

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (10): 2153.doi: 10.7503/cjcu20200370
收稿日期:2020-06-18
出版日期:2020-10-10
发布日期:2020-10-08
通讯作者:
胡文浩
E-mail:huwh9@mail.sysu.edu.cn
Received:2020-06-18
Online:2020-10-10
Published:2020-10-08
Contact:
HU Wenhao
E-mail:huwh9@mail.sysu.edu.cn
摘要:
金属参与的不对称催化反应是制备光学活性化合物的重要途径之一, 其中新型手性配体的设计合成一直是不对称催化领域中十分关键且富有挑战性的课题. 从20世纪90年代末开始, 化学家们尝试在手性配体中引入螺环结构, 创造性地发展了螺[4.4]壬烷骨架、 螺双二氢茚骨架、 螺[4.4]壬二烯骨架和螺二色满骨架等手性螺环单齿配体, 多齿配体及其催化剂, 并成功应用于不对称催化氢化、 不对称碳碳键形成或碳杂键形成等不对称转化反应中, 合成了众多富有价值的手性产品, 有力地推动了不对称催化反应的工业应用化进程. 本文综合评述了手性螺环配体的早期发现、 发展历程以及近期的研究成果, 介绍了螺环配体在药物及天然产物中的应用研究进展, 并对手性螺环结构的小分子催化剂的研究进展进行叙述和说明.
中图分类号:
TrendMD:
许聪, 胡文浩. 手性螺环配体及催化剂在不对称催化反应中的应用研究进展. 高等学校化学学报, 2020, 41(10): 2153.
XU Cong, HU Wenhao. Recent Advances of ChiraI Spiro Ligands and Their Catalysts in Asymmetric Catalysis†. Chem. J. Chinese Universities, 2020, 41(10): 2153.
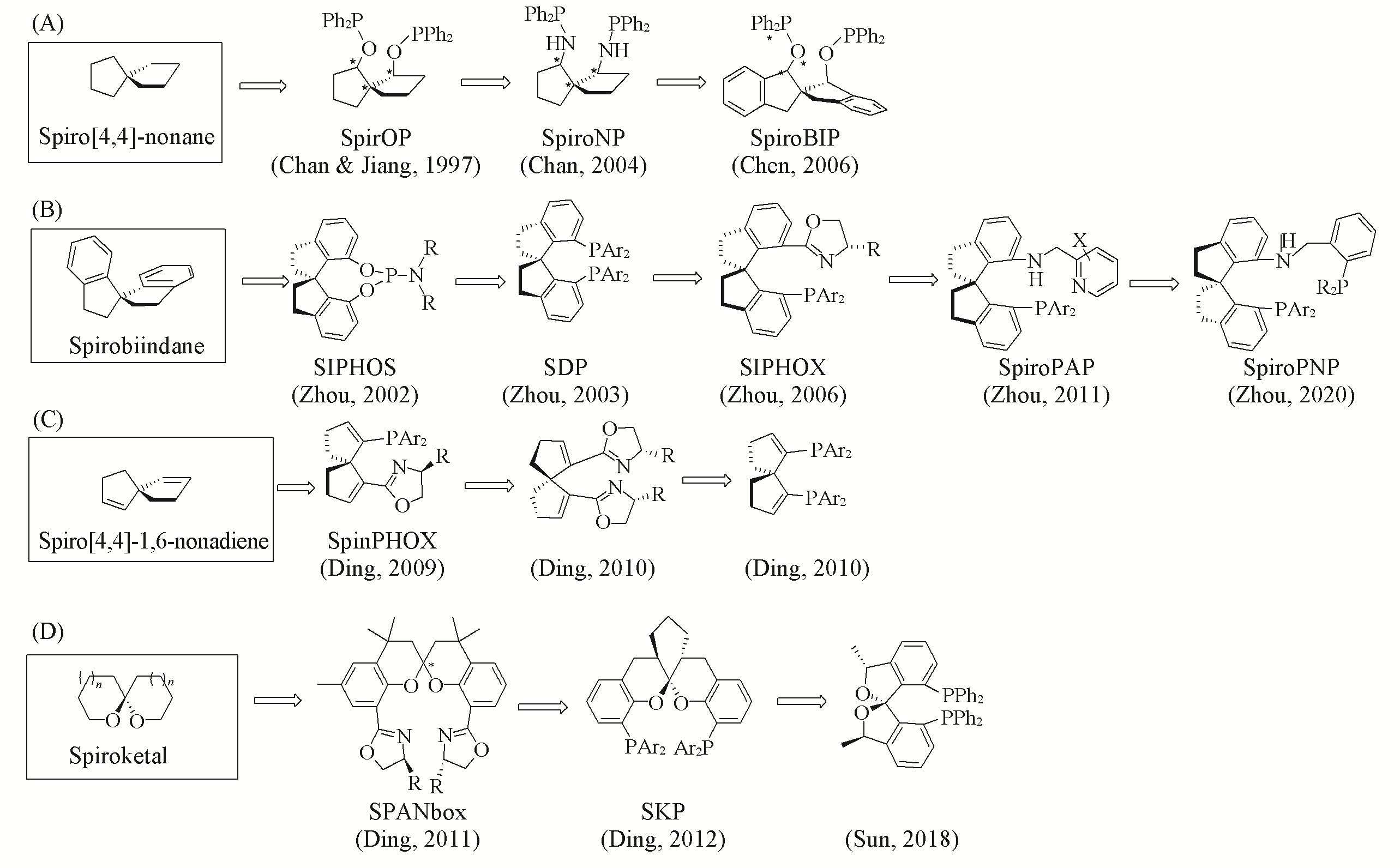
Fig.3 Development of chiral spiro ligandsRepresentative spiro skeleton: (A) spiro[4.4]nonane; (B) spirobiindane; (C) spiro[4,4]nonadiene; (D) spiroketal skeletons.
| 1 | Ojima I., Catalytic Asymmetric Synthesis, Wiley⁃VCH, Weinheim, 2000, 1—796 |
| 2 | Jacobsen E. N., Pfaltz A., Yamamoto H., Comprehensive Asymmetric Catalysis, Springer, Berlin, 1999, 1—1856 |
| 3 | Yamamoto H., Lewis Acids in Organic Synthesis, Wiley⁃VCH, Weinheim, 2001, 1—995 |
| 4 | Lin G. Q., Li Y. M., Chan A. S. C., Priciples and Applications of Asymmetric Synthesis, Wiley, New York, 2001, 1—507 |
| 5 | Xie J. H., Zhou Q. L., Acta Chim. Sinica, 2012, 70(13), 1427—1438(谢建华, 周其林.化学学报, 2012, 70(13), 1427—1438) |
| 6 | Liu Y., Wang Z., Ding K. L., Acta Chim. Sinica, 2012, 70(13), 1464—1470(刘龑, 王正, 丁奎岭.化学学报, 2012, 70(13), 1464—1470) |
| 7 | Zheng K., Lin L. L., Feng X. M., Acta Chim. Sinica, 2012, 70(17), 1785—1790(郑柯, 林丽丽, 冯小明.化学学报, 2012, 70(17), 1785—1790) |
| 8 | Knowles W. S., Sabacky M. J., J. Chem. Soc. Chem. Commun., 1968,(22), 1445—1446 |
| 9 | Xie J. H., Zhou Q. L., Acta Chim. Sinica, 2014, 72(7), 778—797(谢建华, 周其林.化学学报, 2014, 72(7), 778—797) |
| 10 | Tang W., Zhang X., Chem. Rev., 2003, 103(8), 3029—3070 |
| 11 | Hargaden G. C., Guiry P. J., Chem. Rev., 2009, 109(6), 2505—2550 |
| 12 | Liu Y. Y., Li W. B., Zhang J. L., Natl. Sci. Rev., 2017, 4(1), 326—358 |
