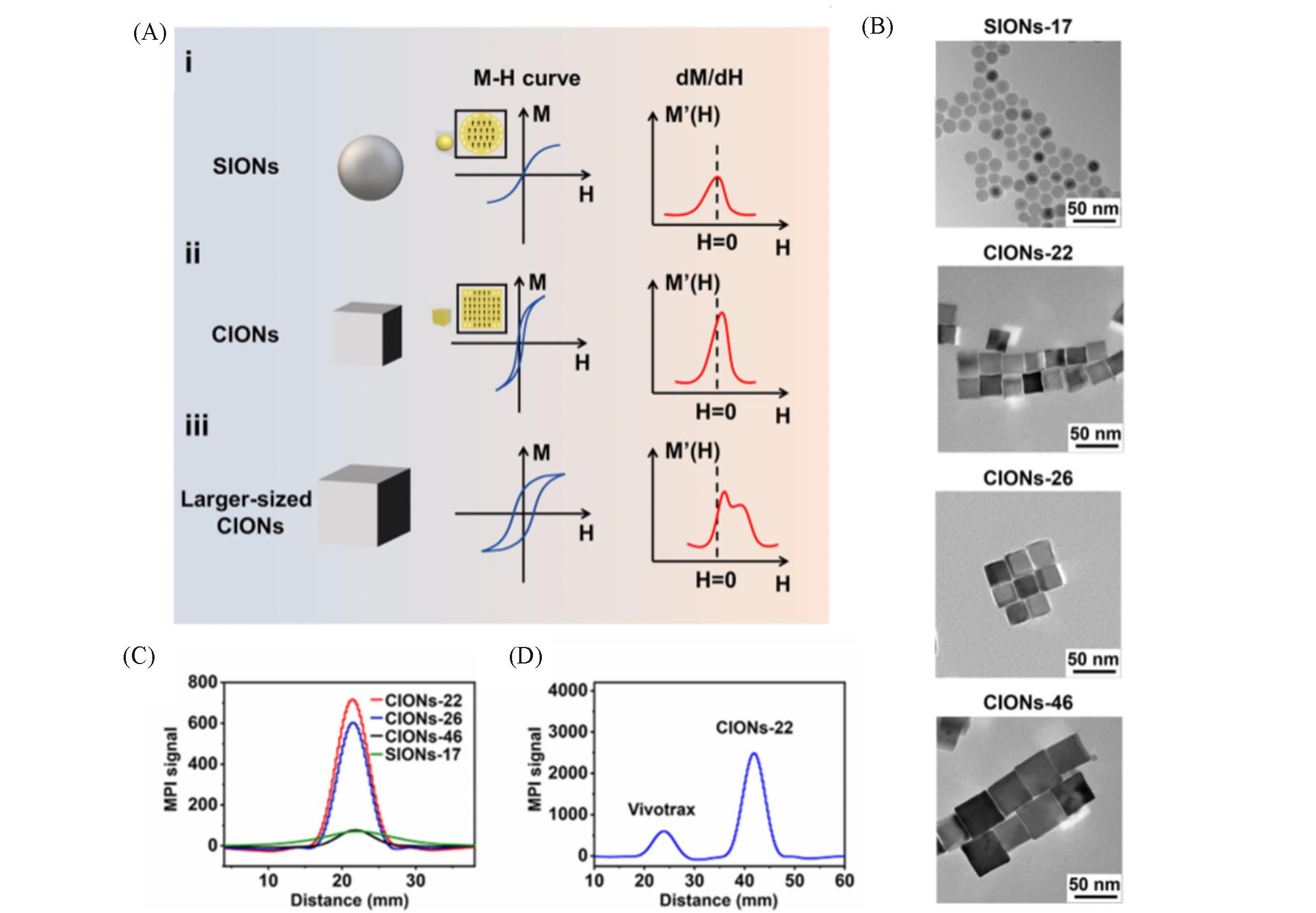
Fig.3 IONPs of different sizes and shapes act as MPI tracers[18](A) Schematic diagram of IONPs shape and size effects on MPI performance; (B) TEM images of SIONs-17 and CIONs of different sizes; (C) MPI performance of SIONs-17 and CIONs of different sizes at 1.7 mmol/L Fe concentration; (D) CIONs-22 versus commercial Vivotrax MPI performance. Copyright 2020, American Chemical Society.
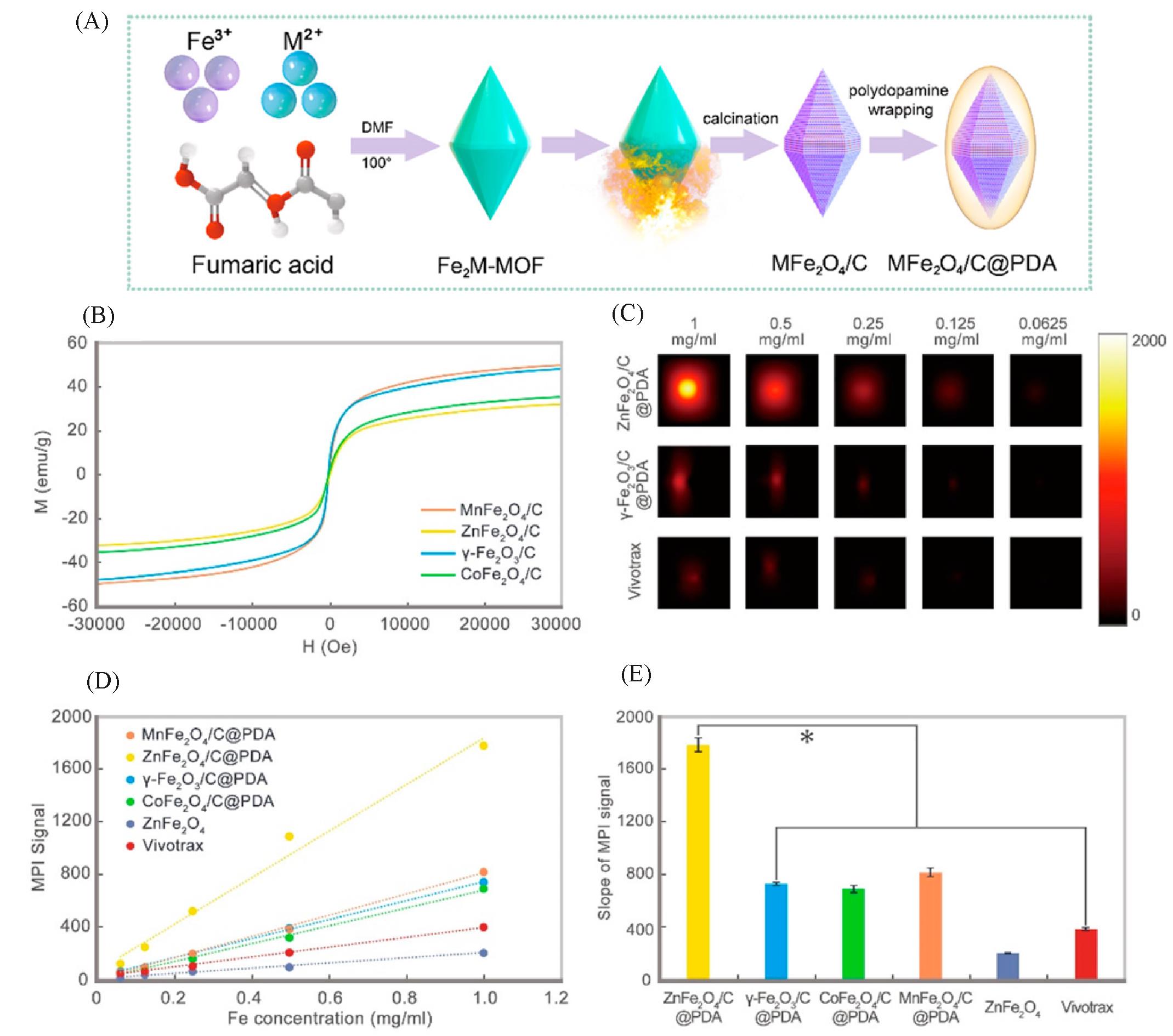
Fig.4 Carbon loading and metal doping improve the performance of MPI tracers[17](A) Schematic diagram of the synthesis progress of MFe2O4/C@PDA; (B) vibrating sample magnetometer (VSM) measurement for all samples; (C) MPI