

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (11): 2356.doi: 10.7503/cjcu20200319
• 庆祝《高等学校化学学报》复刊40周年专栏 • 上一篇 下一篇
邵伟,LEE Jiyoung,李方园,凌代舜
收稿日期:2020-06-02
出版日期:2020-11-10
发布日期:2020-11-06
基金资助:
SHAO Wei, LEE Jiyoung, LI Fangyuan, LING Daishun( )
)
Received:2020-06-02
Online:2020-11-10
Published:2020-11-06
Contact:
LING Daishun
E-mail:lingds@zju.edu.cn
Supported by:摘要:
癌症严重威胁着人类健康, 因此, 急需开发高效的诊断和治疗方法. 基于光敏剂和近红外激光的光学诊疗将诊断和治疗集于一体, 与传统的手术治疗和化学治疗相比, 光学诊疗显示出无创性和高空间选择性的优点. 有机小分子染料具有确定且易于修饰的化学结构、 良好的重现性和优异的生物相容性, 与无机和聚合物材料相比, 它是一类具有前景的可用于光学诊疗的光敏剂. 本文总结了基于传统小分子染料、 给体-受体(D-A)共轭小分子和聚集诱导发光(AIE)分子等有机小分子的纳米粒子在光学诊疗中的应用. 此外, 对于光学诊疗用有机小分子染料纳米粒子未来的挑战和前景也进行了展望.
中图分类号:
TrendMD:
邵伟, LEE Jiyoung, 李方园, 凌代舜. 有机小分子纳米粒子的光学诊疗应用. 高等学校化学学报, 2020, 41(11): 2356.
SHAO Wei, LEE Jiyoung, LI Fangyuan, LING Daishun. Organic Small Molecule Nanoparticles for Phototheranostics. Chem. J. Chinese Universities, 2020, 41(11): 2356.
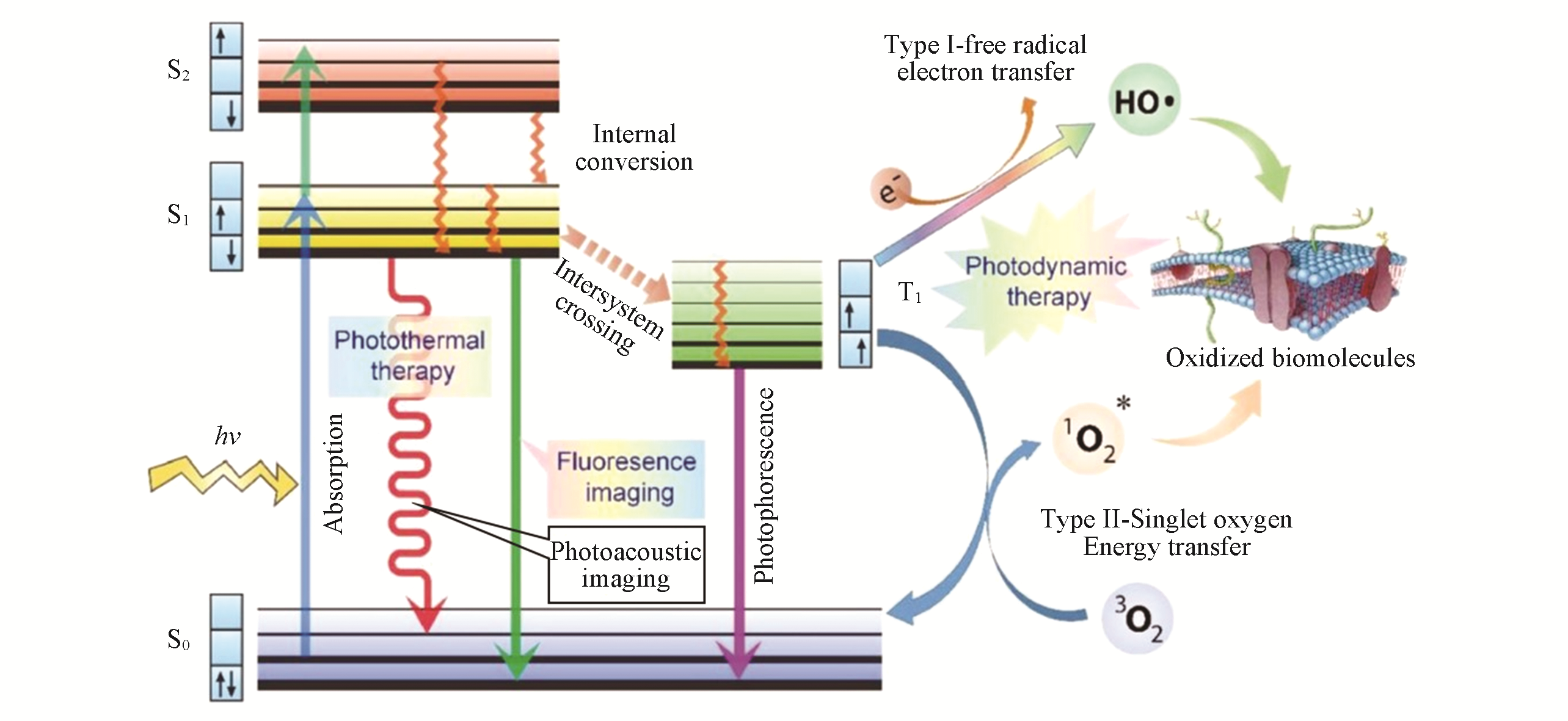
Fig.1 Jablonski diagram showing different photophysical and photochemical processes of photosensitizers under laser excitation employed in phototheranostics[23]Copyright 2017, Wiley?VCH.
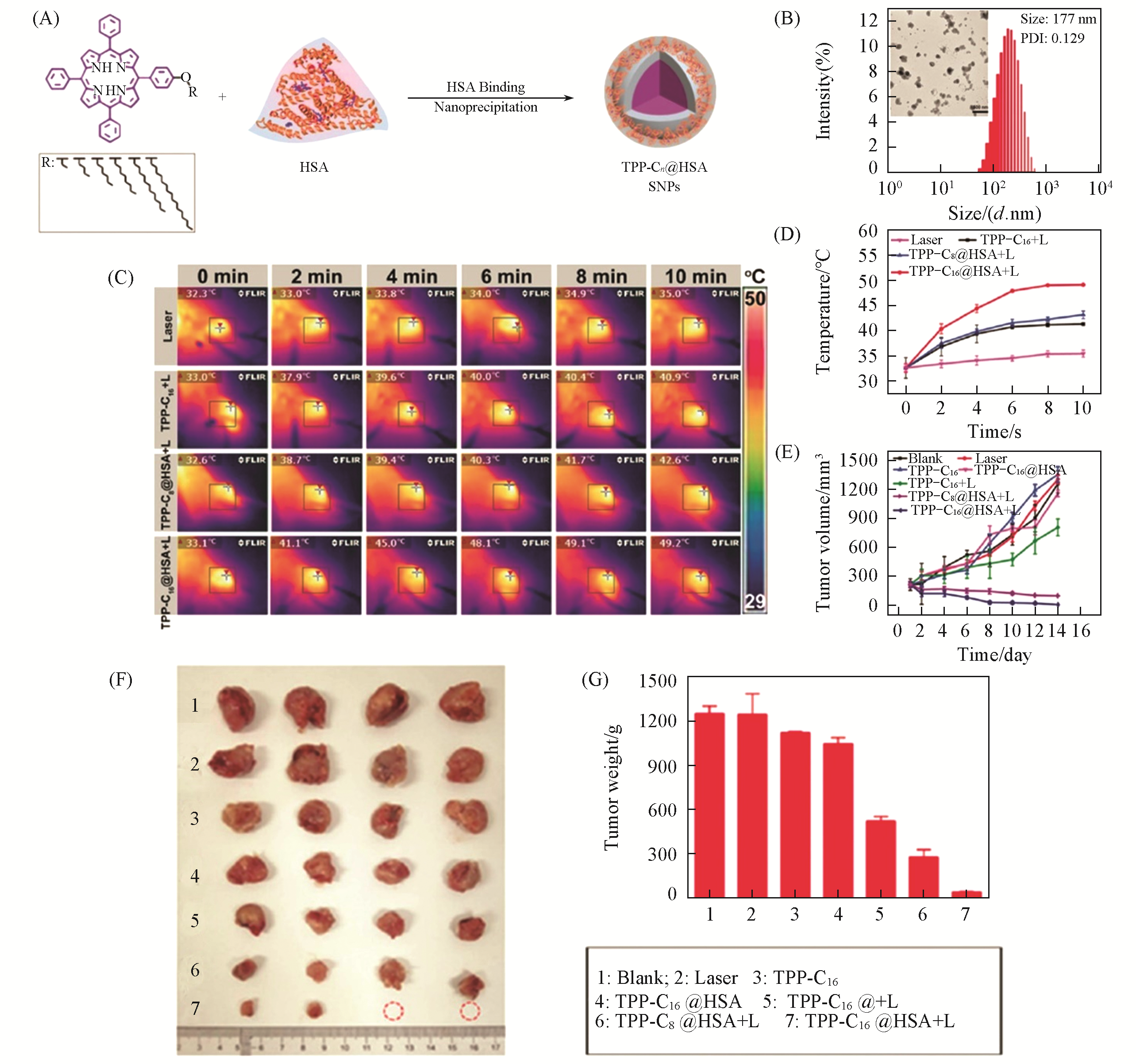
Fig.4 TPP?Cn@HSA SNPs for photothermal therapy[94](A) Self-assembly of the porphyrin derivatives and HSA into NPs (TPP-Cn@HSA SNPs); (B) size distribution and transmission electron microscopy(TEM) image of TPP-C16@HSA SNPs; (C) infrared thermal images of tumor-bearing mice treated with laser only, TPP-C16 NPs, TPP-C8@HSA SNPs, and TPP-C16@HSA SNPs plus irradiation, respectively; (D) temperature changes of tumor locations upon irradiation monitored by an infrared thermal camera in different groups; (E) relative tumor volumes; (F) tumor photographs; (G) tumor weight changes of mice treated with control, light illumination, TPP-C16, TPP-C16@HSA,TPP-C16+L, TPP-C8@HSA+L, and TPP-C16@HSA+L.Copyright 2019, Royal Society of Chemistry.
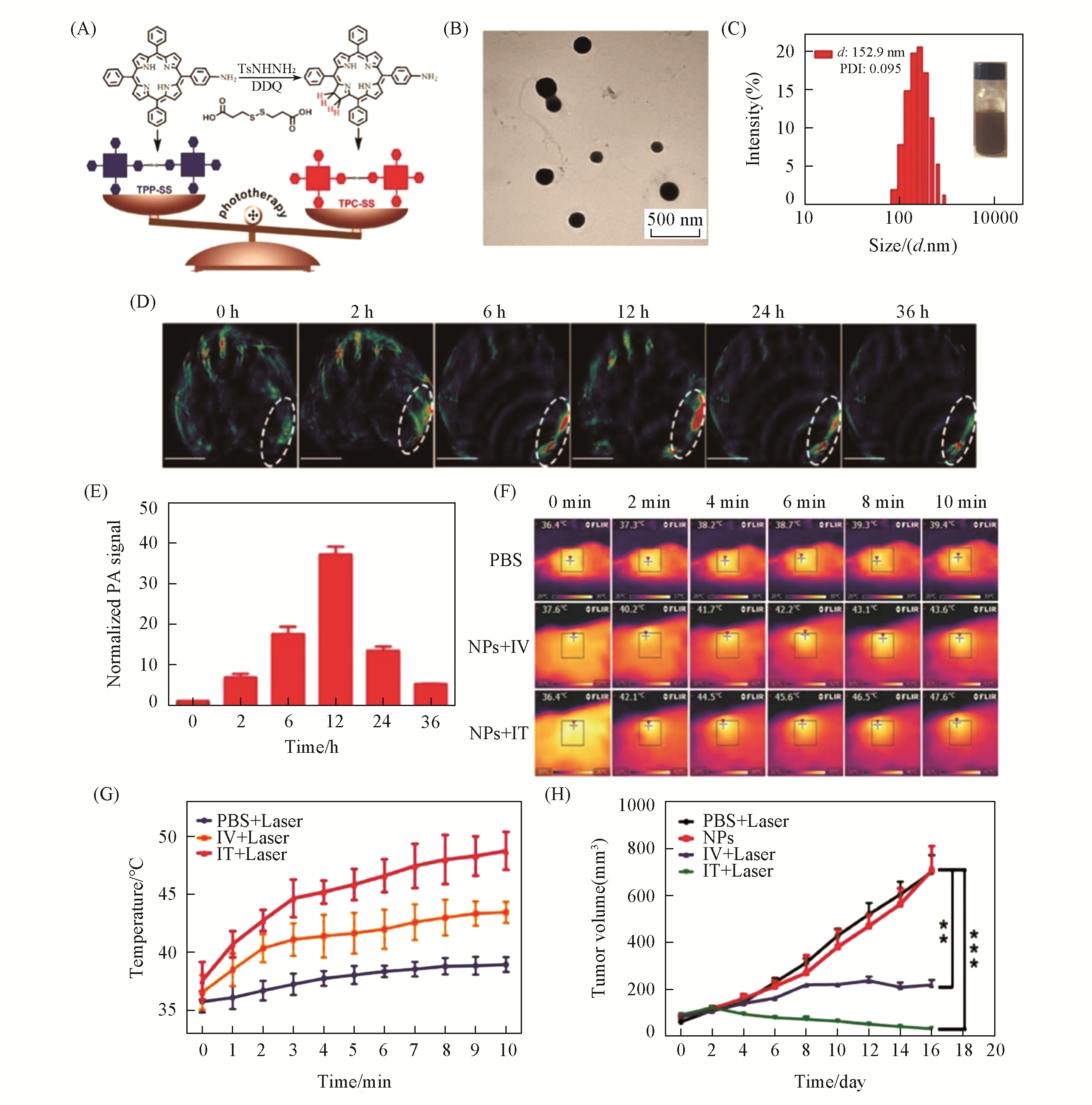
Fig.5 TPC?SS NPs for PAI?guided photothermal therapy[31](A) Schematic illustrations of the synthetic procedures of TPP-SS and TPC-SS; (B) TEM image of TPC-SS NPs; (C) DLS profile of TPC-SS NPs; (D) In vivo photoacoustic(PA) imaging of tumor tissue before and after i.v. injection of TPC-SS NPs upon 680 nm laser irradiation at different time points(0, 2, 6, 12, 24, and 36 h); (E) normalized PA signals in the tumor at different times; (F) infrared thermal images of U14 tumor-bearing mice injected with phosphate buffer saline(PBS) and TPC-SS NPs by intravenous and intratumor injection,respectively, exposed to 635 nm laser at a power density of 360 and 240 J/cm2 recorded at different time intervals, respectively; (G) temperature of tumors monitored by the infrared thermal camera in different groups upon laser irradiation; (H) relative tumor volume changes of mice treated with PBS+laser, only TPC-SS NPs, TPC-SS NPs by intravenous injection+laser and TPC-SS NPs by intratumor injection+Laser, statistical significance: **p≤0.01; ***p≤0.001.Copyright 2018, Wiley-VCH.
