

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (11): 3379.doi: 10.7503/cjcu20210441
收稿日期:2021-06-28
出版日期:2021-11-10
发布日期:2021-08-17
通讯作者:
陈祥军
E-mail:chenxiangjun@zju.edu.cn
基金资助:
LIN Ningqin1, YAO Ke2, CHEN Xiangjun1,2( )
)
Received:2021-06-28
Online:2021-11-10
Published:2021-08-17
Contact:
CHEN Xiangjun
E-mail:chenxiangjun@zju.edu.cn
Supported by:摘要:
白内障是全球致盲率最高的眼科疾病, 发病组织为晶状体. 晶状体内纤维细胞含有高浓度的晶状体蛋白, 晶状体蛋白家族分α?, β?和γ?3大亚家族. α-晶状体蛋白具有小分子伴侣功能, 可识别错误折叠蛋白质, 维持晶状体内蛋白质稳态; β?/γ?晶状体蛋白通过分子内或分子间相互作用, 主要发挥结构蛋白功能. 晶状体蛋白在晶状体纤维细胞内呈瞬时有序排列, 精准分子识别及动态相互作用在维持晶状体透明度中发挥关键作用. 晶状体内蛋白质稳态失衡是白内障的主要致病因素. 晶状体蛋白半衰期长, 且翻译合成后不再更新, 广泛受pH值、 金属离子、 辐射损伤和蛋白质翻译后修饰等细胞内外环境因素和化学因素的干扰, 影响晶状体蛋白间的分子识别和相互作用, 诱发白内障. 理清化学调控的晶状体蛋白分子识别及互作调控, 有助于阐明白内障发病机理, 并发掘防治白内障的创新策略. 本文基于晶状体蛋白识别互作与白内障研究进展, 综合评述了晶状体蛋白的分子识别、 相互作用方式、 调控因素及研究技术创新, 并探讨了晶状体蛋白识别互作调控网络在白内障药物研发的应用价值与挑战.
中图分类号:
TrendMD:
林宁钦, 姚克, 陈祥军. 晶状体蛋白识别互作与白内障的研究进展. 高等学校化学学报, 2021, 42(11): 3379.