images of γ-Fe2O3/C@PDA, ZnFe2O4/C@PDA, and Vivotrax at series concentrations; (D) the plot and (E) slope of MPI signals of all samples. Copyright 2021, American Chemical Society.
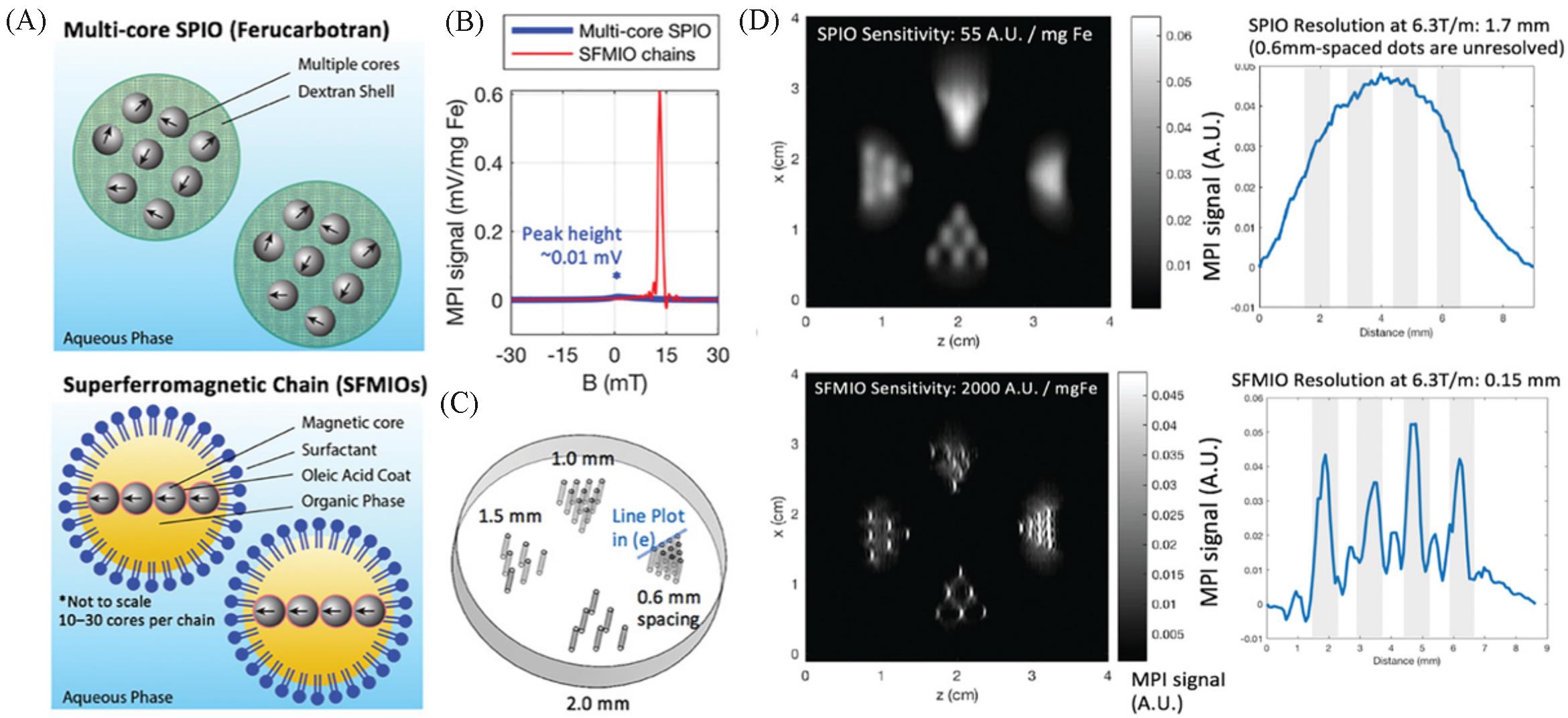
Fig.5 Order⁃of⁃magnitude mass sensitivity and resolution gains using superferro magnetic chains(SFMIOs)[25](A) Schematic of the SFMIO nanoparticle design compared to standard SPIOs; (B) MPI point spread function shows SFMIO signal peak is orders-of-magnitude taller and sharper than standard multi-core SPIO ferucarbotran; (C) imaging phantom of 0.3 mm radii wells with varying well-to-well spacing(mm); (D) MPI images of panel (C). Copyright 2021, Wiley-VCH GmbH.