| 13 | Dai L. X., Hou X. L., Chiral Ferrocenes in Asymmetric Catalysis, Wiley⁃VCH, New York, 2010, 1—396 |
| 14 | Zhu S. F., Zhou Q. L., Privileged Chiral Ligands and Catalysts, Wiley⁃VCH Verlag GmbH & Co. KgaA, Weinheim, 2011, 137—170 |
| 15 | Zhu S. F., Zhou Q. L., Spiro Ligands for Asymmetric Catalysis, in Ligand Design in Metal Chemistry, Wiley⁃VCH, Weinheim, 2016, 66—103 |
| 16 | Zhou Q. L., Xie J. H., Top. Organomet. Chem., 2011, 36, 1—28 |
| 17 | Ding K., Han Z., Wang Z., Chem. Asian J., 2009, 4(1), 32—41 |
| 18 | Xie J. H., Zhou Q. L., Acc. Chem. Res., 2008, 41(5), 581—593 |
| 19 | Zhang Z. H., Chin. J. Org. Chem., 2005, 25(4), 355—363(张占辉.有机化学, 2005, 25(4), 355—363) |
| 20 | Srivastava N., Mital A., Kumar A., Chem. Commun., 1992, 493—494 |
| 21 | Chan A. S. C., Lin C. C., Sun J., Hu W., Li Z., Pan W., Mi A., Jiang Y., Huang T. M., Yang T. K., Chen J. H., Wang Y., Lee G. H., Tetrahedron: Asymmetry, 1995, 6(12), 2953—2959 |
| 22 | Cram D. J., Steinberg H., J. Am. Chem. Soc., 1954, 76(10), 2753—2757 |
| 23 | Ohta T., Takaya H., Noyori R., Inorg. Chem., 1988, 27(3), 566—569 |
| 24 | Chan A. S. C., Hu W., Pai C. C., Lau C. P., Jiang Y., Mi A., Yan M., Sun J., Lou R., Deng J., J. Am. Chem. Soc., 1997, 119(40), 9570—9571 |
| 25 | Hu W., Yan M., Lau C. P., Yang S. M., Chan A. S. C., Jiang Y., Mi A., Tetrahedron Lett., 1999, 40(5), 973—976 |
| 26 | Guo Z. Q., Guan X. Y., Chen Z. Y., Tetrahedron: Asymmetry, 2006, 17(3), 468—473 |
| 27 | Jiang Y., Xue S., Li Z., Deng J., Mi A., Chan A. S. C., Tetrahedron: Asymmetry, 1998, 9(18), 3185—3189 |
| 28 | Jiang Y., Xue S., Yu K., Li Z., Deng J., Mi A., Chan A. S. C., J. Organomet. Chem., 1999, 586(2), 159—165 |
| 29 | Lin C. W., Lin C. C., Lam L. F. L., Au⁃Yeung T. T. L., Chan A. S. C., Tetrahedron Lett., 2004, 45(39), 7379—7381 |
| 30 | Bajracharya G. B., Arai M. A., Koranne P. S., Suzuki T., Takizawa S., Sasai H., Bull. Chem. Soc. Jpn., 2009, 82(3), 285—302 |
| 31 | Zhu G., Cao P., Jiang Q., Zhang X., J. Am. Chem. Soc., 1997, 119(7), 1799—1800 |
| 32 | Arai M. A., Arai T., Sasai H., Org. Lett., 1999, 1(11), 1795—1797 |
| 33 | Tsujihara T., Takenaka K., Onitsuka K., Hatanaka M., Sasai H., J. Am. Chem. Soc., 2009, 131(10), 3452—3453 |