LIN Ningqin, YAO Ke, CHEN Xiangjun. Research Progress of Molecular Recognition and Interaction of Crystallins Linking Cataract. Chem. J. Chinese Universities, 2021, 42(11): 3379.
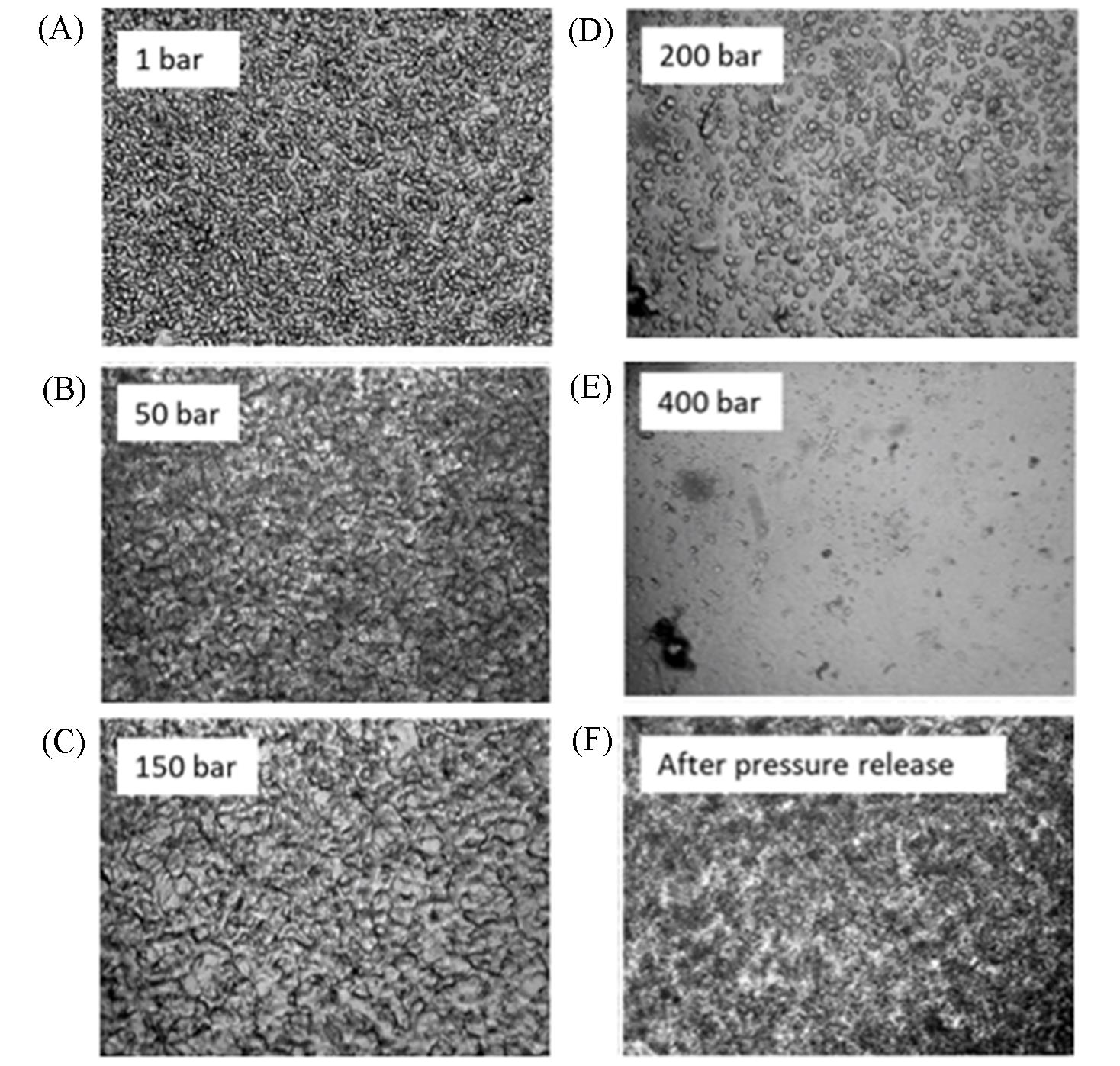
Fig.2 Pressure?dependent droplet formation and dissolution in the LLPS process[28]γD-droplet formation and dissolution in different pressure, (A) 1 bar(1 bar=0.1 MPa); (B) 50 bar; (C) 150 bar; (D) 200 bar; (E) 400 bar; (F) after pressure release, 0 bar. Copyright 2019, American Chemical Society.
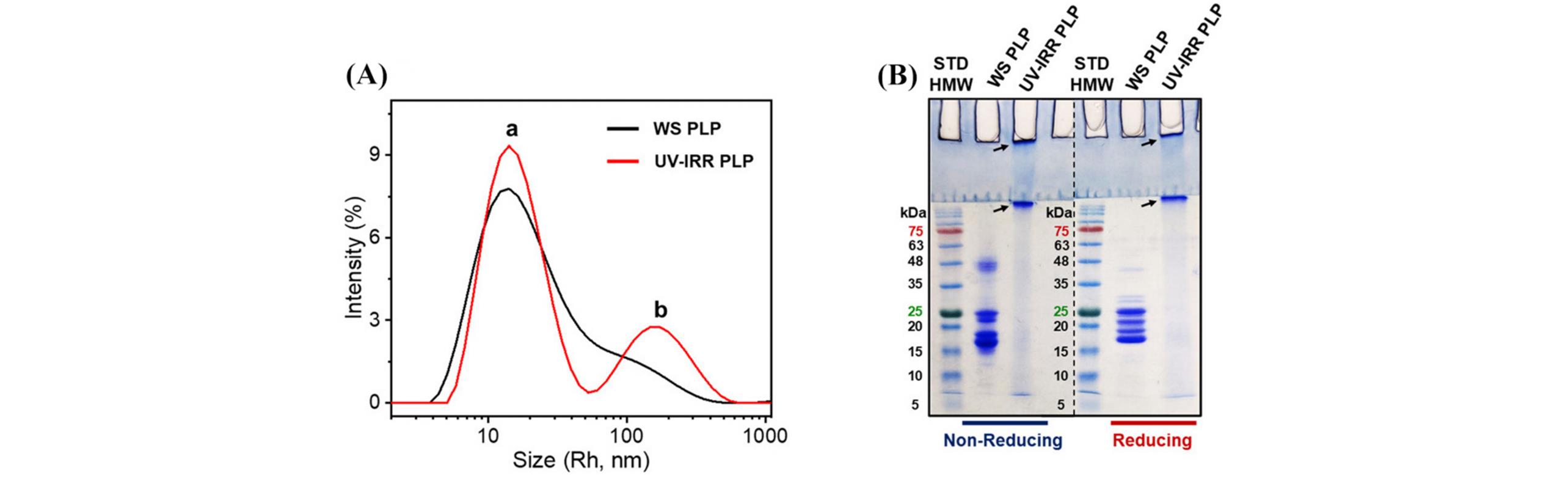
Fig.3 Aggregation of UV?irradiated water?soluble lens proteins[41](A) Dynamic light scattering measurements of water-soluble and UV-irradiated porcine lens proteins; (B) SDS-PAGE analysis of water-soluble porcine lens proteins before and after UV-light irradiation, under reducing and non-reducing conditions.Copyright 2020, American Chemical Society.