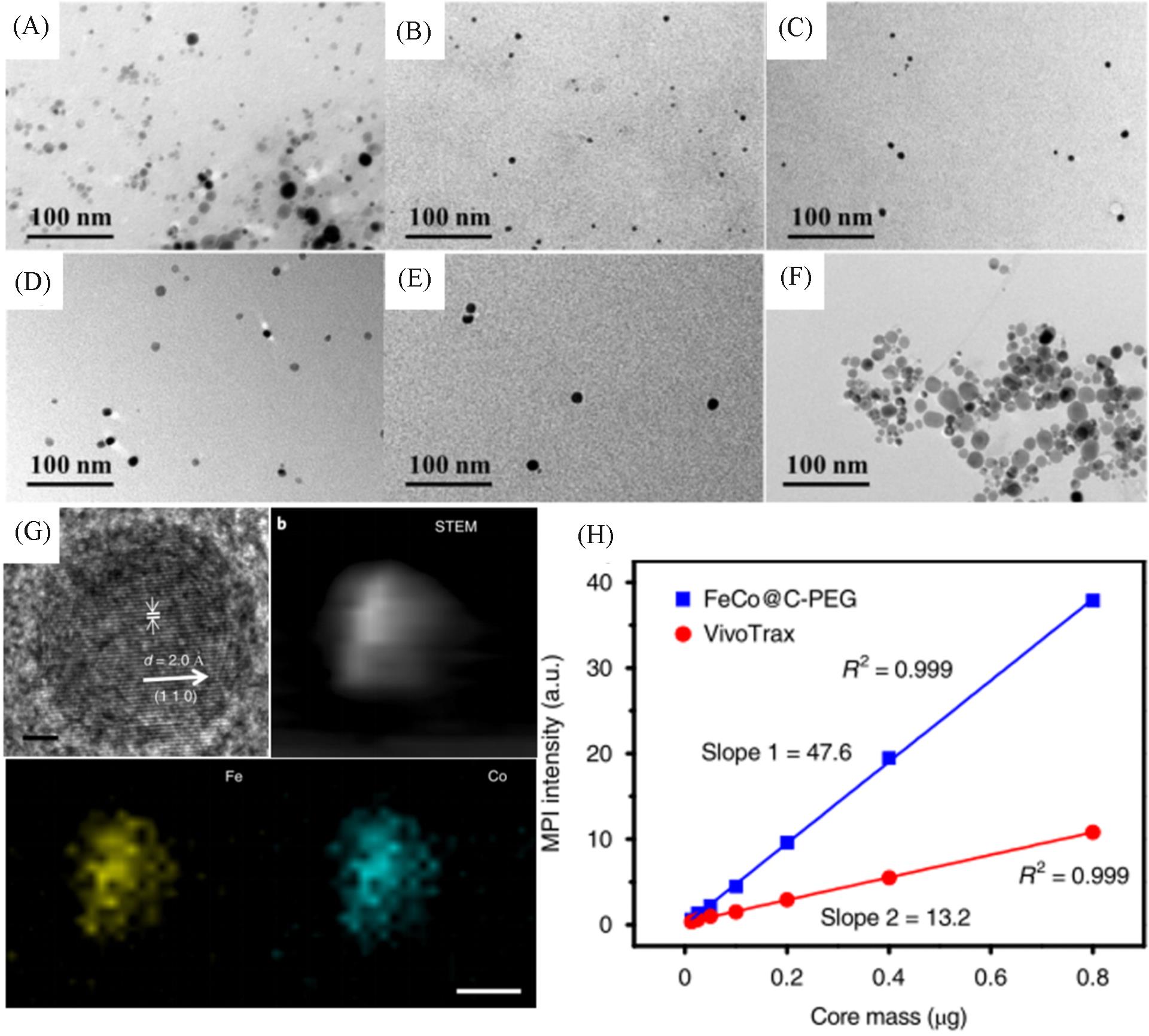
Fig.6 Representation and MPI performance of FeCo@C⁃PEG[19](A) TEM image of FeCo@C-PEG before density gradient separation; (B—F) TEM images of FeCo@C-PEG after density gradient separation; (G) HR-TEM and HAADF-STEM images of FeCo@C and element mapping; (H) MPI signals of FeCo@C-PEG and Vivotrax versus the nanoparticle core mass. Copyright 2020, the authors.