| 34 | Takizawa S., Honda Y., Arai M. A., Kato T., Sasai H., Heterocycles, 2003, 60(11), 2551—2556 |
| 35 | Kato T., Marubayashi K., Takizawa S., Sasai H., Tetrahedron: Asymmetry, 2004, 15(23), 3693—3697 |
| 36 | Birman V. B., Rheingold A. L., Lam K. C., Tetrahedron: Asymmetry, 1999, 10(1), 125—131 |
| 37 | Zhu S. F., Xie J. H., Liu B., Xing L., Zhou Q. L., Tetrahedron: Asymmetry, 2003, 14(20), 3219—3224 |
| 38 | Fu Y., Hou G. H., Xie J. H., Xing L., Wang L. X., Zhou Q. L., J. Org. Chem., 2004, 69(23), 8157—8160 |
| 39 | Fu Y., Guo X. X., Zhu S. F., Hu A. G., Xie J. H., Zhou Q. L., J. Org. Chem., 2004, 69(14), 4648—4655 |
| 40 | Hou G. H., Xie J. H., Yan P. C., Zhou Q. L., J. Am. Chem. Soc., 2009, 131(4), 1366—1367 |
| 41 | Yang Y., Zhu S. F., Duan H. F., Zhou C. Y., Wang L. X., Zhou Q. L., J. Am. Chem. Soc., 2007, 129(8), 2248—2249 |
| 42 | Yang Y., Zhu S. F., Zhou C. Y., Zhou Q. L., J. Am. Chem. Soc., 2008, 130(43), 14052—14053 |
| 43 | Li K., Li M. L., Zhang Q., Zhu S. F., Zhou Q. L., J. Am. Chem. Soc., 2018, 140(24), 7458—7461 |
| 44 | Duan H. F., Xie J. H., Qiao X. C., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2008, 47(23), 4351—4353 |
| 45 | Duan H. F., Xie J. H., Shi W. J., Zhang Q., Zhou Q. L., Org. Lett., 2006, 8(7), 1479—1481 |
| 46 | Duan H. F., Jia Y. X., Wang L. X., Zhou Q. L., Org. Lett., 2006, 8(12), 2567—2569 |
| 47 | Xie J. H., Wang L. X., Fu Y., Zhu S. F., Fan B. M., Duan H. F., Zhou Q. L., J. Am. Chem. Soc.,2003, 125(15), 4404—4405 |
| 48 | Xie J. H., Zhou Z. T., Kong W. L., Zhou Q. L., J. Am. Chem. Soc., 2007, 129(7), 1868—1869 |
| 49 | Xie J. H., Liu S., Huo X. H., Cheng X., Duan H. F., Fan B. M., Wang L. X., Zhou Q. L., J. Org. Chem., 2005, 70(8), 2967—2973 |
| 50 | Liu S., Xie J. H., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2007, 46(39), 7506—7508 |
| 51 | Liu S., Xie J. H., Li W., Kong W. L., Wang L. X., Zhou Q. L., Org. Lett., 2009, 11(21), 4994—4997 |
| 52 | Xie J. H., Liu S., Kong W. L., Bai W. J., Wang X. C., Wang L. X., Zhou Q. L., J. Am. Chem. Soc., 2009, 131(2), 4222—4223 |
| 53 | Bai W. J., Xie J. H., Li Y. L., Liu S., Zhou Q. L., Adv. Synth. Catal., 2010, 352(1), 81—84 |
| 54 | Cheng L. J., Xie J. H., Wang L. X., Zhou Q. L., Adv. Synth. Catal., 2012, 354(6), 1105—1113 |