| Post?translational modification | Target protein | Reference |
|---|---|---|
| Deamidation | βA3 | [ |
| βB1 | [ | |
| βB2 | [ | |
| γS | [ | |
| Oxidation | αA | [ |
| βB2 | [ | |
| γC | [ | |
| γD | [ | |
| γS | [ | |
| Glycosylation | α?Cry | [ |
| γC | [ | |
| γD | [ | |
| Phosphorylation | αA | [ |
| αB | [ | |
| β?Cry | [ | |
| Acetylation | αA | [ |
| γD | [ | |
| Methylation | βA1 | [ |
| γD | [ | |
| Racemization/isomerization | αA | [ |
| βB2 | [ | |
| Truncation | αA | [ |
| βB1 | [ |
表1 汇总了白内障中常见的晶状体蛋白质翻译后修饰类型[36,43~46,49~52,54,56~71].
Table 1 Examples of post-translational modifications and their target protein in cataract
| Post?translational modification | Target protein | Reference |
|---|---|---|
| Deamidation | βA3 | [ |
| βB1 | [ | |
| βB2 | [ | |
| γS | [ | |
| Oxidation | αA | [ |
| βB2 | [ | |
| γC | [ | |
| γD | [ | |
| γS | [ | |
| Glycosylation | α?Cry | [ |
| γC | [ | |
| γD | [ | |
| Phosphorylation | αA | [ |
| αB | [ | |
| β?Cry | [ | |
| Acetylation | αA | [ |
| γD | [ | |
| Methylation | βA1 | [ |
| γD | [ | |
| Racemization/isomerization | αA | [ |
| βB2 | [ | |
| Truncation | αA | [ |
| βB1 | [ |
| 1 | World Health Organization. World Report on Vision, 2019, https://www.who.int/publications/i/item/9789241516570 |
| 2 | Yao K., Wang W., Chin. J. Ophthalmol., 2020,(5), 321—324(姚克, 王玮. 中华眼科杂志, 2020, (5), 321—324) |
| 3 | Feng Z. Q., Li J. H., Chin. J. Ophthalmol. Med., 2019, 9(1), 1—6(冯张青, 李俊红. 中华医学眼科杂志, 2019, 9(1), 1—6) |
| 4 | Yan H., Chen X., Chen Y., Rec. Adv. Ophthalmol., 2019, 39(1), 1—7(严宏, 陈曦, 陈颖. 眼科新进展, 2019, 39(1), 1—7) |
| 5 | Selivanova O. M., Galzitskaya O. V., Biology. Basel., 2020, 9(4), 85 |
| 6 | Slingsby C., Wistow G. J., Clark A. R., Protein Sci., 2013, 22(4), 367—380 |
| 7 | Schey K. L., Wang Z., Friedrich M. G., Garland D. L., Truscott R. J. W., Prog. Retin. Eye Res., 2020, 76, 100802 |
| 8 | Serebryany E., King J. A., Prog. Biophys. Mol. Biol., 2014, 115(1), 32—41 |
| 9 | Tweeddale H. J., Hawkins C. L., Janmie J. F., Truscott R. J., Davies M. J., Free Radic. Res., 2016, 50(10), 1116—1130 |
| 10 | Bloemendal H., de Jong W., Jaenicke R., Lubsen N. H., Slingsby C.,Tardieu A., Prog. Biophys. Mol. Biol., 2004, 86(3), 407—485 |
| 11 | Wu R. B., Li P. L., Chin. Sci. Bull., 2019, 64(22), 2285—2291(吴荣波, 李丕龙. 中国科学通报, 2019, 64(22), 2285—2291) |
| 12 | Bierma J. C., Roskamp K. W., Ledray A. P., Kiss A. J., Cheng C. H. C., Martin R. W., J. Mol. Biol., 2018, 430(24), 5151—5168 |
| 13 | Moreau K. L., King J. A., Trends Mol. Med., 2012, 18(5), 273—282 |