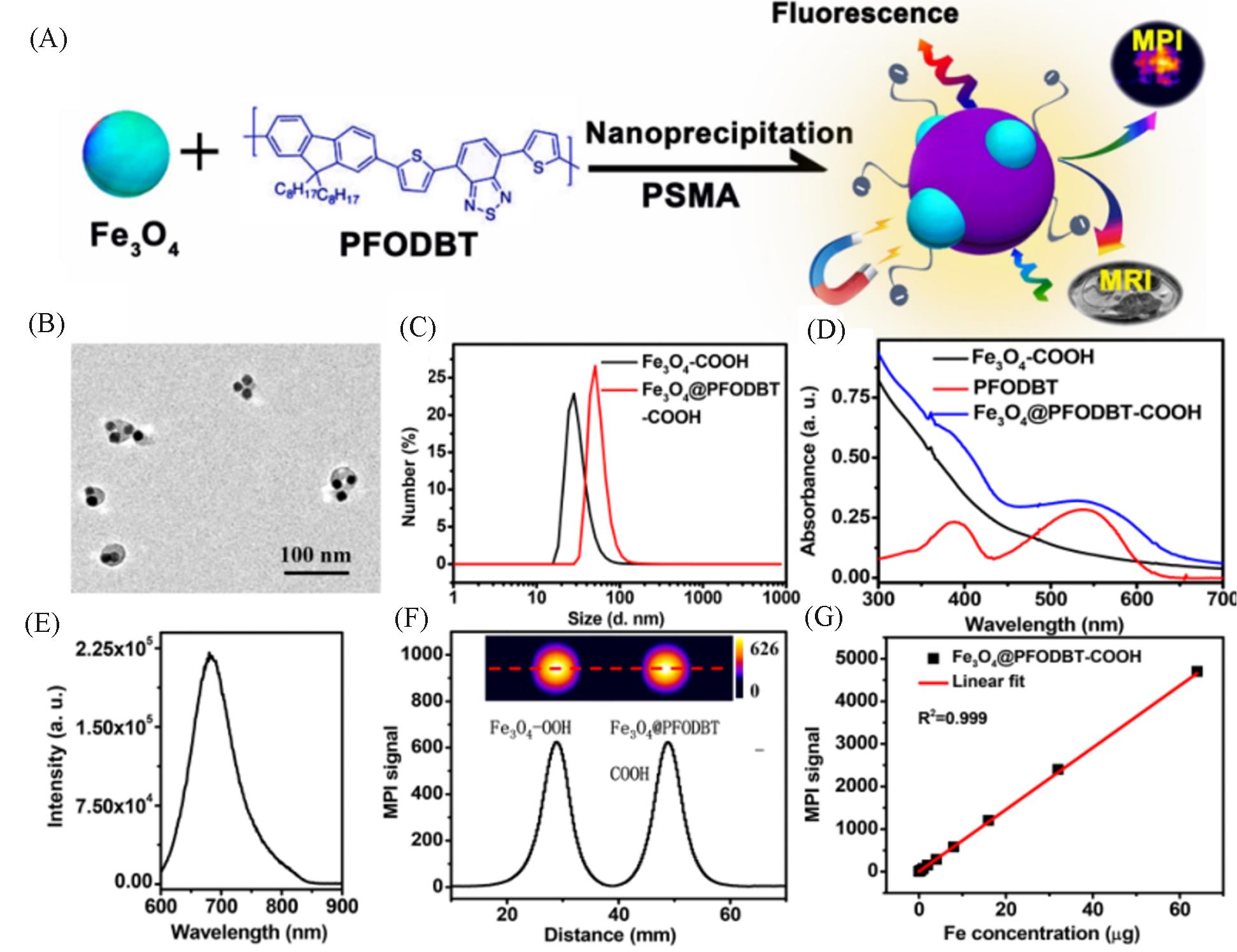
Fig.8 Synthesis and MPI performance of Janus MNPs[35](A) Schematic of the preparation of Fe3O4@PFODBT Janus nanoparticles through nanoprecipitation; (B) TEM image of Fe3O4@PFODBT Janus nanoparticles; (C) DLS size of Fe3O4 and Fe3O4@PFODBT in PBS; (D) UV-Vis absorption spectra of PFODBT in THF, and Fe3O4 and Fe3O4@PFODBT in PBS; (E) fluorescence spectrum of Fe3O4@PFODBT(excited at 540 nm); (F) two-dimensional projection MPI scanning of Fe3O4 and Fe3O4@PFODBT with the same amount of Fe(8 μg) in 200 μL of H2O, and their corresponding linear scanning MPI spectrum; (G) plot of MPI signals of Fe3O4@PFODBT versus amounts of Fe in 200 μL of H2O. Copyright 2018, American Chemical Society.
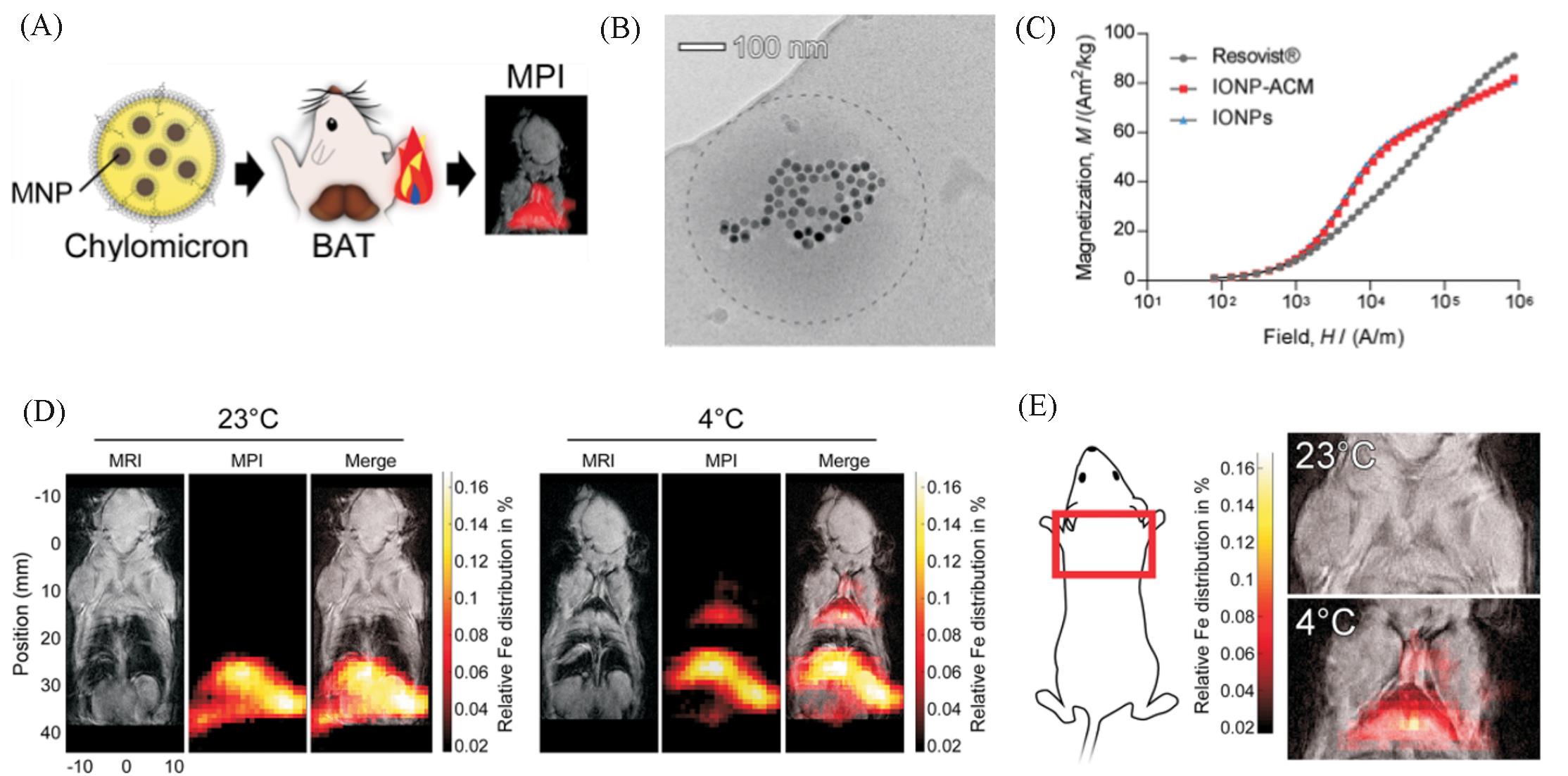
Fig.9 MPI tracers for quantification of lipoprotein uptake in vivo[37](A) Scheme shows quantification of lipoprotein uptake in vivo using MPI; (B) representative cryo-TEM image of IONP-loaded ACM. Dashed line outlines the circumference of the ACM. (C) Representative cryo-TEM image of IONP-loaded ACM. Dashed line outlines the circumference of the ACM. (D) Representative whole-body MRI and MPI images of mice injected with ZnMNP-ACM after exposure to either 23 or 4 ℃; (E) representative MPI images of the interscapular area(red box) with MRI overlay.Copyright 2020 American Chemical Society.