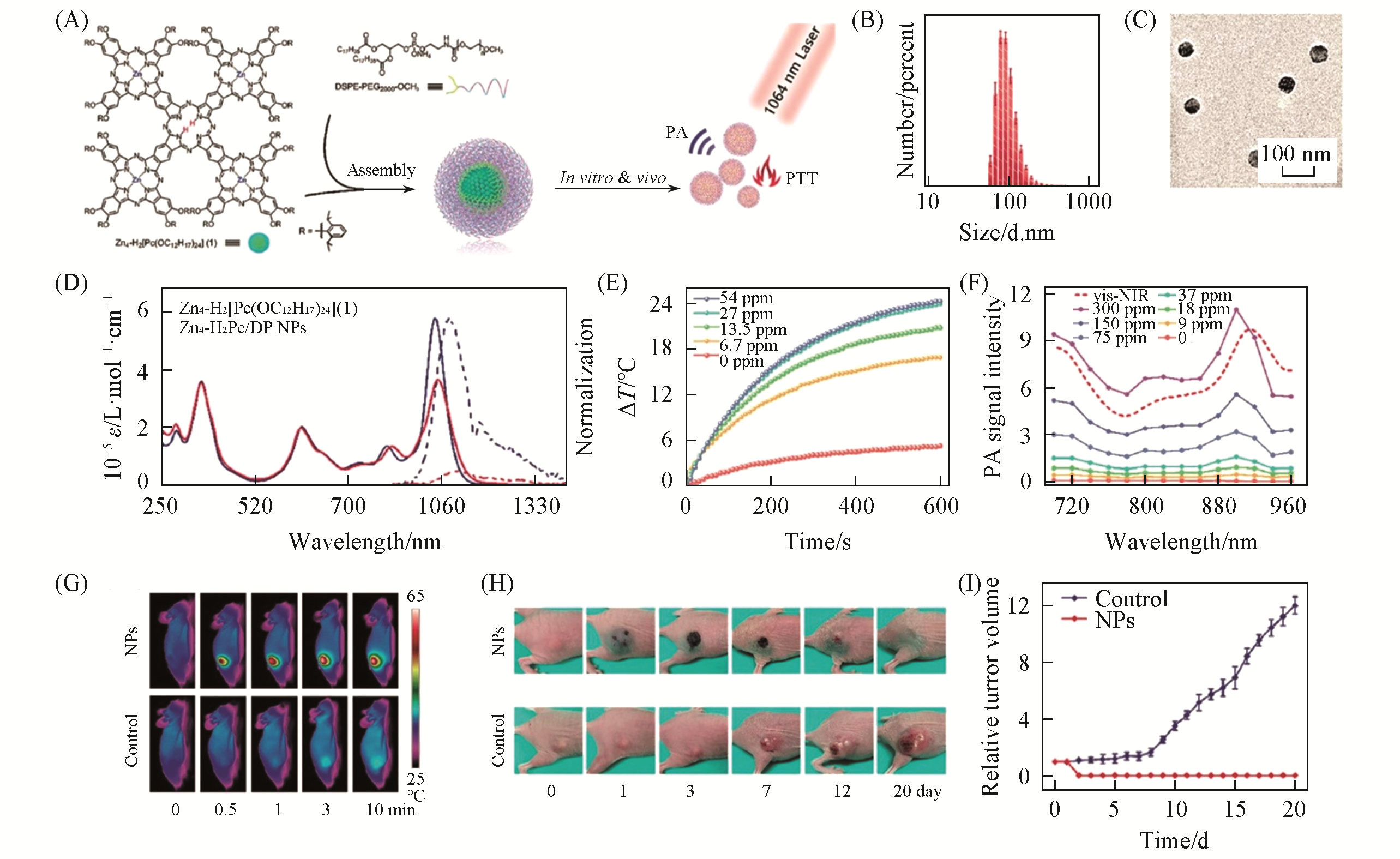
Fig.6 Zn4?H2Pc/DP NP for PAI?guided NIR?Ⅱ photothermal therapy[93](A) Illustration of Zn4-H2Pc/DP NP fabrication for photothermal therapy and photoacoustic imaging; (B) DLS profile of Zn4-H2Pc/DP NP; (C) TEM image of Zn4-H2Pc/DP NP; (D) UV-Vis-NIR absorption and fluorescence emission spectra of Zn4-H2· [Pc(OC12H17)24](1) in CH2Cl2 and Zn4-H2Pc/DP NPs in water, respectively; (E) temperature change curves of Zn4-H2Pc/DP NPs exposed to the 1064 nm laser at various concentrations(0.9 W/cm2, 10 min); (F) photoacoustic spectra of Zn4-H2Pc/DP NPs in the water at various concentrations(dashed line: vis-NIR absorption spectrum of Zn4-H2Pc/DP NPs); (G) IR thermal images of mice under irradiation at varied time intervals(0, 0.5, 1, 3 and 10 min); (H) digital photos of mice before and after treatment at varied time intervals(0, 1, 3, 7, 12 and 20 day); (I) tumor growth curves of mice in control and NPs after treatment (n=5).Copyright 2019, Royal Socity of Chemistry.
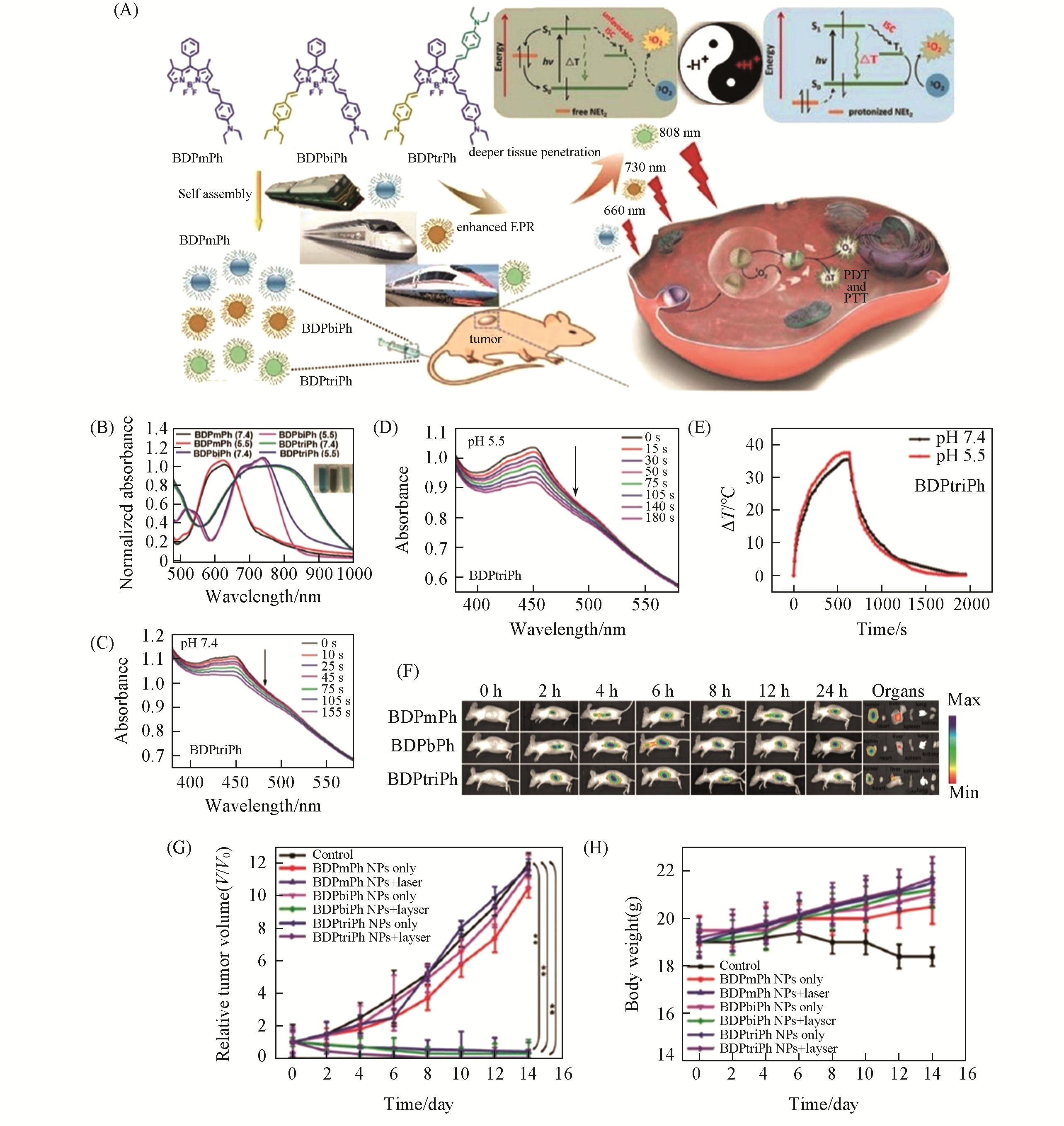
Fig.7 Penetration depth?tunable BODIPY?based nanoparticles for phototherapy[125](A) Schematic illustration of the preparation and application of BDPmPh, BDPbiPh, and BDPtriPh NPs and the mechanism of pH-triggered enhanced PTT/PDT; (B) normalized absorption spectra of BDPmPh, BDPbiPh, and BDPtriPh NPs in PBS at different pH(7.4 and 5.5); (C) degradation of 1,3-diphenylisobenzofuran(DPBF) in the presence of BDPtriPh NPs in PBS at pH of 7.4; (D) degradation of DPBF in the presence of BDPtriPh NPs in PBS at pH of 5.5; (E) photothermal heating curves of BDPtriPh NPs in PBS at different pH(7.4 and 5.5); (F) In vivo fluorescence images of mice treated with BDPmPh, BDPbiPh, and BDPtriPh NPs for different times and biodistribution of the NPs in tumors and major organs;(G) tumor growth curves of the mice in different groups during the treatments;(H) body weight changes of the mice in different groups during the treatments.Copyright 2018, Royal Society of Chemistry.
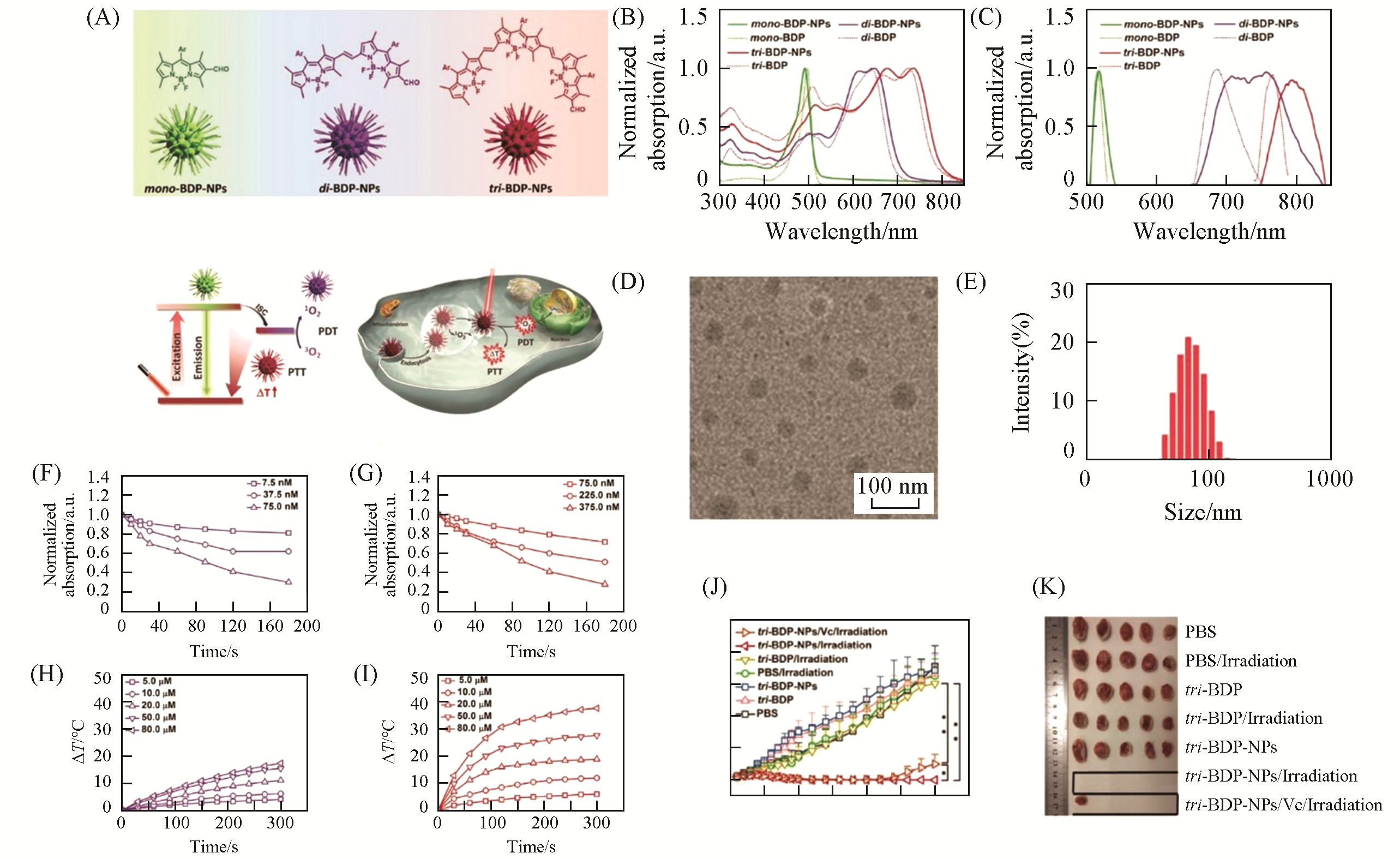
Fig.8 BODIPY?based nanoparticles with tunable phototherapeutic effect[123](A) The chemical structures and nanoparticles of CPs, photoconversion routes of the CPs, and synergistic PTT/PDT of tri-BDP-NPs against tumor cells under laser irradiation; normalized absorption spectra(B) and emission spectra(C) of mono-BDP-NPs, di-BDP-NPs, and tri-BDP-NPs as compared to free mono-BDP, di-BDP, and tri-BDP in DMSO; (D) TEM image of tri-BDP-NPs; (E) size distribution of tri-BDP-NPs determined by DLS; normalized absorbance of DPBF at 410 nm in the solutions of di-BDP-NPs(F) and tri-BDP-NPs(G) at different concentrations under 660 or 785 nm laser irradiation(0.5 W/cm2, 3 min); temperature elevations of di-BDP-NPs(H) and tri-BDP-NPs(I) under 660 or 785 nm laser irradiation(0.5 W/cm2, 5 min); (J) tumor growth curves of the mice in different groups; (K) photograph of tumors of the mice in different groups at the end of treatments.Copyright 2018, Wiley-VCH.