| 14 | Roskamp K. W., Paulson C. N., Brubaker W. D., Martin R. W., Acc. Chem. Res., 2020, 53(4), 863—874 |
| 15 | Leng X. Y., Wang S., Cao N. Q., Qi L. B., Yan Y. B., Biochemistry, 2014, 53(15), 2464—2473 |
| 16 | Gupta R., Chen J., Srivastava O. P., Mol. Vis., 2010, 16(40), 2242—2252 |
| 17 | Hains P. G., Truscott R. J. W., Biochim. Biophys. Acta Proteins Proteom., 2008, 1784(12), 1959—1964 |
| 18 | Hai Y., Lan C.J., Liao X., Int. J. Ophthalm., 2021, 21(6), 1017—1020(海玥, 兰长骏, 廖萱. 国际眼科杂志, 2021, 21(6), 1017—1020) |
| 19 | Boelens W. C., Prog. Biophys. Mol. Biol., 2014, 115(1), 3—10 |
| 20 | Singh B. N., Rao K. S., Ramakrishna T., Rangaraj N., Rao C. M., J. Mol. Biol., 2007, 366(3), 756—767 |
| 21 | Launay N., Goudeau B., Kato K., Vicart P., Lilienbaum A., Exp. Cell Res., 2006, 312(18), 3570—3584 |
| 22 | Bassnett S., Wilmarth P. A., David L. L., Mol. Vis., 2009, 15, 2448—2463 |
| 23 | Tan F. G., Donovan A. K., Ledee D. R., Zelenka P. S., Fariss R. N., Chepelinsky A. B., Invest. Ophthalmol. Vis. Sci., 2004, 45(3), 863—871 |
| 24 | Vendra V. P. R., Khan I., Chandani S., Muniyandi A., Balasubramanian D., Biochim. Biophys. Acta Gen. Subj., 2016, 1860(1), 333—343 |
| 25 | Broide M. L., Berland C. R., Pande J., Ogun O. O., Benedek G. B., Proc. Natl. Acad. Sci. USA, 1991, 88(13), 5660—5664 |
| 26 | Annunziata O., Ogun O., Benedek G. B., Proc. Natl. Acad. Sci. USA, 2003, 100(3), 970—974 |
| 27 | Wang Y., Lomakin A., McManus J. J., Ogun O., Benedek G. B., Proc. Natl. Acad. Sci. U S A, 2010, 107(30), 13282—13287 |
| 28 | Cinar S., Cinar H., Chan H. S., Winter R., J. Am. Chem. Soc., 2019, 141(18), 7347—7354 |
| 29 | Bassnett S., J. Physiol. London, 1990, 431, 445—464 |
| 30 | Wang Y. T., Petty S., Trojanowski A., Knee K., Goulet D., Mukerji I., King J., Invest. Ophthalmol. Vis. Sci., 2010, 51(2), 672—678 |
| 31 | Mohr B. G., Dobson C. M., Garman S. C., Muthukumar M., J. Chem. Phys., 2013, 139(12), 121914 |
| 32 | Cobb B. A., Petrash J. M., J. Biol. Chem., 2000, 275(9), 6664—6672 |
| 33 | Anbarasu K., Sivakumar J., Biochim. Biophys. Acta Gen. Subj., 2016, 1860(1), 222—233 |
| 34 | Aravind P., Mishra A., Suman S. K., Jobby M. K., Sankaranarayanan, R., Sharma Y., Biochemistry, 2009, 48(51), 12180—12190 |
| 35 | Quintanar L., Dominguez Calva J. A., Serebryany E., Rivillas Acevedo L., Haase⁃Pettingell C., Amero C., King J. A., ACS Chem. Biol., 2016, 11(1), 263—272 |
| 36 | Ramkumar S., Fan X. J., Wang B. L., Yang S. C., Monnier V. M., Biochim. Biophys. Acta Mol. Basis Dis., 2018, 1864(11), 3595—3604 |
| 37 | Dominguez Calva J. A., Perez Vazquez M. L., Serebryany E., King J. A., Quintanar L., J. Biol. Inorg. Chem., 2018, 23(7), 1105—1118 |