| 55 | Chen J. Q., Xie J. H., Bao D. H., Liu S., Zhou Q. L., Org. Lett., 2012, 14(11), 2714—2717 |
| 56 | Cheng L. J., Xie J. H., Chen Y., Wang L. X., Zhou Q. L., Org. Lett., 2013, 15(4), 764—767 |
| 57 | Li G., Xie J. H., Hou J., Zhu S. F., Zhou Q. L., Adv. Synth. Catal., 2013, 355(8), 1597—1604 |
| 58 | Liu C., Xie J. H., Li Y. L., Chen J. Q., Zhou Q. L., Angew. Chem. Int. Ed., 2013, 52(2), 593—596 |
| 59 | Cheng X., Zhang Q., Xie J. H., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2005, 44(7), 1118—1121 |
| 60 | Zhu S. F., Xie J. B., Zhang Y. Z., Li S., Zhou Q. L., J. Am. Chem. Soc., 2006, 128(39), 12886—12891 |
| 61 | Guo C., Sun D. W., Yang S., Mao S. J., Xu X. H., Zhu S. F., Zhou Q. L., J. Am. Chem. Soc., 2015, 137(1), 90—93 |
| 62 | Li S., Zhu S. F., Zhang C. M., Song S., Zhou Q. L., J. Am. Chem. Soc., 2008, 130(27), 8584—8585 |
| 63 | Li S., Zhu S. F., Xie J. H., Song S., Zhang C. M., Zhou Q. L., J. Am. Chem. Soc., 2010, 132(3), 1172—1179 |
| 64 | Song S., Zhu S. F., Pu L. Y., Zhou Q. L., Angew. Chem. Int. Ed., 2013, 52(23), 6072—6075 |
| 65 | Xie J. B., Xie J. H., Liu X. Y., Kong W. L., Li S., Zhou Q. L., J. Am. Chem. Soc., 2010, 132(13), 4538—4539 |
| 66 | Xie J. B., Xie J. H., Liu X. Y., Zhang Q. Q., Zhou Q. L., Chem. Asian J., 2011, 6(3), 899—908 |
| 67 | Zhu S. F., Yu Y. B., Li S., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2012, 51(35), 8872—8875 |
| 68 | Yu Y. B., Cheng L., Li Y. P., Fu Y., Zhu S. F., Zhou Q. L., Chem. Commun., 2015, 52(26), 4812—4815 |
| 69 | Xie J. H., Liu X. Y., Xie J. B., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2011, 50(32), 7329—7332 |
| 70 | Xie J. H., Liu X. Y., Yang X. H., Xie J. B., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2012, 51(1), 201—203 |
| 71 | Yang X. H., Xie J. H., Zhou Q. L., Org. Chem. Front., 2014, 1(2), 190—193 |
| 72 | Yang X. H., Wang K., Zhu S. F., Xie J. H., Zhou Q. L., J. Am. Chem. Soc., 2014, 136(50), 17426—17429 |
| 73 | Bao D. H., Wu H. L., Liu C. L., Xie J. H., Zhou Q. L., Angew. Chem. Int. Ed., 2015, 54(30), 8791—8794 |
| 74 | Zhang F. H., Wang C., Xie J. H., Zhou Q. L., Adv. Synth. Catal., 2019, 361(12), 2832—2835 |
| 75 | Gu X. S., Yu N., Yang X. H., Zhu A. T., Xie J. H., Zhou Q. L., Org. Lett., 2019, 21(11), 4111—4115 |