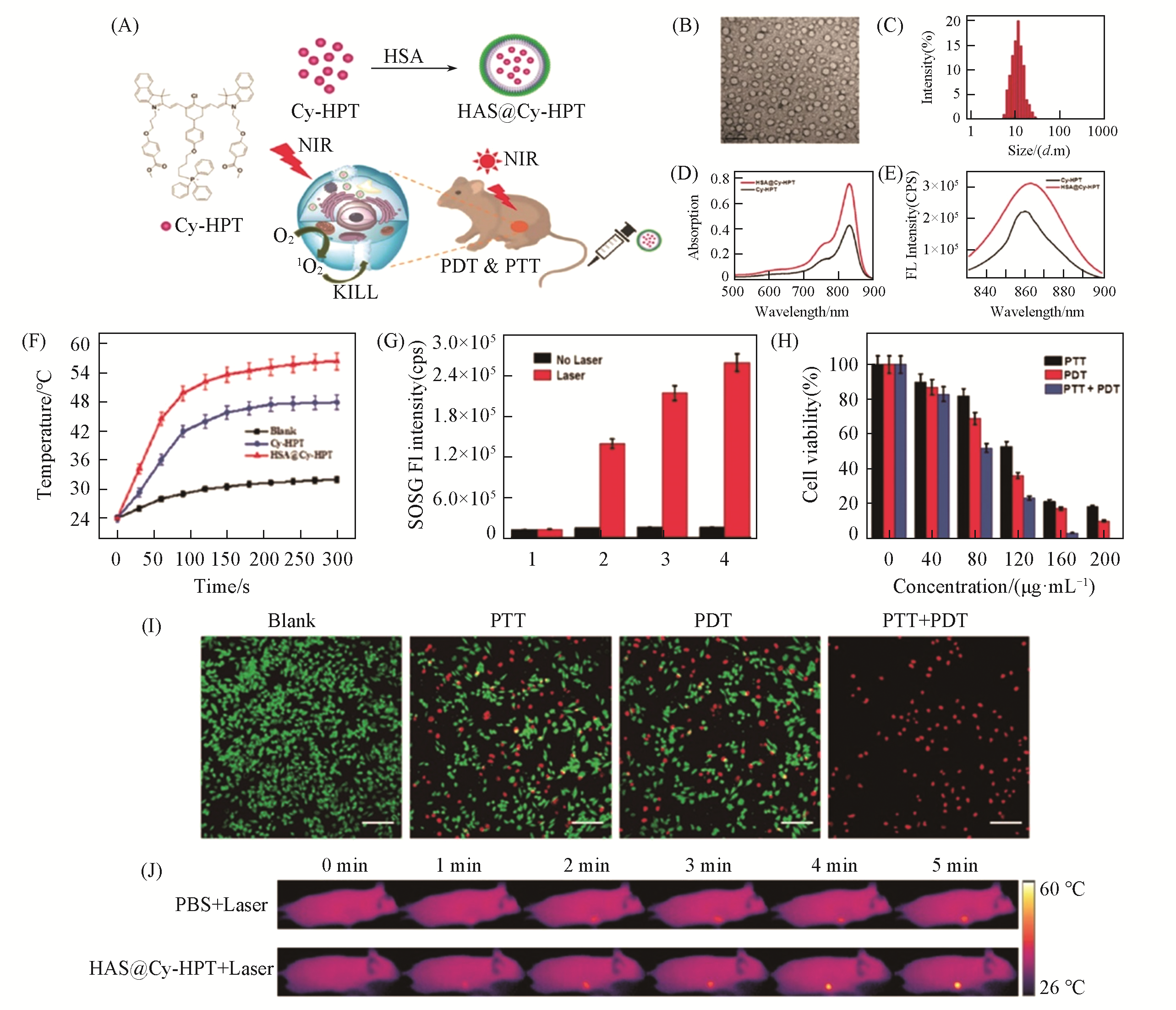
Fig.9 HSA@Cy?HPT for PTT and PDT[152](A) The chemical structure of Cy-HPT, preparation of HSA@Cy-HPT and NIR laser-induced PTT/PDT of HSA@Cy-HPT; (B) TEM image of HSA@Cy-HPT, scale bar: 20 nm; (C) size distribution of HSA@Cy-HPT determined by DLS;(D) absorption spectra of Cy-HPT and HSA@Cy-HPT;(E) fluorescence spectra of Cy-HPT and HSA@Cy-HPT;(F) photothermal heating curves of Cy-HPT, HSA@Cy-HPT and blank sample under laser irradiation;(G) fluorescence intensity of SOSG: 1. blank, 2. ICG,3. Cy-HPT and 4.HSA@Cy-HPT;(H) MTT assay results of PTT, PDT and synergistic PTT/PDT treatments with HSA@Cy-HPT in HepG2 cells; (I) fluorescence images of CalceinAM/PI co-stained HepG2 cells after PTT, PDT or synergistic PTT/PDT treatments with HSA@Cy-HPT; scale bar: 60 μm; (J) infrared thermographs of subcutaneous HepG2 tumor xenograft mice during a 5 min NIR laser irradiation.Copyright 2019, Royal Society of Chemistry.
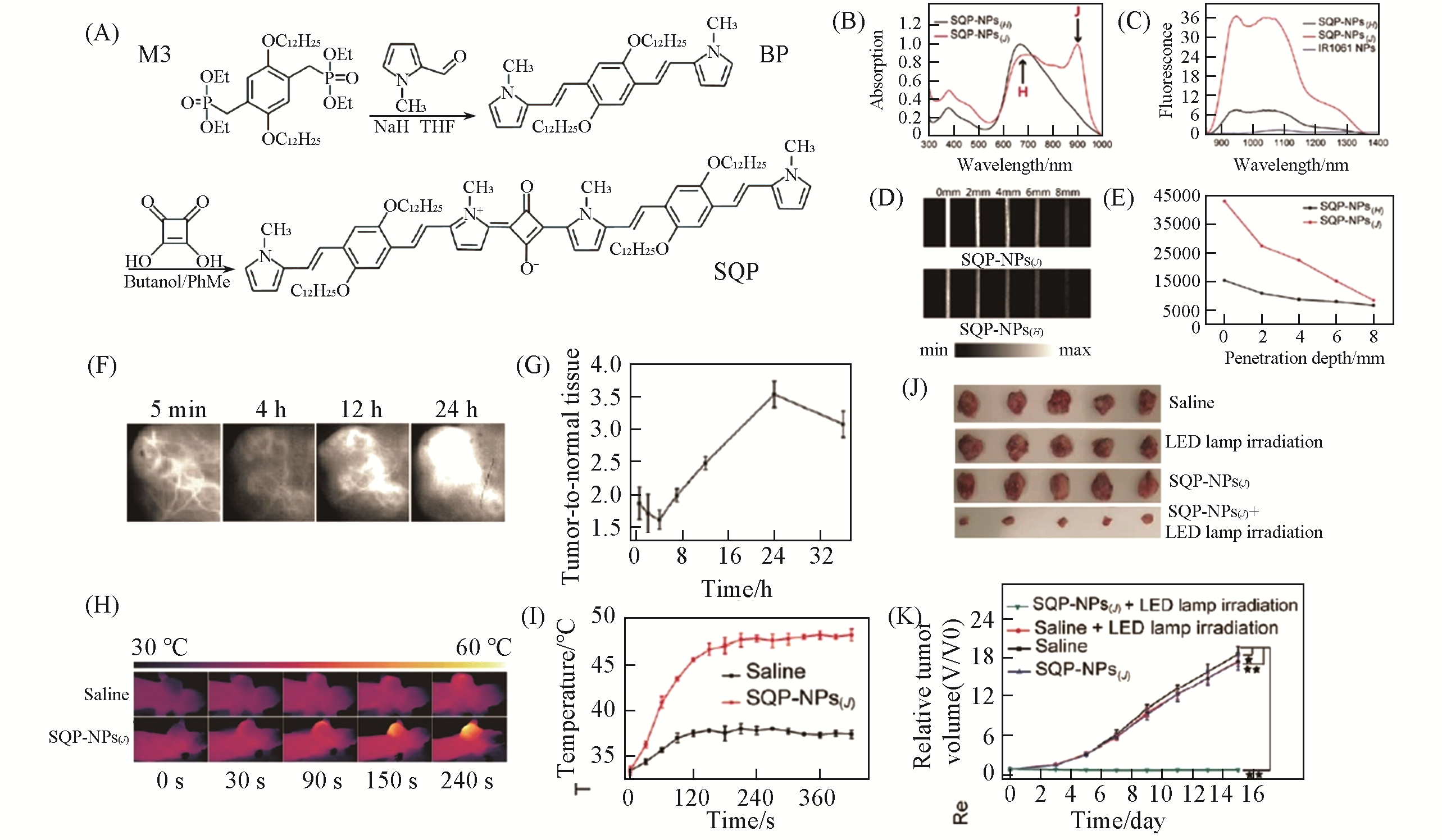
Fig.10 SQP?NPs(J) for NIR?II fluorescence imaging?guided PTT[165](A) Synthesis of SQP; (B) UV-Vis-NIR absorption spectra of the SQP-NPs(J) and SQP-NPs(H); (C) NIR-Ⅱ fluorescence emission spectra of the SQP-NPs(J), SQP-NPs(H), and IR1061 NPs at the same concentration of 10μg/mL under 808 nm excitation; (D) NIR-II fluorescence images of the SQP-NPs(J) andSQP-NPs(H) at different depths; (E) NIR-Ⅱ fluorescence intensity of the SQP-NPs(J) and SQP-NPs(H) at different depths; (F) NIR-Ⅱ fluorescence images of tumor sites at different times after administrating SQP-NPs(J); (G) tumor-to-normal tissue ratio of the tumor sites at different times after intravenous injection of SQP-NPs(J); (H) infrared thermographs of the tumor bearing mice after intravenous injection of saline and SQP-NPs(J) under laser irradiation(810 nm, 0.8 W·cm-2) at indicated time points; (I) temperature elevation curves of the tumors of the mice treated with saline and SQP-NPs(J) under laser irradiation; (J) photographs of the tumors of the mice in different groups after treatments; (K) tumor growth curves of the mice in different groups.Copyright 2018, Royal Society of Chemistry.
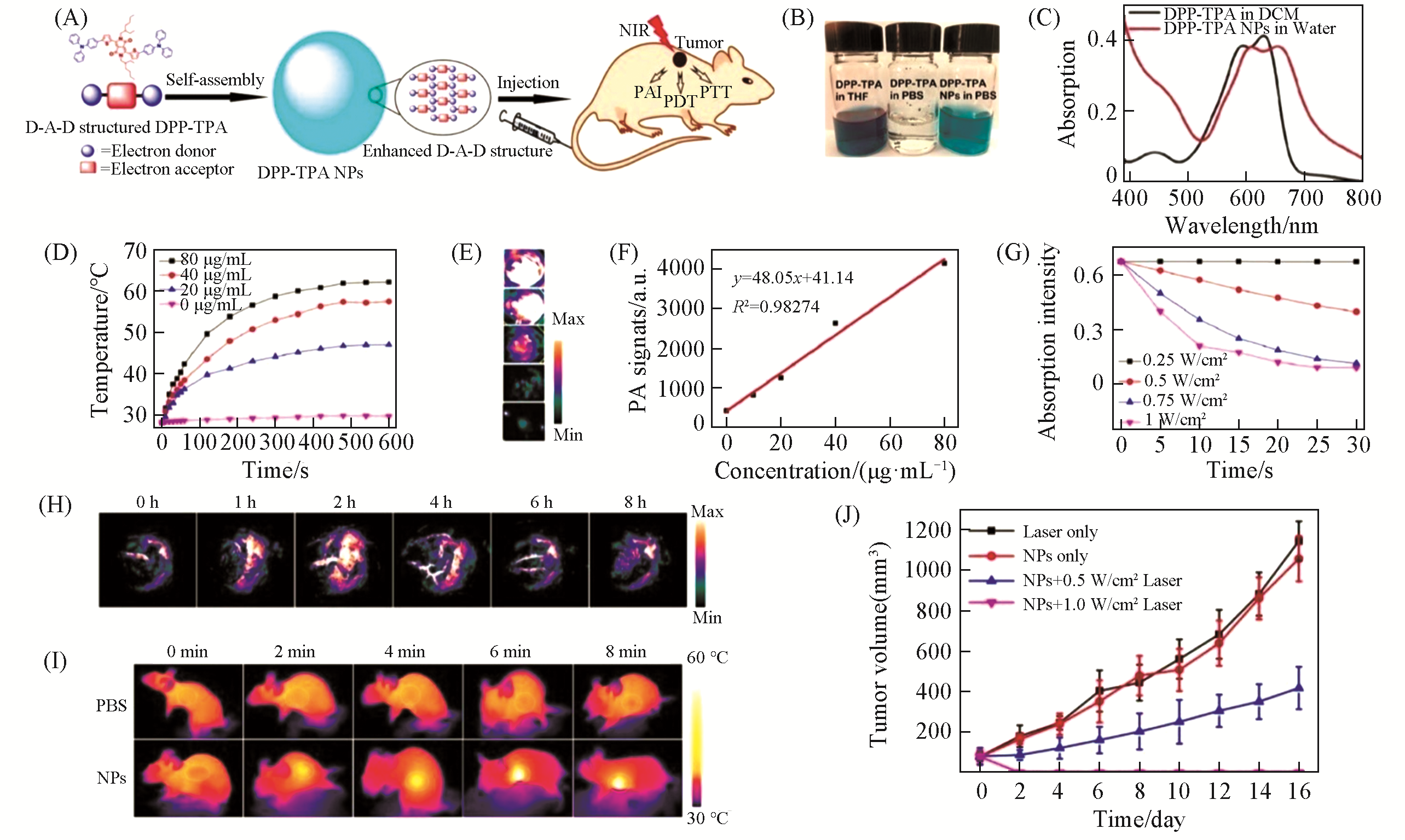
Fig.11 DPP?TPA NPs for PAI?guided synergistic PTT/PDT[58](A) Schematic demonstration of DPP-TPA NPs as phototheranostic agents for PAI-guided PTT/PDT; (B) photographs of DPP-TPA in THF and PBS, and DPP-TPA NPs in PBS; (C) UV-Vis absorption spectra of DPP-TPA in dichloromethane (DCM) and DPP-TPA NPs in PBS; (D) photothermal heating curves of DPP-TPA NPs with different concentrations(660 nm, 1.0 W/cm2); (E) PA images of DPP-TPA NPs with different concentrations; (F) linear relationship between the PA signal intensity and DPP-TPA NPs concentration; (G) absorption of DPP-TPA at 418 nm mixed with DPBF in DCM over time under 660 nm laser irradiation; (H) PA images of tumor sites at different time intervals after intravenous injection of DPP-TPA NPs into tumor-bearing mice; (I) infrared thermographs of tumor sites after injecting PBS and DPP-TPA NPs for 2 h under different laser irradiation times; (J) tumor volume changes of the mice in different groups.Copyright 2016, American Chemical Society.