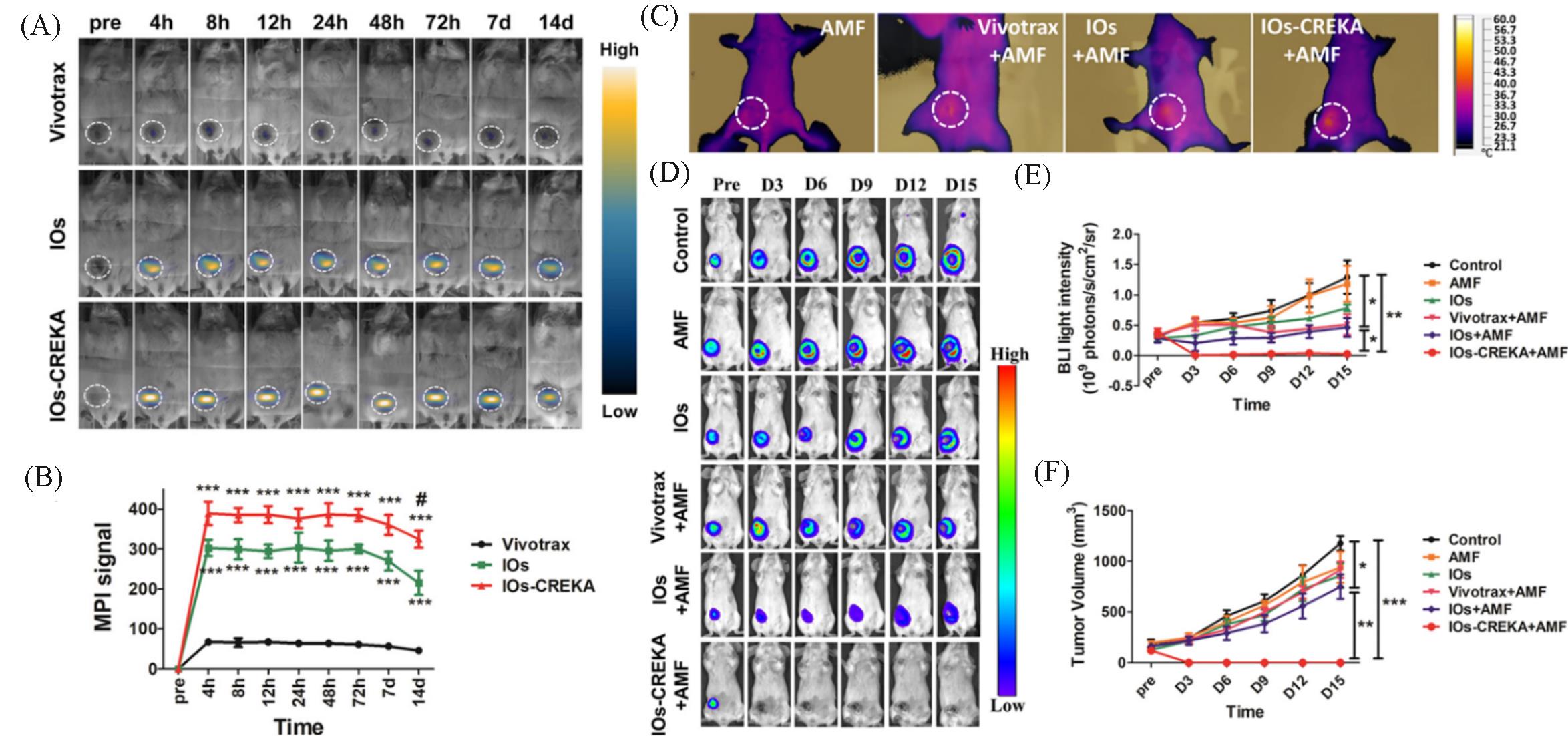
Fig.11 MPI tracers for cancer imaging and MHT[50](A) In vivo dynamic MPI of mice intratumorally injected with Vivotrax, IOs, and IOs-CREKA NPs, respectively; (B) MPI signal calculation and comparison between groups; (C) increase in the temperature induced by the MHT with different NPs treatment; (D) therapeutic efficacy of the magnetic hyperthermia dynamically monitored by BLI in different groups; (E, F) the calculation and comparison of the tumor bioluminescence intensity(E) and tumor volume changes(F). Copyright 2019, American Chemical Society.