| 38 | Kamari F., Hallaj S., Dorosti F., Alinezhad F., Taleschian Tabrizi N., Farhadi F., Aslani H., Graefes Arch. Clin. Exp. Ophthalmol., 2019, 257(10), 2065—2077 |
| 39 | Kim I., Saito T., Fujii N., Kanamoto T., Chatake T., Fujii N., Biochem. Biophys. Res. Commun., 2015, 466(4), 622—628 |
| 40 | Anbaraki A., Ghahramani M., Muranov K. O., Kurganov B. I., Yousefi R., Int. J. Biol. Macromol., 2018, 118, 1120—1130 |
| 41 | Honisch C., Donadello V., Hussain R., Peterle D., de Filippis V., Arrigoni G., Gatto C., Giurgola L., Siligardi G., Ruzza P., ACS Omega, 2020, 5(8), 4293—4301 |
| 42 | Gupta R., Srivastava O. P., J. Biol. Chem., 2004, 279(43), 44258—44269 |
| 43 | Lampi K. J., Fox C. B., David L. L., Exp. Eye. Res., 2012, 104, 48—58 |
| 44 | Lampi K. J., Oxford J. T., Bachinger H. P., Shearer T. R., David L. L., Kapfer D. M., Exp. Eye. Res., 2001, 72(3), 279—288 |
| 45 | Takata T., Oxford J. T., Brandon T. R., Lampi K. J., Biochemistry, 2007, 46(30), 8861—8871 |
| 46 | Lapko V. N., Purkiss A. G., Smith D. L., Smith J. B., Biochemistry, 2002, 41(27), 8638—8648 |
| 47 | Flaugh S. L., Mills I. A., King J., J. Biol. Chem., 2006, 281(41), 30782—30793 |
| 48 | Nye Wood M. G., Spraggins J. M., Caprioli R. M., Schey K. L., Donaldson P. J., Grey A. C., Exp. Eye. Res., 2017, 154, 70—78 |
| 49 | Kaiser C. J. O., Peters C., Schmid P. W. N., Stavropoulou M., Zou J., Dahiya V., Mymrikov E. V., Rockel B., Asami S., Haslbeck M., Rappsilber J., Reif B., Zacharias M., Buchner J., Weinkauf S., Nat. Struct. Mol. Biol., 2019, 26(12), 1141—1150 |
| 50 | Fan X., Zhou S., Wang B., Hom G., Guo M., Li B., Yang J., Vaysburg D., Monnier V., Mol. Cell. Proteom., 2015, 14(12), 3211—3223 |
| 51 | Zhao W. J., Yan Y. B., Int. J. Biol. Macromol., 2018, 108, 665—673 |
| 52 | Thorn D. C., Grosas A. B., Mabbitt P. D., Ray N. J., Jackson C. J., Carver J. A., J. Mol. Biol., 2019, 431(3), 483—497 |
| 53 | Gao C., Wu J.H., Luo Y., Yan Ke Xue Bao, 2020, 35(4), 234—242(高潮, 吴继红, 罗怡.眼科学报, 2020, 35(4), 234—242) |
| 54 | Yousefi R., Javadi S., Amirghofran S., Oryan A., Moosavi Movahedi A. A., Int. J. Biol. Macromol., 2016, 82, 328—338 |
| 55 | Nandi S. K., Nahomi R. B., Rankenberg J., Glomb M. A., Nagaraj R. H., J. Biol. Chem., 2020, 295(17), 5701—5716 |
| 56 | Chaudhury S., Ghosh P., Parveen S., Dasgupta S., Int. J. Biol. Macromol., 2017, 96, 392—402 |
| 57 | Li C. T., How S. C., Chen M. E., Lo C. H., Chun M. C., Chang C. K., Chen W. A., Wu J. W., Wang S. S. S., Int. J. Biol. Macromol., 2018, 118, 442—451 |