| 76 | Bao D. H., Gu X. S., Xie J. H., Zhou Q. L., Org. Lett., 2017, 19(1), 118—121 |
| 77 | Lin H., Xiao L. J., Zhou M. J., Yu H. M., Xie J. H., Zhou Q. L., Org. Lett., 2016, 18(6), 1434—1437 |
| 78 | Yan P. C., Xie J. H., Zhang X. D., Chen K., Li Y. Q., Zhou Q. L., Che D. Q., Chem. Commun., 2014, 50(100), 15987—15990 |
| 79 | Yan P. C., Zhu G. L., Xie J. H., Zhang X. D., Zhou Q. L., Li Y. Q., Shen W. H., Che D. Q., Org. Process Res. Dev., 2013, 17(2), 307—312 |
| 80 | Zhang F. H., Zhang F. J., Li M. L., Xie J. H., Zhou Q. L., Nat. Catal., 2020, DOI: 10.1038/s41929-020-0474-5 |
| 81 | Liu B., Zhu S. F., Wang L. X., Zhou Q. L., Tetrahedron: Asymmetry, 2006, 17(4), 634—641 |
| 82 | Liu B., Zhu S. F., Zhang W., Chen C., Zhou Q. L., J. Am. Chem. Soc., 2007, 129(18), 5834—5835 |
| 83 | Zhu S. F., Xu B., Wang G. P., Zhou Q. L., J. Am. Chem. Soc., 2012, 134(1), 436—442 |
| 84 | Song X. G., Ren Y. Y., Zhu S. F., Zhou Q. L., Adv. Synth. Catal., 2016, 358(15), 2366—2370 |
| 85 | Zhu S. F., Cai Y., Mao H. X., Xie J. H., Zhou Q. L., Nat. Chem., 2010, 2, 546—551 |
| 86 | Zhang Y. Z., Zhu S. F., Wang L. X., Zhou Q. L., Angew. Chem. Int. Ed., 2008, 47(44), 8496—8498 |
| 87 | Han Z., Wang Z., Zhang X., Ding K., Angew. Chem. Int. Ed., 2009, 48(0), 5345—5349 |
| 88 | Shang J., Han Z., Li Y., Wang Z., Ding K., Chem. Commun., 2012, 48(42), 5172—5174 |
| 89 | Han Z., Wang Z., Zhang X., Ding K., Chin. Sci. Bull., 2010, 55(25), 2840—2846 |
| 90 | Han Z., Wang Z., Zhang X., Ding K., Scientia Sinica Chimica, 2010, 40(7), 950—955(韩召斌, 王正, 张绪穆, 丁奎岭.中国科学•化学, 2010, 40(7), 950—955) |
| 91 | Han Z., Wang Z., Zhang X., Ding K., Tetrahedron: Asymmetry, 2010, 21(11—12), 1529—1533 |
| 92 | Wang X., Han Z., Wang Z., Ding K., Angew. Chem. Int. Ed., 2012, 51(4), 936—940 |
| 93 | Freixa Z., Beentjes M. S., Batema G. D., Dieleman C. B., van Strijdonck G. P. F., Reek J. N. H., Kamer P. C. J., Fraanje J., Goubitz K., van Leeuwen P. W. N. M., Angew. Chem. Int. Ed., 2003, 42(11), 1284—1287 |
| 94 | Freixa Z., Kamer P. J., Lutz M., Spek A. L., van Leeuwen P. W. N. M., Angew. Chem. Int. Ed., 2005, 44(28), 4385—4388 |
| 95 | Wang X., Meng F., Wang Y., Han Z., Chen Y. J., Liu L., Wang Z., Ding K., Angew. Chem. Int. Ed., 2012, 51(37), 9276—9282 |