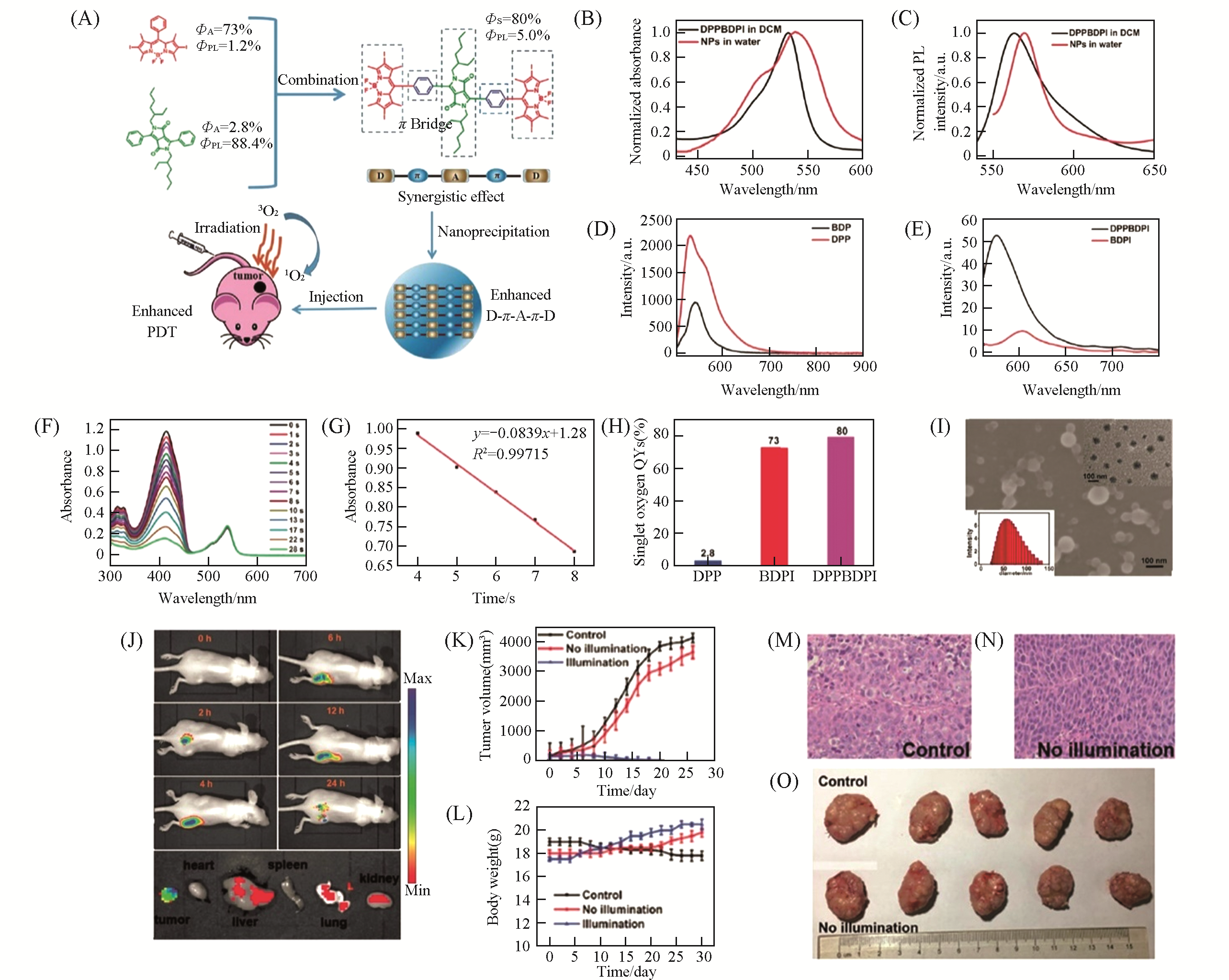
Fig.12 DPPBDPI NPs for enhanced PDT[67](A) Schematic illustration of the DPPBDPI NPs with enhanced 1O2 and fluorescence quantum yields as a theranostic agent for PDT. Normalized absorption(B) and emission spectra(C) of DPPBDPI in DCM and DPPBDPI NPs in water; (D) emission spectra of DPP and BDP in DCM, showing absolute PL quantum yields of 88.4% and 24.7%,respectively; (E) emission spectra of boron dipyrrome-thene(BDPI) and DPPBDPI, showing absolute photoluminescence(PL) quantum yields of 1.2% and 5.0%, respectively; (F) the degradation of DPBF in the presence of DPPBDPI in DCM and xenon lamp irradiation; (G) linear fit of the absorption and the irradiation time; (H) singlet oxygen quantum yields of DPP, BDPI and DPPBDPI; (I) scanning electron microscopy(SEM), transmission electron microscopy(TEM) and dynamic light scattering(DLS) of DPPBDPI NPs; (J) in vivo time-dependent fluorescence imaging and biodistribution of DPPBDPI NPs in the tumor, heart, liver, spleen, lung and kidney after injection for 24 h; (K) tumor volume change during treatment over a month; (L) the body weight change reported every two days; (M, N) H&E staining of the tumor histologic section for the control(M) and no illumination groups(N); (O) photographs of the tumors of the sacrificed mice after treatment.Copyright 2018, Royal Society of Chemistry.
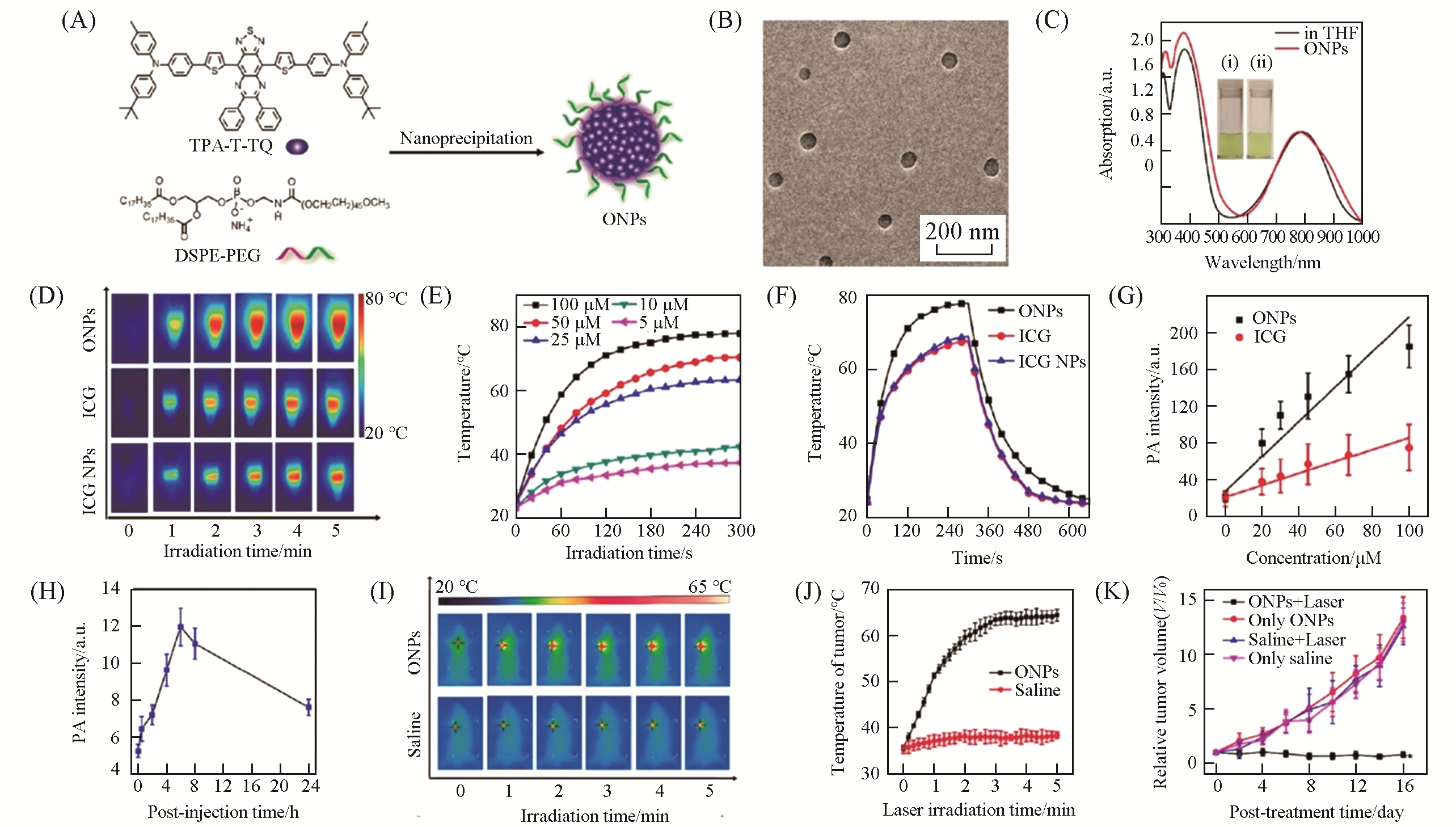
Fig.13 ONPs for PAI?guided PTT[63](A) Chemical structure of TPA-T-TQ and the nanoprecipitation method for the preparation of TPA-T-TQ ONPs; (B) TEM image of TPA-T-TQ ONPs; (C) UV-Vis-NIR absorption spectra of TPA-T-TQ in THF and TPA-T-TQ ONPs in water; (D) infrared thermographs of ICG, ICG NPs, and TPA-T-TQ ONPs under laser irradiation(808 nm, 0.8 W/cm2) for different times; (E) the photothermal heating curves of TPA-T-TQ ONPs with different concentrations(808 nm, 0.8 W/cm2); (F) comparison of the photothermal effects of ICG, ICG NPs, and TPA-T-TQ ONPs with the same concentration (100 μmol/L) under laser irradiation (808 nm 0.8 W/cm2); (G) PA intensities of ICG and TPA-T-TQ ONPs versus their concentrations; (H) PA intensity at the tumor site versus the time post-injection; (I) infrared thermographs of 4T1 tumor-bearing mice under laser irradiation(808 nm, 0.5 W/cm2) for different times; (J) the temperature of tumors of the mice treated with saline and TPA-T-TQ ONPs as a function of laser irradiation time(808 nm, 0.5 W/cm2); (K) tumor growth curves of the mice in different treatment groups.Copyright 2017, American Chemical Society.
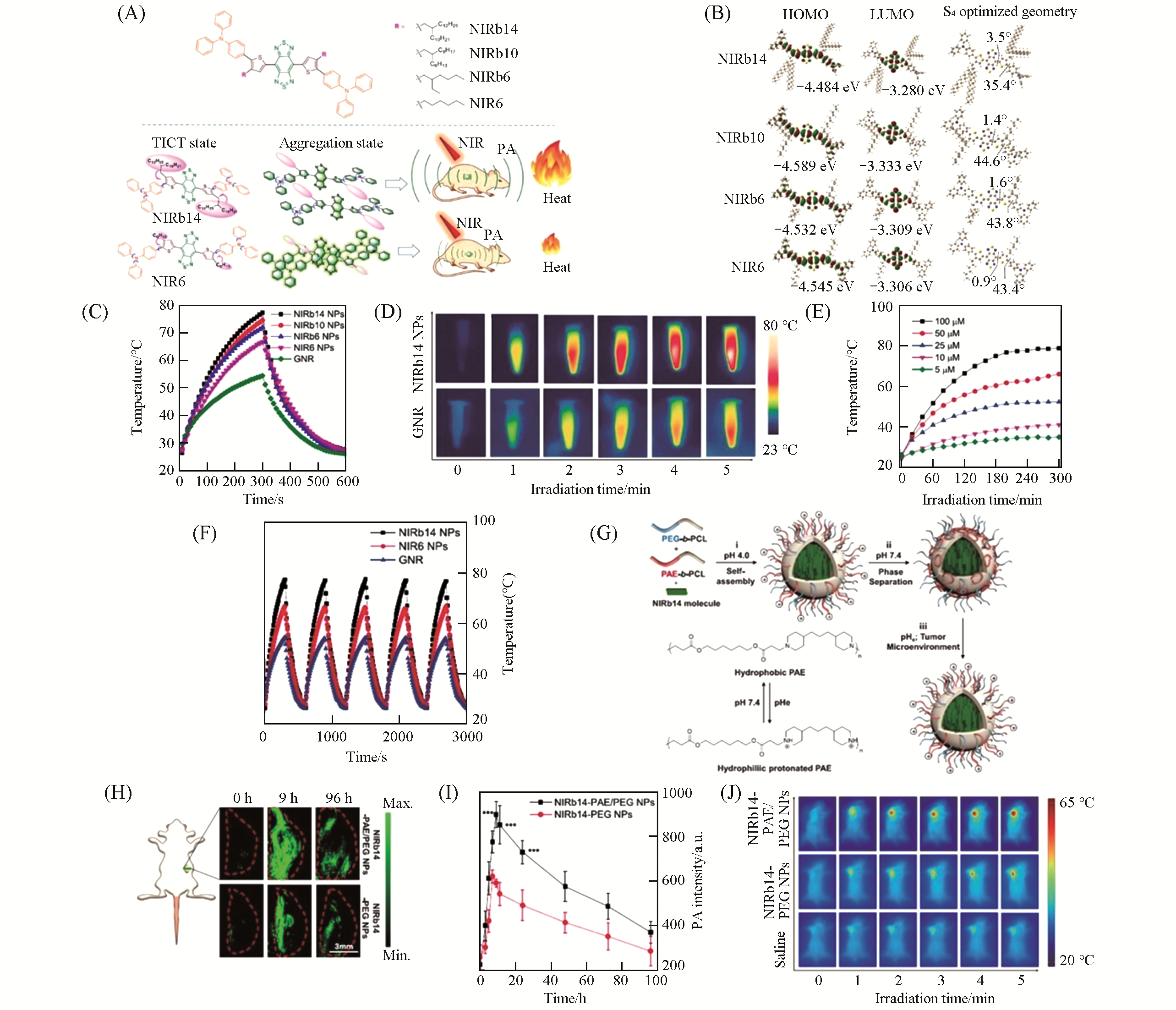
Fig.14 NIRb14 NPs for PAI?guided PTT[62](A) Chemical structures of NIRb14, NIRb10, NIRb6 and NIR6, and the schematic illustration of the TICT states of the molecules in solution and aggregation states;(B) highest occupied molecular orbital(HOMO), lowest unoccupied molecular orbital(LUMO) distributions and optimized S0 geometries of the molecules; (C) photothermal heating curves of NIRb14, NIRb10, NIRb6, NIR6 NPs, and GNRs with the same concentration(100 μmol/L, 808 nm, 0.8 W/cm2); (D) infrared thermographs of NIRb14 NPs(100 μmol/L) and GNRs under laser irradiation(808 nm, 0.8 W/cm2) for different times; (E) photothermal heating curves of NIRb14 NPs with different concentrations(808 nm, 0.8 W/cm2); (F) photothermal stability of NIRb14 NPs, NIR6 NPs, and GNRs during five heating/cooling cycles; (G) schematic illustration of the preparation of pH-responsive NIRb14-PAE/PEG NPs; (H) PA images of the tumor sites of mice before and after intravenous injection of NIRb14-PAE/PEG or NIRb14-PEG NPs; (I) PA intensity at the tumor site versus the time post-injection of NIRb14-PAE/PEG and NIRb14-PEG NPs, respectively; (J) infrared thermographs of 4T1 tumor-bearing mice under laser irradiation(808 nm, 0.8 W/cm2) for different times.Copyright 2019, American Chemical Society.