| 58 | Thornell E., Aquilina A., Cell. Mol. Life Sci., 2015, 72(21), 4127—4137 |
| 59 | Aquilina J. A., Benesch J. L. P., Ding L. L., Yaron O., Horwitz J., Robinson C. V., J. Biol. Chem., 2004, 279(27), 28675—28680 |
| 60 | Huang C. H., Wang Y. T., Tsai C. F., Chen Y. J., Lee J. S., Chiou S. H., Mol. Vis., 2011, 17(22), 186—197 |
| 61 | Kim Y. H., Kapfer D. M., Boekhorst J., Lubsen N. H., Bachinger H. P., Shearer T. R., David L. L., Feix J. B., Lampi K. J., Biochemistry, 2002, 41(47), 14076—14084 |
| 62 | Michiel M., Duprat E., Skouri-Panet F., Lampi J. A., Tardieu A., Lampi K. J., Finet S., Exp. Eye. Res., 2010, 90(6), 688—698 |
| 63 | Vetter C. J., Thorn D. C., Wheeler S. G., Mundorff C. C., Halverson K. A., Wales T. E., Shinde U. P., Engen J. R., David L. L., Carver J. A., Lampi K. J., Protein Sci., 2020, 29(9), 1945—1963 |
| 64 | Nagaraj R. H., Nahomi R. B., Shanthakumar S., Linetsky M., Padmanabha S., Pasupuleti N., Wang B. L., Santhoshkumar P., Panda A. K., Biswas A., Biochim. Biophys. Acta. Mol. Basis Dis., 2012, 1822(2), 120—129 |
| 65 | DiMauro M. A., Nandi S. K., Raghavan C. T., Kar R. K., Wang B. L., Bhunia,A., Nagaraj R. H., Biswas A., Biochemistry, 2014, 53(46), 7269—7282 |
| 66 | Lapko V. N., Cerny R. L., Smith D. L., Smith J. B., Protein Sci., 2005, 14(1), 45—54 |
| 67 | Lapko V. N., Smith D. L., Smith J. B., Protein Sci., 2003, 12(8), 1762—1774 |
| 68 | Takata T., Ha S., Koide T., Fujii N., Protein Sci., 2020, 29(4), 955—965 |
| 69 | Takata T., Murakami K., Toyama A., Fujii N., Biochim. Biophys. Acta Proteins. Proteom., 2018, 1866(7), 767—774 |
| 70 | Kallur L. S., Aziz A., Abraham E. C., Mol. Cell. Biochem., 2008, 308(1), 85—91 |
| 71 | van Montfort R. L. M., Bateman O. A., Lubsen N. H., Slingsby C., Protein Sci., 2003, 12(11), 2606—2612 |
| 72 | Wang K. J., Liao X. Y., Lin K., Xi Y. B., Wang S., Wan X. H., Yan Y. B., Int. J. Biol. Macromol., 2021, 172, 475—482 |
| 73 | Liang J. J. N., Protein Sci., 2004, 13(9), 2476—2482 |
| 74 | Khago D., Wong E. K., Kingsley C. N., Freites J. A., Tobias D. J., Martin R. W., Biochim. Biophys. Acta Gen. Subj., 2016, 1860(1), 325—332 |
| 75 | Liang J. J. N., Fu L., Biochem. Biophys. Res. Commun., 2002, 293(1), 7—12 |
| 76 | Fu L., Liang J. J. N., Biochem. Biophys. Res. Commun., 2003, 302(4), 710—714 |
| 77 | Barnwal R. P., Devi K. M., Agarwal G., Sharma Y., Chary K. V. R., Proteins, 2011, 79(2), 569—580 |
| 78 | Kingsley C. N., Brubaker W. D., Markovic S., Diehl A., Brindley A. J., Oschkinat H., Martin R. W., Structure, 2013, 21(12), 2221—2227 |