| 96 | Wang X., Guo P., Wang X., Wang Z., Ding K., Adv. Synth. Catal., 2013, 355(14—15), 2900—2907 |
| 97 | Wang X., Guo P., Han Z., Wang X., Wang Z., Ding K., J. Am. Chem. Soc., 2014, 136(1), 405—411 |
| 98 | Li J., Chen G., Wang Z., Zhang R., Zhang X., Ding K., Chem. Sci. 2011, 2(6), 1141—1144 |
| 99 | Li J., Pan W., Wang Z., Zhang X., Ding K., Adv. Synth. Catal., 2012, 354(10), 1980—1986 |
| 100 | Jacquet O., Clément N. D., Freixa Z., Ruiz A., Claver C., van Leeuwen P. W. N. M., Tetrahedron: Asymmetry, 2011, 22(14/15), 1490—1498 |
| 101 | Zheng Z., Cao Y., Chong Q., Han Z., Ding J., Luo C., Wang Z., Zhu D., Zhou Q. L., Ding K., J. Am. Chem. Soc., 2018, 140(32), 10374—10381 |
| 102 | Chen G. Q., Lin B. J., Huang J. M., Zhao L. Y., Chen Q. S., Jia S. P., Yin Q., Zhang X., J. Am. Chem. Soc., 2018, 140(26), 8064—8068 |
| 103 | Huang J., Hong M., Wang C. C., Kramer S., Lin G. Q., Sun X. W., J. Org. Chem., 2018, 83(20), 12838—12846 |
| 104 | Yin L., Xing J., Wang Y., Shen Y., Lu T., Hayashi T., Dou X., Angew. Chem. Int. Ed., 2019, 58(8), 2474—2478 |
| 105 | Liu N., Zhu W., Yao J., Yin L., Lu T., Dou X., ACS Catal., 2020, 10(4), 2596—2602 |
| 106 | Rahmana A., Lin X., Org. Biomol. Chem., 2018, 16(26), 4753—4777 |
| 107 | Chung Y. K., Fu G. C., Angew. Chem. Int. Ed., 2009, 48(12), 2225—2227 |
| 108 | Lundgren R. J., Wilsily A., Marion N., Ma C., Chung Y. K., Fu G. C., Angew. Chem. Int. Ed., 2013, 52(9), 2525—2528 |
| 109 | Wang D., Wang G. P., Sun Y. L., Zhu S. F., Wei Y., Zhou Q. L., Shi M., Chem. Sci., 2015, 6(12), 7319—7325 |
| 110 | Wang Q. G., Zhu S. F., Ye L. W., Zhou C. Y., Sun X. L., Tang Y., Zhou Q. L., Adv. Synth. Catal., 2010, 352(11/12), 1914—1919 |
| 111 | Takizawa S., Kishi K., Yoshida Y., Mader S., Arteaga F. A., Lee S., Hoshino M., Rueping M., Fujita M., Sasai H., Angew. Chem. Int. Ed., 2015, 54(51), 15511—15515 |
| 112 | Sankar M. G., Garcia⁃Castro M., Golz C., Strohmann C., Kumar K., Angew. Chem. Int. Ed., 2016, 55(33), 9709—9713 |
| 113 | Corić I., Müller S., List B., J. Am. Chem. Soc., 2010, 132(49), 17370—17373 |
| 114 | Xu F., Huang D., Han C., Shen W., Lin X., Wang Y., J. Org. Chem., 2010, 75(24), 8677—8680 |
| 115 | Huang D., Xu F. X., Lin X., Wang Y., Chem. Eur. J., 2012, 18(11), 3148—3152 |