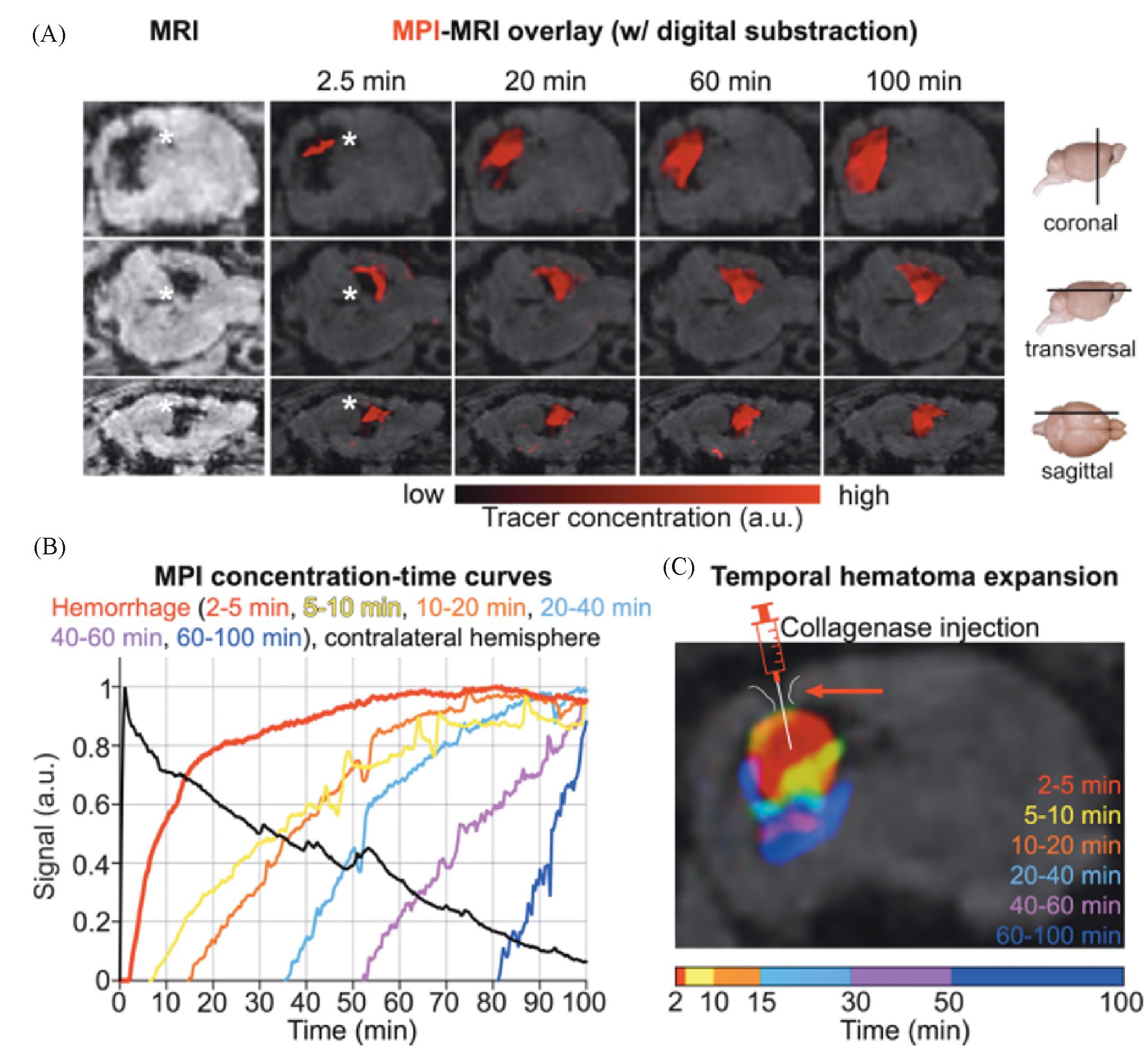
Fig.12 Rapid detection of intracranial hemorrhage with MPI[54](A) Early detection and expansion of the intracranial hemorrhage after the tracer injection at several time points on fused MPI/MRI slices(upper row: coronal sections; middle row: transversal sections; lower row: sagittal sections; the white asterisk indicates the hemorrhage in MRI and MPI; the signal information from these data sets was converted into a concentration⁃time curve on a voxel by pixel basis); (B) only (1.80±0.3) min passed between the tracer bolus arrival in the brain and the hemorrhage detection (bleeding continued up to 100 min; the extravasation of the tracer and expansion of the hemorrhage could be monitored in real time); (C) color coding the tracer arrival time allowed for the differentiation of bleeding areas of different age(the needle tip of the syringe marks the collagenase injection site for hemorrhage induction). Copyright 2020, American Chemical Society.
| 1 | Smith B. R., Gambhir S. S., Chem. Rev., 2017, 117(3), 901—986 |
| 2 | Rudin M., Weissleder R., Nat. Rev. Drug Discovery, 2003, 2(2), 123—131 |
| 3 | Chen F., Teng L., Lu C., Zhang C., Rong Q., Zhao Y., Yang Y., Wang Y., Song G., Zhang X., Anal. Chem., 2020, 92(19), 13452—13461 |
| 4 | Gleich B., Weizenecker J., Nature, 2005, 435(7046), 1214—1217 |
| 5 | Pablico⁃Lansigan M. H., Situ S. F., Samia A. C. S., Nanoscale, 2013, 5(10), 4040—4055 |
| 6 | Wu L. C., Zhang Y., Steinberg G., Qu H., Huang S., Cheng M., Bliss T., Du F., Rao J., Song G., Pisani L., Doyle T., Conolly S., Krishnan K., Grant G., Wintermark M., Am. J. Neuroradiol., 2019, 40(2), 206 |
| 7 | Bulte J. W. M., Adv. Drug Del. Rev., 2019, 138, 293—301 |
| 8 | Knopp T., Gdaniec N., Möddel M., Physics in Medicine & Biology, 2017, 62(14), R124—R178 |
| 9 | Sun C., Lee J. S. H., Zhang M., Adv. Drug Del. Rev., 2008, 60(11), 1252—1265 |
| 10 | Lu C., Han L., Wang J., Wan J., Song G., Rao J., Chem. Soc. Rev., 2021, 50(14), 8102—8146 |
| 11 | Yu E. Y., Chandrasekharan P., Berzon R., Tay Z. W., Zhou X. Y., Khandhar A. P., Ferguson R. M., Kemp S. J., Zheng B., Goodwill P. W., Wendland M. F., Krishnan K. M., Behr S., Carter J., Conolly S. M., ACS Nano, 2017, 11(12), 12067—12076 |
| 12 | Bauer L. M., Situ S. F., Griswold M. A., Samia A. C. S., J. Phys. Chem. Lett., 2015, 6(13), 2509—2517 |
| 13 | Borgert J., Schmidt J. D., Schmale I., Rahmer J., Bontus C., Gleich B., David B., Eckart R., Woywode O., Weizenecker J., Schnorr J., Taupitz M., Haegele J., Vogt F. M., Barkhausen J., J. Cardiovasc. Comput. Tomogr., 2012, 6(3), 149—153 |
| 14 | Biederer S., Knopp T., Sattel T. F., Lüdtke⁃Buzug K., Gleich B., Weizenecker J., Borgert J., Buzug T. M., J. Phys. D: Appl. Phys., 2009, 42(20), 205007 |
| 15 | Khandhar A. P., Ferguson R. M., Arami H., Krishnan K. M., Biomaterials, 2013, 34(15), 3837—3845 |
| 16 | Goodwill P. W., Saritas E. U., Croft L. R., Kim T. N., Krishnan K. M., Schaffer D. V., Conolly S. M., Adv. Mater., 2012, 24(28), 3870—3877 |
| 17 | Jiang Z., Han X., Du Y., Li Y., Li Y., Li J., Tian J., Wu A., Nano Lett., 2021, 21(7), 2730—2737 |
| 18 | Wang Q., Ma X., Liao H., Liang Z., Li F., Tian J., Ling D., ACS Nano, 2020, 14(2), 2053—2062 |