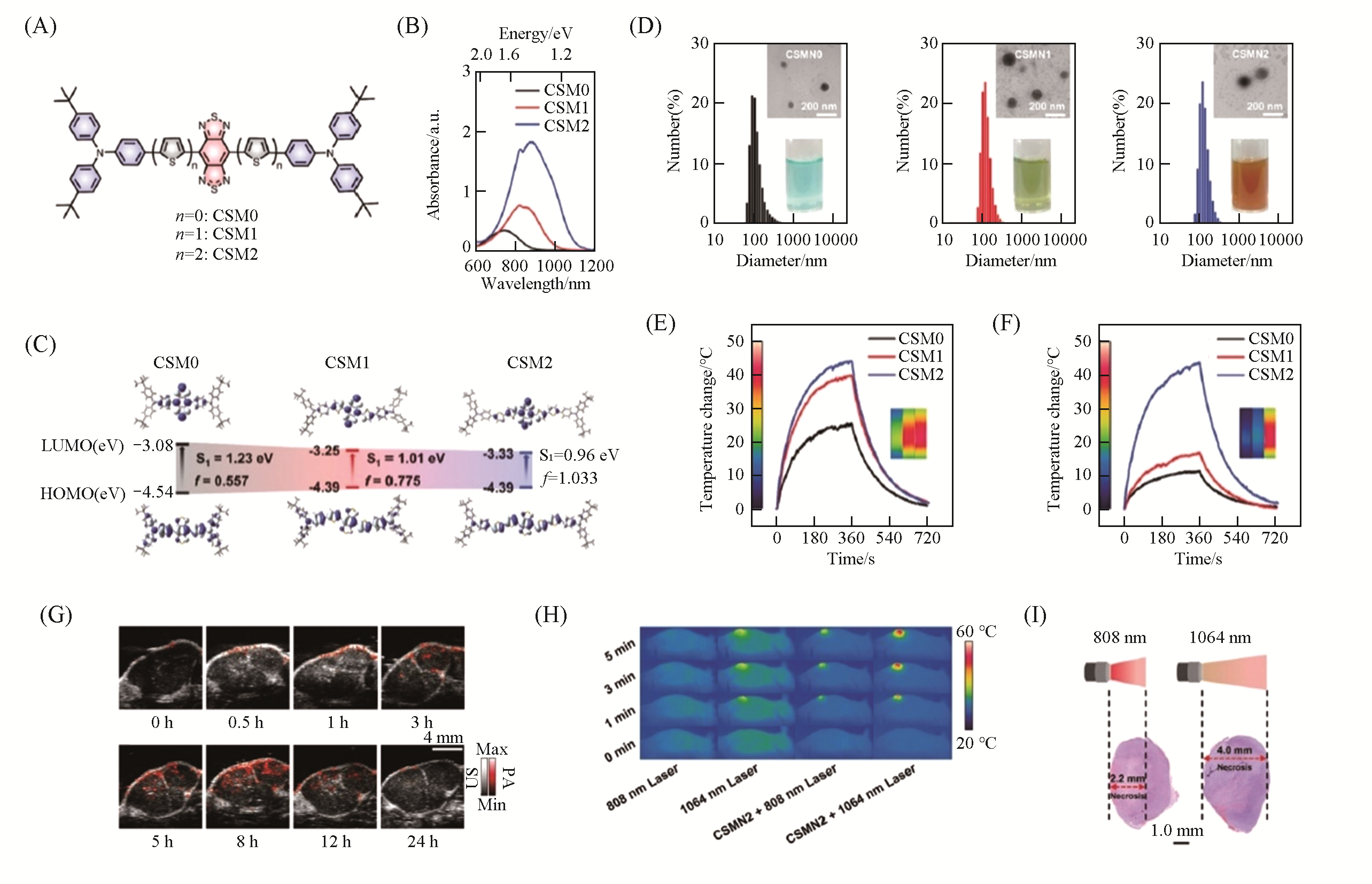
Fig.15 Molecularly engineered CSMN2 for PAI?guided NIR?II PTT[188](A) Chemical structures of CSM0—2; (B) Vis-NIR absorption spectra of CSM0—2 in chloroform; (C) frontier molecular orbital distributions, energy levels, and corresponding oscillator strengths for CSM0—2 calculated at the B3LYP/6-31G* level; (D) DLS profiles of CSMN0—2(insets are the TEM images and digital photographs of CSMN0—2 in water);photothermal heating curves of CSMN0—2(100 μmol/L) under 808(E) and 1064(F) nm laser irradiation with a power density of 1.0 W/cm2(inset shows the infrared thermographs of the samples after irradiating for 6 min, from left to right: CSMN0, CSMN1 and CSMN2, respectively); (G) PA images of the tumor site at different time intervals post-injection; (H) the infrared thermographs at the tumor sites in the groups of 808 nm laser, 1064 nm laser, CSMN2+808 nm laser and CSMN2+1064 nm laser during laser irradiation at different time intervals; (I) H&E staining of the whole tumors of the mice after the treatments of CSMN2+808 nm laser and CSMN2+1064 nm laser.Copyright 2020, Royal Society of Chemistry.
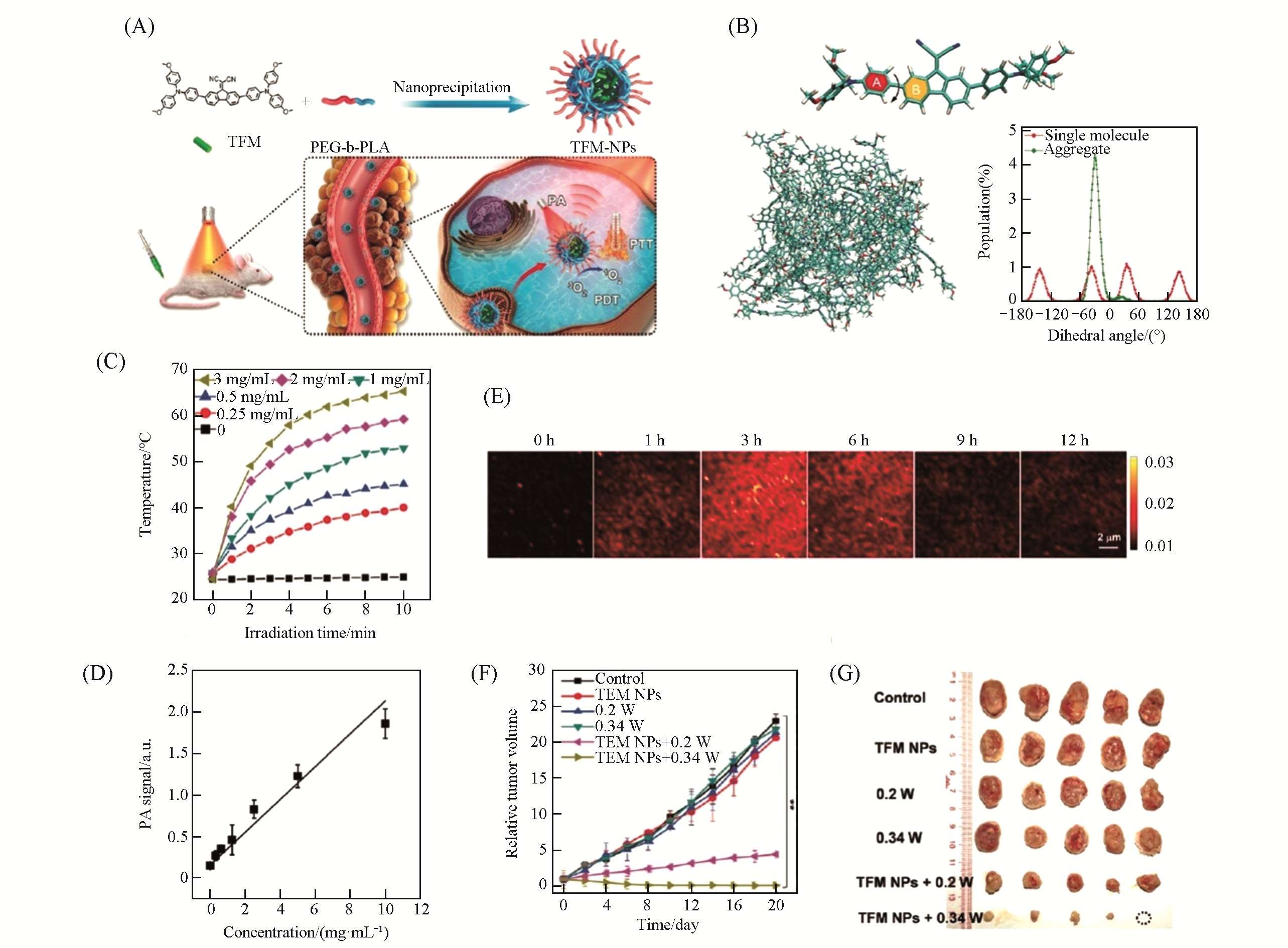
Fig.16 TFM NPs for PAI?guided PTT/PDT[72](A) Preparation of TFM NPs using nanoprecipitation method and the schematic illustration of the use of TFM NPs for PAI-guided PTT/PDT; (B) molecular dynamics(MD) simulations to study the molecular geometry of TFM in single molecular state and the packing style of TFM molecules in aggregation state; (C) photothermal heating curves of TFM NPs with different concentrations under laser irradiation(633 nm, 0.5 W/cm2); (D) PA intensities of TFM NPs at 680 nm at different concentrations; (E) PA images of EMT-6 tumor sites after intravenous injection of TFM NPs; (F) tumor growth curves of the mice in different groups; (G) tumor images collected from the mice in different groups after treatments.Copyright 2019, Wiley-VCH.
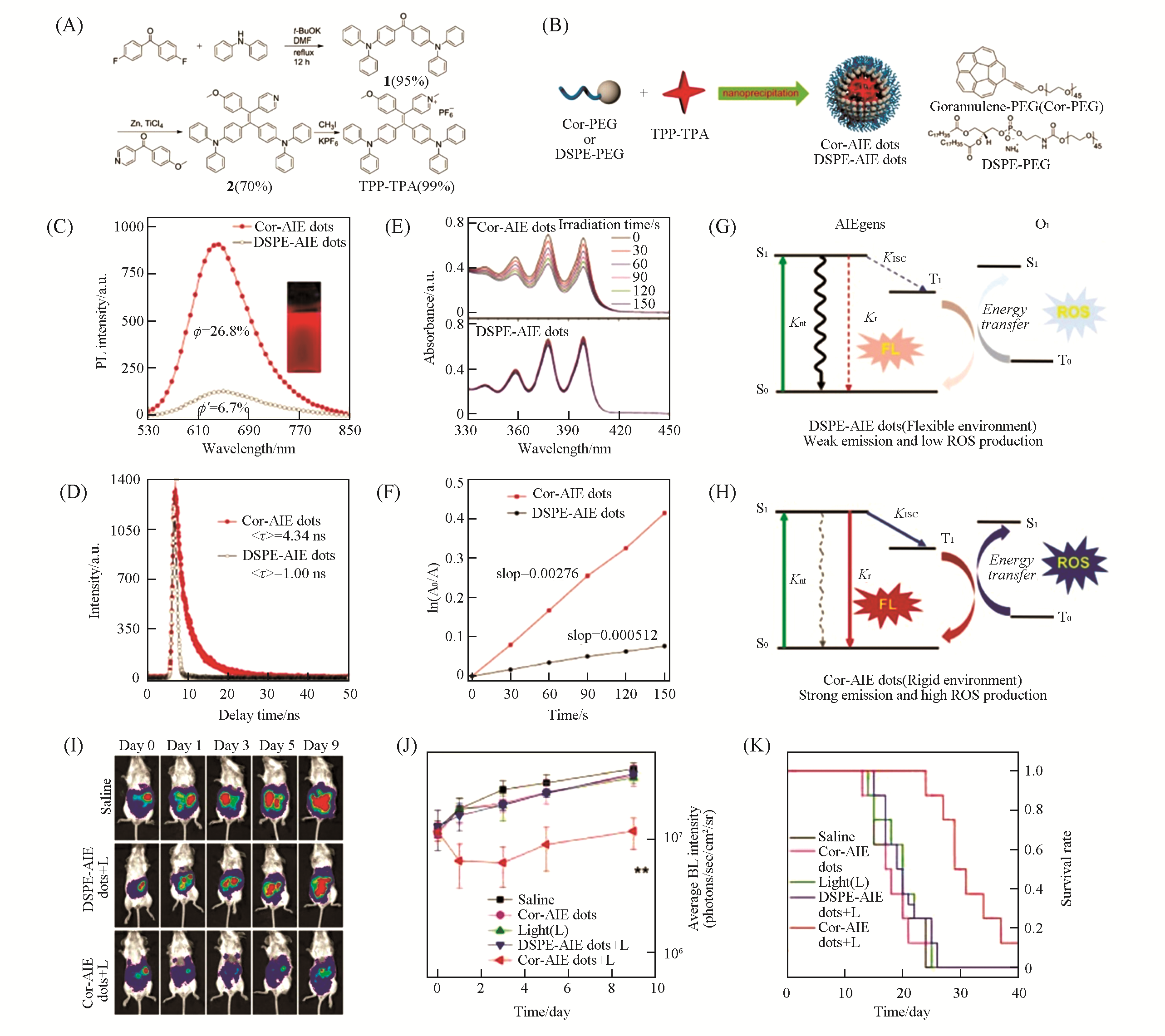
Fig.17 Cor?AIE dots for enhanced PDT[70](A) Synthesis of NIR-emissive TPP-TPA with indicated yield for each step; (B) schematic depiction of the preparation of Cor-AIE dots and DSPE-AIE dots using the nanoprecipitation method; PL(C) and fluorescence lifetime spectra(D) of Cor-AIE dots and DSPE-AIE dots; UV-Vis absorption spectra(E) and decomposition rate(F) of 9,10-anthracenediyl-bis(methylene)-dimalonic acid(ABDA) for Cor-AIE dots and DSPE-AIE dots under light irradiation; (G, H) Jablonski diagram showing the nonradiative, radiative, and ISC processes for DSPE-AIE dots and Cor-AIE dots; (I) time-dependent fluorescence images of peritoneal carcinomatosis-bearing mice in “Saline”, “DSPE-AIE dots+L” and “Cor-AIE dots+L” groups; (J) average fluorescence intensities of intraperito-neal tumors on days 0, 1, 3, 5 and 9 in different groups; (K) survival rate curves of the mice in different groups.Copyright 2018, Wiley-VCH.