| 116 | Luo J., Zhang T., Wang L., Liao G., Yao Q. J., Wu Y. J., Zhan B. B., Lan Y., Lin X. F., Shi B. F., Angew. Chem. Int. Ed., 2019, 58(20), 6708—6712 |
| 117 | Xu B., Zhu S. F., Xie X. L., Shen J. J., Zhou Q. L., Angew. Chem. Int. Ed., 2011, 50(48), 11483—11486 |
| 118 | Xing C. H., Liao Y. X., Ng J., Hu Q. S., J. Org. Chem., 2011, 76(10), 4125—4131 |
| 119 | Li Y. P., Zhu S. F., Zhou Q. L., Org. Lett., 2019, 21(23), 9391—9395 |
| 120 | Tian J. M., Yuan Y. H., Tu Y. Q., Zhang F. M., Zhang X. B., Zhang S. H., Wang S. H., Zhang X. M., Chem. Commun., 2015, 51(49), 9979—9982 |
| 121 | Wang Q., Han C., Feng X., Du H., Chin. J. Org. Chem., 2019, 39(8), 2257—2263(王桥天, 韩彩芳, 冯向青, 杜海峰.有机化学, 2019, 39(8), 2257—2263) |
| 122 | Chang X., Ma P. L., Chen H. C., Li C. Y., Wang P., Angew. Chem. Int. Ed., 2019, 59(23), 8937—8940 |
| [1] | 穆宏文, 吴昊, 高莹莹, 金瑛, 王黎明. 有机催化吲哚碳环上的不对称Friedel⁃Crafts烷基化反应[J]. 高等学校化学学报, 2022, 43(2): 20210571. |
| [2] | 黄明耀, 朱守非. 催化不对称碳硼成键反应研究进展[J]. 高等学校化学学报, 2020, 41(7): 1426. |
| [3] | 郭庆君. 手性磷酰胺类配体不对称催化串联反应合成手性3-取代苯酞化合物[J]. 高等学校化学学报, 2019, 40(10): 2104. |
| [4] | 李霄, 高立国, 弓莹, 马亚军, 马向荣. 杂双金属复合物ZABDP催化芳香酮与芳香醛的直接不对称Aldol反应[J]. 高等学校化学学报, 2017, 38(5): 778. |
| [5] | 李鑫, 韩玉, 谈柏轩, 王斌, 程津培. 苯并呋喃-2,3-二酮和丙酮的不对称Aldol反应[J]. 高等学校化学学报, 2014, 35(9): 1908. |
| [6] | 刘健, 石鑫. 利用"Click"反应制备含有(R)-(+)-BINOL的有机聚合物及其钛配合物在不对称加成反应中的催化性能[J]. 高等学校化学学报, 2013, 34(5): 1052. |
| [7] | 孙杨, 张雷, 徐飞, 龚波林. 原子转移自由基聚合技术制备新型手性配体交换色谱固定相及对手性化合物的拆分[J]. 高等学校化学学报, 2012, 33(12): 2644. |
| [8] | 张庆友, 张丹丹, 索净洁, 李静亚, 龙海林, 许禄. 不对称反应的对映体过量值预测[J]. 高等学校化学学报, 2012, 33(07): 1413. |
| [9] | 张东岩, 汪权, 陈福欣, 袁野, 王锐. C2轴对称樟脑磺酰胺基醇配体催化环己烯乙炔对酮的不对称加成反应[J]. 高等学校化学学报, 2008, 29(9): 1750. |
| [10] | 任奇志, 丁晓健, 原鲜霞, 王爱琴 . 单面桥联手性金属卟啉的合成、构象分析及不对称催化氧化性能[J]. 高等学校化学学报, 2007, 28(11): 2128. |
| [11] | 程司堃 张生勇 王平安 孙晓莉 赵燕. 一种新型可溶性高聚物负载手性配体的合成及其催化烯烃的不对称双羟化反应研究[J]. 高等学校化学学报, 2006, 27(2): 250. |
| [12] | 樊保敏,谢建华,周章涛,张齐,涂永强,周其林 . 手性螺环单磷配体在不对称氢甲酰化反应中的应用 |
| [13] | 范青华, 杨夕强, 刘国华, 陈晓闽, 陈新滋. 新型树状结构手性联二萘酚衍生物的合成及催化性能研究[J]. 高等学校化学学报, 2003, 24(2): 274. |
| [14] | 周懿波, 段学欣, 周其林. 新型手性氢化喹啉噁唑啉配体的合成[J]. 高等学校化学学报, 2001, 22(S1): 130. |
| [15] | 袁建超, 张玉华, 陈敏东, 吕士杰, 王冬梅. 主链光学活性ω-十一烯酸-一氧化碳共聚物的合成与表征[J]. 高等学校化学学报, 2001, 22(7): 1152. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