| 19 | Song G., Kenney M., Chen Y. S., Zheng X., Deng Y., Chen Z., Wang S. X., Gambhir S. S., Dai H., Rao J., Nat. Biomed. Eng., 2020, 4(3), 325—334 |
| 20 | Gloag L., Mehdipour M., Ulanova M., Mariandry K., Nichol M. A., Hernández⁃Castillo D. J., Gaudet J., Qiao R., Zhang J., Nelson M., Thierry B., Alvarez⁃Lemus M. A., Tan T. T., Gooding J. J., Braidy N., Sachdev P. S., Tilley R. D., Chem. Commun., 2020, 56(24), 3504—3507 |
| 21 | Kim D., Lee N., Park M., Kim B. H., An K., Hyeon T., J. Am. Chem. Soc., 2009, 131(2), 454—455 |
| 22 | Wu L., Mendoza⁃Garcia A., Li Q., Sun S., Chem. Rev., 2016, 116(18), 10473—10512 |
| 23 | Ferguson R. M., Minard K. R., Khandhar A. P., Krishnan K. M., Med. Phys., 2011, 38(3), 1619—1626 |
| 24 | Ferguson R. M., Khandhar A. P., Krishnan K. M., J. Appl. Phys., 2012, 111(7), 07B318 |
| 25 | Tay Z. W., Savliwala S., Hensley D. W., Fung K. L. B., Colson C., Fellows B. D., Zhou X., Huynh Q., Lu Y., Zheng B., Chandrasekharan P., Rivera‐Jimenez S. M., Rinaldi‐Ramos C. M., Conolly S. M., Small Methods, 2021, 5(11), 2100796 |
| 26 | Seo W. S., Lee J. H., Sun X., Suzuki Y., Mann D., Liu Z., Terashima M., Yang P. C., Mcconnell M. V., Nishimura D. G., Dai H., Nat. Mater., 2006, 5(12), 971—976 |
| 27 | Yu J., Yang C., Li J., Ding Y., Zhang L., Yousaf M. Z., Lin J., Pang R., Wei L., Xu L., Sheng F., Li C., Li G., Zhao L., Hou Y., Adv. Mater., 2014, 26(24), 4114—4120 |
| 28 | Gao J., Liang G., Cheung J. S., Pan Y., Kuang Y., Zhao F., Zhang B., Zhang X., Wu E. X., Xu B., J. Am. Chem. Soc., 2008, 130(35), 11828—11833 |
| 29 | Zeng J., Jing L., Hou Y., Jiao M., Qiao R., Jia Q., Liu C., Fang F., Lei H., Gao M., Adv. Mater., 2014, 26(17), 2694—2698 |
| 30 | Zeng H., Li J., Wang Z. L., Liu J. P., Sun S., Nano Lett., 2004, 4(1), 187—190 |
| 31 | Meiklejohn W. H., J. Appl. Phys., 1962, 33(3), 1328—1335 |
| 32 | Chai Y., Feng F., Li Q., Yu C., Feng X., Lu P., Yu X., Ge M., Wang X., Yao L., J. Am. Chem. Soc., 2019, 141(8), 3366—3370 |
| 33 | Chien C. C., Chen H. H., Lai S. F., Wu K. C., Cai X., Hwu Y., Petibois C., Chu Y., Margaritondo G., J. Nanobiotechnol., 2012, 10(1), 10 |
| 34 | Landgraf L., Christner C., Storck W., Schick I., Krumbein I., Dähring H., Haedicke K., Heinz⁃Herrmann K., Teichgräber U., Reichenbach J. R., Tremel W., Tenzer S., Hilger I., Biomaterials, 2015, 68, 77—88 |
| 35 | Song G., Chen M., Zhang Y., Cui L., Qu H., Zheng X., Wintermark M., Liu Z., Rao J., Nano Lett., 2017, 18(1), 182—189 |
| 36 | Huang B., Law M. W. M., Khong P. L., Radiology, 2009, 251(1), 166—174 |
| 37 | Hildebrand S., Löwa N., Paysen H., Fratila R. M., Reverte⁃Salisa L., Trakoolwilaiwan T., Niu Z., Kasparis G., Preuss S. F., Kosch O., M. De La Fuente J., Thanh N. T. K., Wiekhorst F., Pfeifer A., ACS Nano, 2020, 15(1), 434—446 |
| 38 | Hong H., Yang Y., Zhang Y., Cai W., Curr. Top. Med. Chem., 2010, 10(12), 1237—1248 |
| 39 | Srinivas M., Aarntzen E. H. J. G., Bulte J. W. M., Oyen W. J., Heerschap A., de Vries I. J. M., Figdor C. G., Adv. Drug Del. Rev., 2010, 62(11), 1080—1093 |
| 40 | Gu E., Chen W. Y., Gu J., Burridge P., Wu J. C., Theranostics, 2012, 2(4), 335—345 |
| 41 | Hong G., Antaris A. L., Dai H., Nat. Biomed. Eng., 2017, 1(1), 0010 |