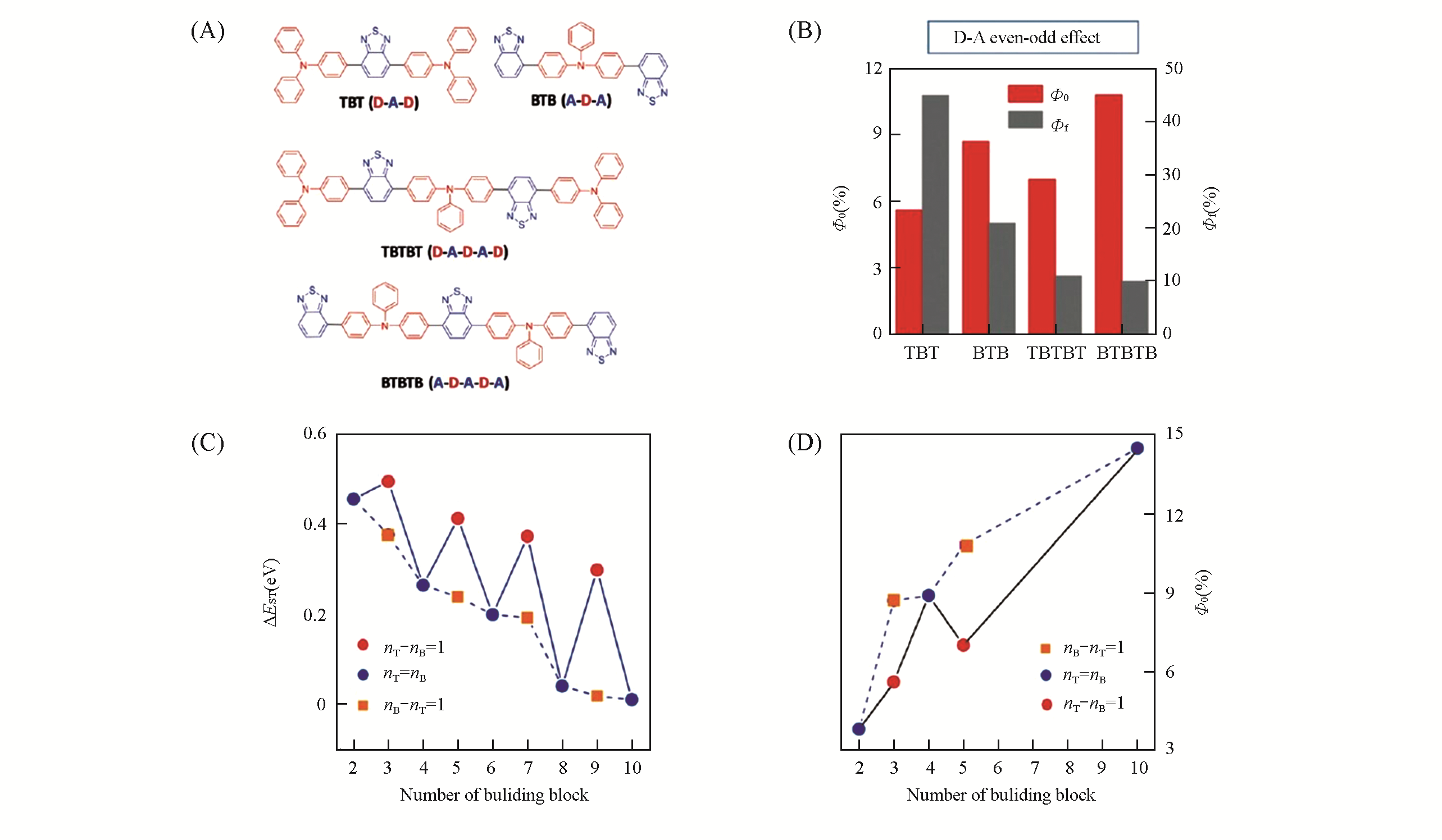
Fig.18 D?A even?odd effect on the photosensitization of the AIEgens[71]Chemical structures(A) and 1O2 and fluorescence quantum yields(B) of TBT, BTB, TBTBT and BTBTB;dependence of ΔEST(C) and 1O2 quantum yield(D) on the number of building blocks; nT =number of donor units(T); nB=number of acceptor units (B).Copyright 2018, Wiley-VCH.
| 1 | Ferrari M., Nat. Rev. Cancer, 2005, 5, 161—171 |
| 2 | Hong G., Diao S., Antaris A. L., Dai H., Chem. Rev., 2015, 115, 10816—10906 |
| 3 | Huang X., Zhang W., Guan G., Song G., Zou R., Hu J., Acc. Chem. Res., 2017, 50, 2529—2538 |
| 4 | Kim H., Beack S., Han S., Shin M., Lee T., Park Y., Kim K. S., Yetisen A. K., Yun S. H., Kwon W., Hahn S. K., Adv. Mater., 2018, 30, 1701460 |
| 5 | Li J., Rao J., Pu K., Biomaterials, 2018, 155, 217—235 |
| 6 | Lim E. K., Kim T., Paik S., Haam S., Huh Y. M., Lee K., Chem. Rev., 2015, 115, 327—394 |
| 7 | Liu Y., Bhattarai P., Dai Z., Chen X., Chem. Soc. Rev., 2019, 48, 2053—2108 |
| 8 | Ng K. K., Zheng G., Chem. Rev., 2015, 115, 11012—11042 |
| 9 | Qian J., Tang B. Z., Chem., 2017, 3, 56—91 |
| 10 | Yang B., Chen Y., Shi J., Chem., 2018, 4, 1284—1313 |
| 11 | Yang X., Yang M., Pang B., Vara M., Xia Y., Chem. Rev., 2015, 115, 10410—10488 |
| 12 | Zhu H., Cheng P., Chen P., Pu K., Biomater. Sci., 2018, 6, 746—765 |
| 13 | Cai Y., Wei Z., Song C., Tang C., Han W., Dong X., Chem. Soc. Rev., 2019, 48, 22—37 |
| 14 | He S., Song J., Qu J., Cheng Z., Chem. Soc. Rev., 2018, 47, 4258—4278 |
| 15 | Zhao J., Zhong D., Zhou S., J. Mater. Chem. B, 2018, 6, 349—365 |
| 16 | Wang L. V., Hu S., Science, 2012, 335, 1458—1462 |
| 17 | Weber J., Beard P. C., Bohndiek S. E., Nat. Methods, 2016, 13, 639—650 |
| 18 | Guo B., Chen J., Chen N., Middha E., Xu S., Pan Y., Wu M., Li K., Liu C., Liu B., Adv. Mater., 2019, 31, 1808355 |
| 19 | Jiang Y., Upputuri P. K., Xie C., Zeng Z., Sharma A., Zhen X., Li J., Huang J., Pramanik M., Pu K., Adv. Mater., 2019, 31, 1808166 |
| 20 | Lyu Y., Li J., Pu K., Small Methods, 2019, 3, 1900553 |
| 21 | Cheng L., Wang C., Feng L., Yang K., Liu Z., Chem. Rev., 2014, 114, 10869—10939 |
| 22 | Gai S., Yang G., Yang P., He F., Lin J., Jin D., Xing B., Nano Today, 2018, 19, 146—187 |
| 23 | Meng Z., Hou W., Zhou H., Zhou L., Chen H., Wu C., Macromol. Rapid Commun., 2018, 39, 1700614 |
| 24 | Guo M., Mao H., Li Y., Zhu A., He H., Yang H., Wang Y., Tian X., Ge C., Peng Q., Wang X., Yang X., Chen X., Liu G., Chen H., Biomaterials, 2014, 35, 4656—4666 |
| 25 | Li L., Liu Y., Hao P., Wang Z., Fu L., Ma Z., Zhou J., Biomaterials, 2015, 41, 132—140 |
| 26 | Jang B., Park J. Y., Tung C. H., Kim I. H., Choi Y., ACS Nano, 2011, 5, 1086—1094 |
| 27 | Lin J., Wang S., Huang P., Wang Z., Chen S., Niu G., Li W., He J., Cui D., Lu G., Chen X., Nie Z., ACS Nano, 2013, 7, 5320—5329 |
| 28 | Pan J., Yang Y., Fang W., Liu W., Le K., Xu D., Li X., ACS Appl. Nano Mater., 2018, 1, 2785—2795 |
| 29 | Guo L., Niu G., Zheng X., Ge J., Liu W., Jia Q., Zhang P., Zhang H., Wang P., Adv. Sci., 2017, 4, 1700085 |
| 30 | Wang Q., Dai Y., Xu J., Cai J., Niu X., Zhang L., Chen R., Shen Q., Huang W., Fan Q., Adv. Funct. Mater., 2019, 29, 1901480 |
| 31 | Zheng X., Wang L., Liu S., Zhang W., Liu F., Xie Z., Adv. Funct. Mater., 2018, 28, 1706507 |
| 32 | Ngoune R., Peters A., von Elverfeldt D., Winkler K., Pütz G., J. Controlled Release, 2016, 238, 58—70 |
| 33 | Chen M., Tang S., Guo Z., Wang X., Mo S., Huang X., Liu G., Zheng N., Adv. Mater., 2014, 26, 8210—8216 |
| 34 | Liu Y., Wang Z., Liu Y., Zhu G., Jacobson O., Fu X., Bai R., Lin X., Lu N., Yang X., Fan W., Song J., Wang Z., Yu G., Zhang F., Kalish H., Niu G., Nie Z., Chen X., ACS Nano, 2017, 11, 10539—10548 |
| 35 | Zhou J., Jiang Y., Hou S., Upputuri P. K., Wu D., Li J., Wang P., Zhen X., Pramanik M., Pu K., Duan, H. ACS Nano, 2018, 12, 2643—2651 |
| 36 | Guo Z., Zhu S., Yong Y., Zhang X., Dong X., Du J., Xie J., Wang Q., Gu Z., Zhao Y., Adv. Mater., 2017, 29, 1704136 |
| 37 | Huang C. C., Chang P. Y., Liu C. L., Xu J. P., Wu S. P., Kuo W. C., Nanoscale, 2015, 7, 12689—12697 |
| 38 | Jiang X., Zhang S., Ren F., Chen L., Zeng J., Zhu M., Cheng Z., Gao M., Li Z., ACS Nano, 2017, 11, 5633—5645 |
| 39 | Liu J., Zheng X., Yan L., Zhou L., Tian G., Yin W., Wang L., Liu Y., Hu Z., Gu Z., Chen C., Zhao Y., ACS Nano, 2015, 9, 696—707 |
| 40 | Zhang S., Sun C., Zeng J., Sun Q., Wang G., Wang Y., Wu Y., Dou S., Gao M., Li Z., Adv. Mater., 2016, 28, 8927—8936 |
| 41 | Lv G., Guo W., Zhang W., Zhang T., Li S., Chen S., Eltahan A. S., Wang D., Wang Y., Zhang J., Wang P. C., Chang J., Liang X. J., ACS Nano, 2016, 10, 9637—9645 |
| 42 | Dai C., Chen Y., Jing X., Xiang L., Yang D., Lin H., Liu Z., Han X., Wu R., ACS Nano, 2017, 11, 12696—12712 |
| 43 | Ji X., Kong N., Wang J., Li W., Xiao Y., Gan S. T., Zhang Y., Li Y., Song X., Xiong Q., Shi S., Li Z., Tao W., Zhang H., Mei L., Shi J., Adv. Mater., 2018, 30, 1803031 |
| 44 | Lin H., Gao S., Dai C., Chen Y., Shi J., J. Am. Chem. Soc., 2017, 139, 16235—16247 |
| 45 | Lin H., Wang Y., Gao S., Chen Y., Shi J., Adv. Mater., 2018, 30, 1703284 |
| 46 | Tao W., Ji X., Zhu X., Li L., Wang J., Zhang Y., Saw P. E., Li W., Kong N., Islam M. A., Gan T., Zeng X., Zhang H., Mahmoudi M., Tearney G. J., Farokhzad O. C., Adv. Mater., 2018, 30, 1802061 |
| 47 | Li S., Chen Y., Liu H., Wang Y., Liu L., Lv F., Li Y., Wang S., Chem. Mater., 2017, 29, 6087—6094 |
| 48 | Robinson J. T., Welsher K., Tabakman S. M., Sherlock S. P., Wang H., Luong R., Dai H., Nano Res., 2010, 3, 779—793 |
| 49 | Sun W., Zhang X., Jia H. R., Zhu Y. X., Guo Y., Gao G., Li Y. H., Wu F. G., Small, 2019, 15, 1804575 |
| 50 | Jung H. S., Verwilst P., Sharma A., Shin J., Sessler J. L., Kim J. S., Chem. Soc. Rev., 2018, 47, 2280—2297 |
| 51 | Luo S., Zhang E., Su Y., Cheng T., Shi C., Biomaterials, 2011, 32, 7127—7138 |
| 52 | Abrahamse H., Hamblin M. R., Biochem. J., 2016, 473, 347—364 |
| 53 | Ethirajan M., Chen Y., Joshi P., Pandey R. K., Chem. Soc. Rev., 2011, 40, 340—362 |
| 54 | Goncalves M. S., Chem. Rev., 2009, 109, 190—212 |
| 55 | Kowada T., Maeda H., Kikuchi K., Chem. Soc. Rev., 2015, 44, 4953—4972 |
| 56 | Zhang Y., Lovell J. F., Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2017, 9, e1420 |
| 57 | Cai X., Liu J., Liew W. H., Duan Y., Geng J., Thakor N., Yao K., Liao L. D., Liu B., Mater. Chem. Front., 2017, 1, 1556—1562 |
| 58 | Cai Y., Liang P., Tang Q., Yang X., Si W., Huang W., Zhang Q., Dong X., ACS Nano, 2017, 11, 1054—1063 |
| 59 | Cai Y., Wei Z., Song C., Tang C., Huang X., Hu Q., Dong X., Han W., Chem. Commun., 2019, 55, 8967—8970 |
| 60 | Huang S., Upputuri P. K., Liu H., Pramanik M., Wang M., J. Mater. Chem. B, 2016, 4, 1696—1703 |
| 61 | Liang P., Wang Y., Wang P., Zou J., Xu H., Zhang Y., Si W., Dong X., Nanoscale, 2017, 9, 18890—18896 |
| 62 | Liu S., Zhou X., Zhang H., Ou H., Lam J. W. Y., Liu Y., Shi L., Ding D., Tang B. Z., J. Am. Chem. Soc., 2019, 141, 5359—5368 |
| 63 | Qi J., Fang Y., Kwok R. T. K., Zhang X., Hu X., Lam J. W. Y., Ding D., Tang B. Z., ACS Nano, 2017, 11, 7177—7188 |
| 64 | Wang Q., Xia B., Xu J., Niu X., Cai J., Shen Q., Wang W., Huang W., Fan Q., Mater. Chem. Front., 2019, 3, 650—655 |
| 65 | Zhang R., Xu Y., Zhang Y., Kim H. S., Sharma A., Gao J., Yang G., Kim J. S., Sun Y., Chem. Sci., 2019, 10, 8348—8353 |
| 66 | Zhao Z., Chen C., Wu W., Wang F., Du L., Zhang X., Xiong Y., He X., Cai Y., Kwok R. T. K., Lam J. W. Y., Gao X., Sun P., Phillips D. L., Ding D., Tang B. Z., Nat. Commun., 2019, 10, 768 |
| 67 | Zou J., Yin Z., Wang P., Chen D., Shao J., Zhang Q., Sun L., Huang W., Dong X., Chem. Sci., 2018, 9, 2188—2194 |
| 68 | Alifu N., Zebibula A., Qi J., Zhang H., Sun C., Yu X., Xue D., Lam J. W. Y., Li G., Qian J., Tang B. Z., ACS Nano, 2018, 12, 11282—11293 |
| 69 | Feng G., Liu B., Acc. Chem. Res., 2018, 51, 1404—1414 |
| 70 | Gu X., Zhang X., Ma H., Jia S., Zhang P., Zhao Y., Liu Q., Wang J., Zheng X., Lam J. W. Y., Ding D., Tang B. Z., Adv. Mater., 2018, 30, 1801065 |