| 79 | He Y., Kang J., Song J. X., Biochem. Biophys. Res. Commun., 2020, 526(4), 1112—1117 |
| 80 | Park H., Lee H., Seok C., Curr. Opin. Struct. Biol., 2015, 35, 24—31 |
| 81 | Das P., King J. A., Zhou R. H., Proc. Natl. Acad. Sci. USA, 2011, 108(26), 10514—10519 |
| 82 | Chang C. K., Wang S. S. S., Lo C. H., Hsiao H. C., Wu J. W., J. Biomol. Struct. Dyn., 2017, 35(5), 1042—1054 |
| 83 | Zhang C.S., Lai L.H., Acta Phys. Chim. Sin., 2020, 36(1), 154—168(张长胜, 来鲁华. 物理化学学报, 2020, 36(1), 154—168) |
| 84 | Orlando G., Raimondi D., Tabaro F., Codice F., Moreau Y., Vranken W. F., Bioinformatics, 2019, 35(22), 4617—4623 |
| 85 | Chen T. Y., Lei X., Li T. T., Chin. J. Biochem. Mol. Biol., 2020, 36(10), 1129—1137(陈韬宇, 雷颀, 李婷婷. 中国生物化学与分子生物学学报, 2020, 36(10), 1129—1137) |
| 86 | Kang H. S., Yang Z. X., Zhou R. H., J. Am. Chem. Soc., 2018, 140(27), 8479—8486 |
| 87 | Chen P., Liu S. B., Ma W. Y., Chem. J. Chinese Universities, 2020,41(12), 2658—2666(陈鹏, 刘士博, 马维绎. 高等学校化学学报, 2020, 41(12), 2658—2666) |
| 88 | Cheng S. H., Wang H. D., Wang H. L., Yu Y., Zhao G.F., Zou T. Y., Chem. J. Chinese Universities, 2020, 41(11), 2335—2344(程思航, 王宏达, 王慧利, 于洋, 赵关芳, 邹天一. 高等学校化学学报, 2020, 41(11), 2335—2344) |
| [1] | 王隆杰, 范鸿川, 秦渝, 曹秋娥, 郑立炎. 金属有机框架材料在分离分析领域的研究进展[J]. 高等学校化学学报, 2021, 42(4): 1167. |
| [2] | 张晓荣, 陈岚岚, 胡善文. 基于分子识别的细菌检测研究进展[J]. 高等学校化学学报, 2021, 42(11): 3468. |
| [3] | 吉采灵, 程兴, 谈洁, 袁荃. 功能化核酸适体的筛选及分子识别应用[J]. 高等学校化学学报, 2021, 42(11): 3457. |
| [4] | 黄玲, 庄梓健, 李翔, 石沐玲, 刘高强. 基于核酸适体的外泌体分子识别研究进展[J]. 高等学校化学学报, 2021, 42(11): 3493. |
| [5] | 刘学娇, 杨帆, 刘爽, 张春娟, 刘巧玲. 核酸适体靶向的膜蛋白识别与功能调控研究进展[J]. 高等学校化学学报, 2021, 42(11): 3277. |
| [6] | 刘园, 邓瑾琦, 赵帅, 田飞, 李轶, 孙佳姝, 刘超. 基于分子识别的免疫层析技术用于新冠肺炎感染的快速诊断[J]. 高等学校化学学报, 2021, 42(11): 3390. |
| [7] | 彭与煜,王煜,于鑫垚,曾巨澜,肖忠良,曹忠. 基于单(6-巯基-6-去氧)-β-环糊精修饰金电极对L-半胱氨酸的快速灵敏检测[J]. 高等学校化学学报, 2020, 41(2): 268. |
| [8] | 马玉聪, 樊保民, 郝华, 吕金玉, 冯云皓, 杨彪. 十八胺基分子组装体在碳钢表面的作用机理与模拟[J]. 高等学校化学学报, 2019, 40(1): 96. |
| [9] | 沈晓琴, 李智, 王刚林, 王莉, 孙权洪, 罗序成, 马楠. 基于氧化石墨烯和DNA量子点组装体的DNA二元逻辑检测及循环可逆设计[J]. 高等学校化学学报, 2017, 38(12): 2176. |
| [10] | 宋春霞, 羊小海, 王柯敏, 王青, 刘剑波, 黄晋, 李文山, 黄海花, 刘卫. 聚合物在荧光检测领域的应用[J]. 高等学校化学学报, 2016, 37(2): 201. |
| [11] | 周婷, 曹忠, 戴云林, 曹婷婷, 何婧琳, 徐雷涛, 龙姝. 基于氢键作用的杯芳烃超分子识别乙醇的传感机理及分析应用[J]. 高等学校化学学报, 2013, 34(6): 1339. |
| [12] | 顾金英, 朱明莉, 施宪法. 治疗铜中毒的新型高效络合剂对叔丁基硫杂杯[4]芳烃[J]. 高等学校化学学报, 2012, 33(10): 2229. |
| [13] | 王凯, 杨英威, 张晓安. 柱芳烃的合成及主客体化学研究进展[J]. 高等学校化学学报, 2012, 33(01): 1. |
| [14] | 张瑀健, 何振峰, 李国文. 含氮杂冠醚和核酸碱基双亲聚合物的合成及性能[J]. 高等学校化学学报, 2010, 31(7): 1456. |
| [15] | 张铁莉, 刘锋, 李克安. 模板分子中作用基团的数目及位置对印迹聚合物印迹效应的影响[J]. 高等学校化学学报, 2010, 31(6): 1126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||