| 42 | Sutton E. J., Henning T. D., Pichler B. J., Bremer C., Daldrup⁃Link H. E., Eur. Radiol., 2008, 18(10), 2021—2032 |
| 43 | Bulte J. W. M., Am. J. Roentgenol., 2009, 193(2), 314—325 |
| 44 | Hong G., Lee J. C., Robinson J. T., Raaz U., Xie L., Huang N. F., Cooke J. P., Dai H., Nat. Med., 2012, 18(12), 1841—1846 |
| 45 | Liu X. L., Yang Y., Ng C. T., Zhao L. Y., Zhang Y., Bay B. H., Fan H. M., Ding J., Adv. Mater., 2015, 27(11), 1939—1944 |
| 46 | Carrey J., Mehdaoui B., Respaud M., J. Appl. Phys., 2011, 109(8), 083921 |
| 47 | Lee J. H., Jang J. T., Choi J. S., Moon S. H., Noh S. H., Kim J. W., Kim J. G., Kim I. S., Park K. I., Cheon J., Nat. Nanotechnol., 2011, 6(7), 418—422 |
| 48 | Kossatz S., Grandke J., Couleaud P., Latorre A., Aires A., Crosbie⁃Staunton K., Ludwig R., Dähring H., Ettelt V., Lazaro⁃Carrillo A., Calero M., Sader M., Courty J., Volkov Y., Prina⁃Mello A., Villanueva A., Somoza Á., Cortajarena A. L., Miranda R., Hilger I., Breast Cancer Res., 2015, 17(1), 66 |
| 49 | Moroz P., Jones S. K., Gray B. N., Int. J. Hyperth., 2002, 18(4), 267—284 |
| 50 | Du Y., Liu X., Liang Q., Liang X. J., Tian J., Nano Lett., 2019, 19(6), 3618—3626 |
| 51 | Ludewig P., Gdaniec N., Sedlacik J., Forkert N. D., Szwargulski P., Graeser M., Adam G., Kaul M. G., Krishnan K. M., Ferguson R. M., Khandhar A. P., Walczak P., Fiehler J., Thomalla G., Gerloff C., Knopp T., Magnus T., ACS Nano, 2017, 11(10), 10480—10488 |
| 52 | Möddel M., Meins C., Dieckhoff J., Knopp T., New J. Phys., 2018, 20(8), 083001 |
| 53 | Murase K., Song R., Hiratsuka S., Appl. Phys. Lett., 2014, 104(25), 252409 |
| 54 | Szwargulski P., Wilmes M., Javidi E., Thieben F., Graeser M., Koch M., Gruettner C., Adam G., Gerloff C., Magnus T., Knopp T., Ludewig P., ACS Nano, 2020, 14(10), 13913—13923 |
| [1] | 沙蒙, 许维庆, 吴志超, 顾文玲, 朱成周. 单原子材料类酶催化及生物医学应用研究进展[J]. 高等学校化学学报, 2022, 43(5): 20220077. |
| [2] | 张钤, 刘雅薇, 王帆, 刘凯, 张洪杰. 稀土纳米材料在高分辨活体成像及诊疗中的应用[J]. 高等学校化学学报, 2022, 43(12): 20220552. |
| [3] | 丁奕如, 张超颖, 谢贺新. 肾小球滤过率活体实时监测荧光示踪剂的研究进展[J]. 高等学校化学学报, 2022, 43(12): 20220686. |
| [4] | 张志杰, 龚江, 谭海英, 姚坤, 唐涛. PS-b-P2VP/Fe3O4纳米复合材料的制备与表征[J]. 高等学校化学学报, 2014, 35(5): 1080. |
| [5] | 王岩, 王笑英, 吕言云, 许文彬, 郭轶, 徐力. 共同装载吴茱萸碱和Fe3O4纳米粒子的磁性药物载体的制备[J]. 高等学校化学学报, 2013, 34(12): 2866. |
| [6] | 蒋泽权, 宋晟, 窦红静, 孙康, 王一鸣, 黄超凡, 魏振华, 曲冠雄. 配体交换法制备水中高分散稳定性的多羧基修饰Fe3O4纳米颗粒[J]. 高等学校化学学报, 2012, 33(12): 2609. |
| [7] | 付昱, 杨敏, 李妍, 仲崇斌, 焦永华, 林奥雷. 基于天然高分子基元的阻隔层对磁性载药聚乳酸微球的控释作用[J]. 高等学校化学学报, 2012, 33(12): 2779. |
| [8] | 孙术国, 马美湖, 覃芳, 徐娅茹, 余文珍, 罗章. 共价固定亲和素的磁性纳米粒子用于快速测定蛋白A含量[J]. 高等学校化学学报, 2012, 33(07): 1426. |
| [9] | 曹丽燕 王海霞 石娟 周叶红 刘文娟 张国梅 双少敏. 磺丁醚-β-环糊精/ Fe3O4杂化的磁性纳米复合体的合成与表征[J]. 高等学校化学学报, 2011, 32(9): 2152. |
| [10] | 苏鹏飞 陈国 赵珺. 表面羧基化Fe3O4磁性纳米粒子的快捷制备及表征[J]. 高等学校化学学报, 2011, 32(7): 1472. |
| [11] | 高倩 张吉林 洪广言 倪嘉缵. 不同形貌的Fe3O4微-纳米粒子的溶剂热合成[J]. 高等学校化学学报, 2011, 32(3): 552. |
| [12] | 陶磊, 金伟, 张莹, 闫飞, 王琳琳, 牟颖, 金钦汉. 表面等离子体子共振传感器用于研究不同材质包裹的磁性纳米粒子性质[J]. 高等学校化学学报, 2009, 30(5): 882. |
| [13] | 李建平,高会玲,熊志刚 . 磁性纳米金共价固定癌胚抗原单克隆抗体的电流型免疫传感器[J]. 高等学校化学学报, 2008, 29(11): 2149. |
| [14] | 刘洪娜,李松, ,王志飞,何农跃,贺全国 . 一种基于磁性纳米粒子PCR的高通量SNP分型方法[J]. 高等学校化学学报, 2007, 28(6): 1035. |
| [15] | 柯诗剑, 计剑 . 万古霉素修饰磁性纳米粒子的制备及其细菌分离功能[J]. 高等学校化学学报, 2007, 28(1): 26. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