| 71 | Liu S., Zhang H., Li Y., Liu J., Du L., Chen M., Kwok R. T. K., Lam J. W. Y., Phillips D. L., Tang B. Z., Angew. Chem. Int. Ed., 2018, 57, 15189—15193 |
| 72 | Wang D., Lee M. M. S., Xu W., Shan G., Zheng X., Kwok R. T. K., Lam J. W. Y., Hu X., Tang B. Z., Angew. Chem. Int. Ed., 2019, 58, 5628—5632 |
| 73 | Zhang L., Li Y., Che W., Zhu D., Li G., Xie Z., Song N., Liu S., Tang B. Z., Liu X., Su Z., Bryce M. R., Adv. Sci., 2019, 6, 1802050 |
| 74 | Zhen S., Wang S., Li S., Luo W., Gao M., Ng L. G., Goh C. C., Qin A., Zhao Z., Liu B., Tang B. Z., Adv. Funct. Mater., 2018, 28, 1706945 |
| 75 | Feng L., Zhu C., Yuan H., Liu L., Lv F., Wang S., Chem. Soc. Rev., 2013, 42, 6620—6633 |
| 76 | Kaeser A., Schenning A. P., Adv. Mater., 2010, 22, 2985—2997 |
| 77 | Li K., Liu B., Chem. Soc. Rev., 2014, 43, 6570—6597 |
| 78 | Wu C., Chiu D. T., Angew. Chem. Int. Ed., 2013, 52, 3086—3109 |
| 79 | Xu X., Liu R., Li L., Chem. Commun., 2015, 51, 16733—16749 |
| 80 | Ehlerding E. B., Chen F., Cai W., Adv. Sci., 2016, 3, 1500223 |
| 81 | Guo R., Peng H., Tian Y., Shen S., Yang W., Small, 2016, 12, 4541—4552 |
| 82 | Jayaraman P., Gandhimathi C., Venugopal J. R., Becker D. L., Ramakrishna S., Srinivasan D. K., Adv. Drug Delivery Rev., 2015, 94, 77—95 |
| 83 | Mackowiak S. A., Schmidt A., Weiss V., Argyo C., von Schirnding C., Bein T., Brauchle C., Nano Lett., 2013, 13, 2576—2583 |
| 84 | Gao D., Guo X., Zhang X., Chen S., Wang Y., Chen T., Huang G., Gao Y., Tian Z., Yang Z., Mater. Today Bio., 2020, 5, 100035 |
| 85 | Chen D., Zhong Z., Ma Q., Shao J., Huang W., Dong X., ACS Appl. Mater. Interfaces, 2020, 12, 26914—26925 |
| 86 | Yang Z., Chen X., Acc. Chem. Res., 2019, 52, 1245—1254 |
| 87 | Cai Y., Si W., Huang W., Chen P., Shao J., Dong X., Small, 2018, 14, 1704247 |
| 88 | Gottfried J. M., Surf. Sci. Rep., 2015, 70, 259—379 |
| 89 | Du L., Qin H., Ma T., Zhang T., Xing D., ACS Nano, 2017, 11, 8930—8943 |
| 90 | Jin H. G., Zhong W., Yin S., Zhang X., Zhao Y. H., Wang Y., Yuan L., Zhang X. B., ACS Appl. Mater. Interfaces, 2019, 11, 3800—3808 |
| 91 | Li L., Yang Q., Shi L., Zheng N., Li Z., Li K., Qiao S., Jia T., Sun T., Wang Y., J. Mater. Chem. B, 2019, 7, 2247—2251 |
| 92 | Li X., Peng X. H., Zheng B. D., Tang J., Zhao Y., Zheng B. Y., Ke M. R., Huang J. D., Chem. Sci., 2018, 9, 2098—2104 |
| 93 | Pan H., Li S., Kan J. L., Gong L., Lin C., Liu W., Qi D., Wang K., Yan X., Jiang J., Chem. Sci., 2019, 10, 8246—8252 |
| 94 | Zheng X., Wang L., Lei Z., Pei Q., Liu S., Xie Z., Mater. Chem. Front., 2019, 3, 1892—1899 |
| 95 | Zou Q., Abbas M., Zhao L., Li S., Shen G., Yan X., J. Am. Chem. Soc., 2017, 139, 1921—1927 |
| 96 | Tian B., Wang C., Zhang S., Feng L., Liu Z., ACS Nano, 2011, 5, 7000—7009 |
| 97 | Younis M. R., Wang C., An R., Wang S., Younis M. A., Li Z. Q., Wang Y., Ihsan A., Ye D., Xia X. H., ACS Nano, 2019, 13, 2544—2557 |
| 98 | Cao Y., Dong H., Yang Z., Zhong X., Chen Y., Dai W., Zhang X., ACS Appl. Mater. Interfaces, 2017, 9, 159—166 |
| 99 | Han H. S., Choi K. Y., Lee H., Lee M., An J. Y., Shin S., Kwon S., Lee D. S., Park J. H., ACS Nano, 2016, 10, 10858—10868 |
| 100 | Jia Q., Ge J., Liu W., Liu S., Niu G., Guo L., Zhang H., Wang P., Nanoscale, 2016, 8, 13067—13077 |
| 101 | Kim Y. K., Na H. K., Kim S., Jang H., Chang S. J., Min D. H., Small, 2015, 11, 2527—2535 |
| 102 | Jiang Y., Li J., Zhen X., Xie C., Pu K., Adv. Mater., 2018, 30, 1705980 |
| 103 | Guo B., Sheng Z., Kenry K., Hu D., Lin X., Xu S., Liu C., Zheng H., Liu B., Mater. Horiz., 2017, 4, 1151—1156 |
| 104 | Jiang Y., Upputuri P. K., Xie C., Lyu Y., Zhang L., Xiong Q., Pramanik M., Pu K., Nano Lett., 2017, 17, 4964—4969 |
| 105 | Wu J., You L., Lan L., Lee H. J., Chaudhry S. T., Li R., Cheng J. X., Mei J., Adv. Mater., 2017, 29, 1703403 |
| 106 | Yin C., Wen G., Liu C., Yang B., Lin S., Huang J., Zhao P., Wong S. H. D., Zhang K., Chen X., Li G., Jiang X., Huang J., Pu K., Wang L., Bian L., ACS Nano, 2018, 12, 12201—12211 |
| 107 | Guo B., Sheng Z., Hu D., Liu C., Zheng H., Liu B., Adv. Mater., 2018, 30, 1802591 |
| 108 | Sun T., Han J., Liu S., Wang X., Wang Z. Y., Xie Z., ACS Nano, 2019, 13, 7345—7354 |
| 109 | Ding X., Liow C. H., Zhang M., Huang R., Li C., Shen H., Liu M., Zou Y., Gao N., Zhang Z., Li Y., Wang Q., Li S., Jiang J., J. Am. Chem. Soc., 2014, 136, 15684—15693 |
| 110 | Zhou M., Ku G., Pageon L., Li C., Nanoscale, 2014, 6, 15228—15235 |
| 111 | Wang C., Dai C., Hu Z., Li H., Yu L., Lin H., Bai J., Chen Y., Nanoscale Horiz., 2019, 4, 415—425 |
| 112 | Boens N., Leen V., Dehaen W., Chem. Soc. Rev., 2012, 41, 1130—1172 |
| 113 | Loudet A., Burgess K., Chem. Rev., 2007, 107, 4891—4932 |
| 114 | Li Y., Liu T., Liu H., Tian M. Z., Li Y., Acc. Chem. Res., 2014, 47, 1186—1198 |
| 115 | Banuelos J., Martin V., Gomez⁃Duran C. F., Arroyo Cordoba I. J., Pena⁃Cabrera E., Garcia⁃Moreno I., Costela A., Perez⁃Ojeda M. E., Arbeloa T., Lopez Arbeloa I., Chem. Eur. J., 2011, 17, 7261—7270 |
| 116 | Zhu H., Fan J., Du J., Peng X., Acc. Chem. Res., 2016, 49, 2115—2126 |
| 117 | Atilgan S., Ozdemir T., Akkaya E. U., Org. Lett., 2008, 10, 4065—4067 |
| 118 | Bozdemir O. A., Guliyev R., Buyukcakir O., Selcuk S., Kolemen S., Gulseren G., Nalbantoglu T., Boyaci H., Akkaya E. U., J. Am. Chem. Soc., 2010, 132, 8029—8036 |
| 119 | Ekmekci Z., Yilmaz M. D., Akkaya E. U., Org. Lett., 2008, 10, 461—464 |
| 120 | Qi X., Jun E. J., Xu L., Kim S. J., Hong J. S., Yoon Y. J., Yoon J., J. Org. Chem., 2006, 71, 2881—2884 |
| 121 | Hu W., Ma H., Hou B., Zhao H., Ji Y., Jiang R., Hu X., Lu X., Zhang L., Tang Y., Fan Q., Huang W., ACS Appl. Mater. Interfaces, 2016, 8, 12039—12047 |
| 122 | Huang L., Li Z., Zhao Y., Zhang Y., Wu S., Zhao J., Han G., J. Am. Chem. Soc., 2016, 138, 14586—14591 |
| 123 | Ye S., Rao J., Qiu S., Zhao J., He H., Yan Z., Yang T., Deng Y., Ke H., Yang H., Zhao Y., Guo Z., Chen H., Adv. Mater., 2018, 30, 1801216 |
| 124 | Zhang Y., Song N., Li Y., Yang Z., Chen L., Sun T., Xie Z., J. Mater. Chem. B, 2019, 7, 4717—4724 |
| 125 | Zou J., Wang P., Wang Y., Liu G., Zhang Y., Zhang Q., Shao J., Si W., Huang W., Dong X., Chem. Sci., 2019, 10, 268—276 |
| 126 | Lu H., Mack J., Yang Y., Shen Z., Chem. Soc. Rev., 2014, 43, 4778—4823 |
| 127 | Awuah S. G., You Y., RSC Adv., 2012, 2, 11169—11183 |
| 128 | Zhang X. F., Yang X., J. Phys. Chem. B, 2013, 117, 5533—5539 |
| 129 | Zou J., Yin Z., Ding K., Tang Q., Li J., Si W., Shao J., Zhang Q., Huang W., Dong X., ACS Appl. Mater. Interfaces, 2017, 9, 32475—32481 |
| 130 | Vegesna G. K., Sripathi S. R., Zhang J., Zhu S., He W., Luo F. T., Jahng W. J., Frost M., Liu H., ACS Appl. Mater. Interfaces, 2013, 5, 4107—4112 |
| 131 | Wu B., Xu L., Wang S., Wang Y., Zhang W., Polym. Chem., 2015, 6, 4279—4289 |
| 132 | Zhu S., Zhang J., Vegesna G., Luo F. T., Green S. A., Liu H., Org. Lett., 2011, 13, 438—441 |
| 133 | Liu L., Ruan Z., Li T., Yuan P., Yan L., Biomater. Sci., 2016, 4, 1638—1645 |
| 134 | Trofymchuk K., Valanciunaite J., Andreiuk B., Reisch A., Collot M., Klymchenko A. S., J. Mater. Chem. B, 2019, 7, 5199—5210 |
| 135 | Tang Q., Xiao W., Huang C., Si W., Shao J., Huang W., Chen P., Zhang Q., Dong X., Chem. Mater., 2017, 29, 5216—5224 |
| 136 | Guo Z., Nam S., Park S., Yoon J., Chem. Sci., 2012, 3, 2760—2765 |
| 137 | Yapici I., Lee K. S., Berbasova T., Nosrati M., Jia X., Vasileiou C., Wang W., Santos E. M., Geiger J. H., Borhan B., J. Am. Chem. Soc., 2015, 137, 1073—1080 |
| 138 | Mishra A., Behera R. K., Behera P. K., Mishra B. K., Behera G. B., Chem. Rev., 2000, 100, 1973—2012 |
| 139 | Lavis L. D., Raines R. T., ACS Chem. Biol., 2008, 3, 142—155 |
| 140 | Guieu V., Payrastre C., Madaule Y., Garcia⁃Alonso S., Lacroix P. G., Nakatani K., Chem. Mater., 2006, 18, 3674—3681 |
| 141 | Lacroix P. G., Malfant I., Payrastre C., Wolf J. G., Bonvoisin J., Nakatani K., Chem. Mater.,1998, 10, 1135—1140 |
| 142 | Bouit P. A., Rauh D., Neugebauer S., Delgado J. L., Di Piazza E., Rigaut S., Maury O., Andraud C., Dyakonov V., Martin N., Org. Lett., 2009, 11, 4806—4809 |
| 143 | Sun W., Guo S., Hu C., Fan J., Peng X., Chem. Rev., 2016, 116, 7768—7817 |
| 144 | Karton⁃Lifshin N., Segal E., Omer L., Portnoy M., Satchi⁃Fainaro R., Shabat D., J. Am. Chem. Soc., 2011, 133, 10960—10965 |
| 145 | Liu Y., Zhou J., Wang L., Hu X., Liu X., Liu M., Cao Z., Shangguan D., Tan W., J. Am. Chem. Soc., 2016, 138, 12368—12374 |
| 146 | Yin J., Kwon Y., Kim D., Lee D., Kim G., Hu Y., Ryu J. H., Yoon J., J. Am. Chem. Soc., 2014, 136, 5351—5358 |
| 147 | Jian W. H., Yu T. W., Chen C. J., Huang W. C., Chiu H. C., Chiang W. H., Langmuir, 2015, 31, 6202—6210 |
| 148 | Pan J., Wang Y., Zhang C., Wang X., Wang H., Wang J., Yuan Y., Wang X., Zhang X., Yu C., Sun S. K., Yan X. P., Adv. Mater., 2018, 30, 1704408 |
| 149 | Sheng Z., Hu D., Zheng M., Zhao P., Liu H., Gao D., Gong P., Gao G., Zhang P., Ma Y., Cai L., ACS Nano, 2014, 8, 12310—12322 |
| 150 | Yoon H. J., Lee H. S., Lim J. Y., Park J. H., ACS Appl. Mater. Interfaces, 2017, 9, 5683—5691 |
| 151 | Strekowski L., Heterocyclic Polymethine Dyes: Synthesis, Properties and Applications, Springer, Berlin, Heidelberg, 2008, 1—10 |
| 152 | Huang Y., He N., Wang Y., Shen D., Kang Q., Zhao R., Chen L., J. Mater. Chem. B, 2019, 7, 1149—1159 |
| 153 | Chen X., Peng X., Cui A., Wang B., Wang L., Zhang R., J. Photochem. Photobiol., A, 2006, 181, 79—85 |
| 154 | Kovalska V. B., Volkova K. D., Losytskyy M. Y., Tolmachev O. I., Balanda A. O., Yarmoluk S. M., Spectrochim. Acta, Part A, 2006, 65, 271—277 |
| 155 | Volkova K. D., Kovalska V. B., Tatarets A. L., Patsenker L. D., Kryvorotenko D. V., Yarmoluk S. M., Dyes Pigm., 2007, 72, 285—292 |
| 156 | Escobedo J. O., Rusin O., Lim S., Strongin R. M., Curr. Opin. Chem. Biol., 2010, 14, 64—70 |
| 157 | Alagumalai A., FairoosM. K. M., Vellimalai P., Sil M. C., Nithyanandhan J., ACS Appl. Mater. Interfaces, 2016, 8, 35353—35367 |
| 158 | Oswald B., Patsenker L., Duschl J., Szmacinski H., Wolfbeis O. S., Terpetschnig E., Bioconjugate Chem.,1999, 10, 925—931 |
| 159 | Karpenko I. A., Collot M., Richert L., Valencia C., Villa P., Mely Y., Hibert M., Bonnet D., Klymchenko A. S., J. Am. Chem. Soc., 2015, 137, 405—412 |
| 160 | Sreejith S., Joseph J., Lin M., Menon N. V., Borah P., Ng H. J., Loong Y. X., Kang Y., Yu S. W., Zhao Y., ACS Nano, 2015, 9, 5695—5704 |
| 161 | Wu B., Lin Y., Li B., Zhan C., Zeng F., Wu S., Anal. Chem., 2018, 90, 9359—9365 |
| 162 | Umezawa K. C., D., Suzuki K., Anal. Sci., 2008, 24, 213—217 |
| 163 | Nakazumi H., Ohta T., Etoh H., Uno T., Colyer C. L., Hyodo Y., Yagi S., Synth. Met., 2005, 153, 33—36 |
| 164 | Gassensmith J. J., Baumes J. M., Smith B. D., Chem. Commun., 2009, 6329—6338 |
| 165 | Sun P., Wu Q., Sun X., Miao H., Deng W., Zhang W., Fan Q., Huang W., Chem. Commun., 2018, 54, 13395—13398 |
| 166 | Dou L., Liu Y., Hong Z., Li G., Yang Y., Chem. Rev., 2015, 115, 12633—12665 |
| 167 | Bin H., Yang Y., Zhang Z. G., Ye L., Ghasemi M., Chen S., Zhang Y., Zhang C., Sun C., Xue L., Yang C., Ade H., Li Y., J. Am. Chem. Soc., 2017, 139, 5085—5094 |
| 168 | Chen Z., Li J., Li M., Chen C., Xu S., Tang X., Chen L., Chen R., Huang W., Org. Lett., 2018, 20, 6376—6379 |
| 169 | Dutta P., Yang W., Lee W.H., Kang I. N., Lee S. H., J. Mater. Chem., 2012, 22, 10840—10851 |
| 170 | Tang A., Zhan C., Yao J., Chem. Mater., 2015, 27, 4719—4730 |
| 171 | Tang A., Zhan C., Yao J., Zhou E., Adv. Mater., 2017, 29, 1600013 |
| 172 | Yin Q. R., Miao J. S., Wu Z., Chang Z. F., Wang J. L., Wu H. B., Cao Y., J. Mater. Chem. A, 2015, 3, 11575—11586 |
| 173 | Chavez Iii R., Cai M., Tlach B., Wheeler D. L., Kaudal R., Tsyrenova A., Tomlinson A. L., Shinar R., Shinar J., Jeffries⁃El M., J. Mater. Chem. C, 2016, 4, 3765—3773 |
| 174 | Thirion D., Rault⁃Berthelot J., Vignau L., Poriel C., Org. Lett., 2011, 13, 4418—4421 |
| 175 | Yang Y., Zhou Y., He Q., He C., Yang C., Bai F., Li Y., J. Phys. Chem. B, 2009, 113, 7745—7752 |
| 176 | Bulumulla C., Gunawardhana R., Kularatne R. N., Hill M. E., McCandless G. T., Biewer M. C., Stefan M. C., ACS Appl. Mater. Interfaces, 2018, 10, 11818—11825 |
| 177 | Huang Y. F., Chang S. T., Wu K. Y., Wu S. L., Ciou G. T., Chen C. Y., Liu C. L., Wang C. L., ACS Appl. Mater. Interfaces, 2018, 10, 8869—8876 |
| 178 | Kang W., Jung M., Cha W., Jang S., Yoon Y., Kim H., Son H. J., Lee D. K., Kim B., Cho J. H., ACS Appl. Mater. Interfaces, 2014, 6, 6589—6597 |
| 179 | Lee J., Ko S.J., Lee H., Huang J., Zhu Z., Seifrid M., Vollbrecht J., Brus V. V., Karki A., Wang H., Cho K., Nguyen T. Q., Bazan G. C., ACS Energy Lett., 2019, 4, 1401—1409 |
| 180 | Lv L., Yu J., Sui X., Wu J., Dong X., Lu G., Liu X., Peng A., Huang H., J. Mater. Chem. C, 2019, 7, 5739—5747 |
| 181 | Qi J., Han J., Zhou X., Guo C., Yang D., Qiao W., Li Y., Ma D., Wang Z. Y., J. Phys. Chem. C, 2015, 119, 25243—25251 |
| 182 | Cheng K., Chen H., Jenkins C. H., Zhang G., Zhao W., Zhang Z., Han F., Fung J., Yang M., Jiang Y., Xing L., Cheng Z., ACS Nano, 2017, 11, 12276—12291 |
| 183 | Lan M., Zhang J., Zhu X., Wang P., Chen X., Lee C. S., Zhang W., Nano Res., 2015, 8, 2380—2389 |
| 184 | Kaur M., Choi D. H., Chem. Soc. Rev., 2015, 44, 58—77 |
| 185 | He H., Zheng X., Liu S., Zheng M., Xie Z., Wang Y., Yu M., Shuai X., Nanoscale, 2018, 10, 10991—10998 |
| 186 | Huang S., Liu W., Huang J., Wang X., Yang C., Bohra H., Liu Q., Wang M., ACS Appl. Bio. Mater., 2018, 1, 1109—1117 |
| 187 | Wu C., Huang X., Tang Y., Xiao W., Sun L., Shao J., Dong X., Chem. Commun., 2019, 55, 790—793 |
| 188 | Shao W., Wei Q., Wang S., Li F., Wu J., Ren J., Cao F., Liao H., Gao J., Zhou M., Ling D., Mater. Horiz., 2020, 7, 1379—1386 |
| 189 | Ding D., Li K., Liu B., Tang B. Z., Acc. Chem. Res., 2013, 46, 2441—2453 |
| 190 | Mei J., Leung N. L., Kwok R. T., Lam J. W., Tang B. Z., Chem. Rev., 2015, 115, 11718—11940 |
| 191 | Li K., Qin W., Ding D., Tomczak N., Geng J., Liu R., Liu J., Zhang X., Liu H., Liu B., Tang B. Z., Sci. Rep., 2013, 3, 1150 |
| 192 | Ding D., Goh C. C., Feng G., Zhao Z., Liu J., Liu R., Tomczak N., Geng J., Tang B. Z., Ng L. G., Liu B., Adv. Mater., 2013, 25, 6083—6088 |
| 193 | Qi J., Li J., Liu R., Li Q., Zhang H., Lam J. W. Y., Kwok R. T. K., Liu D., Ding D., Tang B. Z., Chem., 2019, 5, 2657—2677 |
| 194 | Kenry Duan Y., Liu B., Adv. Mater., 2018, 30, 1802394 |
| 195 | Fateminia S. M., Wang Z., Goh C. C., Manghnani P. N., Wu W., Mao D., Ng L. G., Zhao Z., Tang B. Z., Liu B., Adv. Mater., 2017, 29, 1604100 |
| 196 | Ji S., Ge J., Escudero D., Wang Z., Zhao J., Jacquemin D., J. Org. Chem., 2015, 80, 5958—5963 |
| 197 | Xu S., Yuan Y., Cai X., Zhang C. J., Hu F., Liang J., Zhang G., Zhang D., Liu B., Chem. Sci., 2015, 6, 5824—5830 |
| 198 | Wu W., Mao D., Xu S., Kenry, Hu F., Li X., Kong D., Liu B., Chem., 2018, 4, 1937—1951 |
| [1] | 刘树威, 晋皓, 尹万忠, 张皓. 用于卵巢癌化疗-光热联合治疗的吉西他滨/聚吡咯复合纳米粒子[J]. 高等学校化学学报, 2022, 43(8): 20220345. |
| [2] | 王雪丽, 宋相伟, 解颜宁, 杜妮阳, 王振新. 部分还原氧化石墨烯的制备、 表征及对人宫颈癌HeLa细胞的体外杀伤作用[J]. 高等学校化学学报, 2022, 43(2): 20210595. |
| [3] | 刘苗, 刘瑞波, 刘巴蒂, 钱鹰. 溶酶体靶向吲哚氟硼二吡咯光敏剂的合成、 双光子荧光成像及光动力治疗[J]. 高等学校化学学报, 2022, 43(10): 20220326. |
| [4] | 陈宏达, 张婳, 王振新. 用于小动物活体的荧光-光热双模成像系统[J]. 高等学校化学学报, 2021, 42(3): 725. |
| [5] | 陆峰, 贡祎, 赵婷, 赵宁, 琚雯雯, 范曲立, 黄维. 二元混合表面活性剂用于窄吸收金纳米棒的无种子合成[J]. 高等学校化学学报, 2021, 42(3): 700. |
| [6] | 王萌萌, 栾天骄, 杨铭焱, 吕佳佳, 高杰, 李洪玉, 卫钢, 袁泽利. 肿瘤乏氧靶向响应的罗丹明荧光探针及其成像介导手术治疗[J]. 高等学校化学学报, 2021, 42(10): 3071. |
| [7] | 梁钰昕, 赵容, 梁馨月, 方晓红. 细胞膜上信号转导蛋白的单分子成像与分析[J]. 高等学校化学学报, 2020, 41(6): 1127. |
| [8] | 白翠婷, 岳仁叶, 罗列高, 马楠. 基于双色荧光传感器的癌细胞成像及microRNA定量检测[J]. 高等学校化学学报, 2020, 41(6): 1252. |
| [9] | 邬丰任,刘永佳,陆学民,朱邦尚. 聚多巴胺修饰金纳米花的可控制备及在光热治疗中的应用[J]. 高等学校化学学报, 2020, 41(3): 465. |
| [10] | 张勇,申城,幸志荣,陈归柒,卢资,侯志兵,陈雪梅. 可视化检测次氯酸的苯并咪唑类荧光增强型探针[J]. 高等学校化学学报, 2019, 40(12): 2480. |
| [11] | 刘晔, 姚顺雨, 方超, 赵外欧, 王静媛, 李亚鹏. 靶向髓过氧化酶的双模态探针分子的合成与表征[J]. 高等学校化学学报, 2018, 39(7): 1573. |
| [12] | 张书鹏, 程友星, 任磊, 文凯, 吕晓林, 叶社房, 周樨. 不同形貌普鲁士蓝纳米粒子的合成及光热性能[J]. 高等学校化学学报, 2018, 39(2): 359. |
| [13] | 宋文植, 李慧, 张艳, 何丹, 李英姿, 黄臻臻, 刘新, 尹万忠. 具有近红外光热转换性能的双层氧化硅包覆金纳米花复合材料的制备及体内代谢研究[J]. 高等学校化学学报, 2018, 39(12): 2644. |
| [14] | 张涛, 汤永嘉, 徐亮, 刘克良. 新型可用于荧光标记的α-氰基丙烯酸酯单体的合成及在小鼠活体成像中的应用[J]. 高等学校化学学报, 2016, 37(6): 1168. |
| [15] | 米小龙, 焦晓洁, 刘畅, 何松, 曾宪顺. 基于罗丹明荧光信号报告基团的细胞通透性铜离子荧光探针[J]. 高等学校化学学报, 2016, 37(10): 1784. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||