

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (12): 2556.doi: 10.7503/cjcu20190370
收稿日期:2019-07-03
出版日期:2019-12-04
发布日期:2019-12-04
通讯作者:
张光华
E-mail:zhanggh@sust.edu.cn
基金资助:
Qiuchen DONG1,Guanghua ZHANG1,*( ),Wanbin ZHANG2,Xue ZHANG1,Jing LIU1
),Wanbin ZHANG2,Xue ZHANG1,Jing LIU1
Received:2019-07-03
Online:2019-12-04
Published:2019-12-04
Contact:
Guanghua ZHANG
E-mail:zhanggh@sust.edu.cn
Supported by:摘要:
以甲基丙烯酸二甲氨基乙酯为母体、 对氯甲基苯乙烯为季铵化试剂, 合成了一种具有疏水结构的甲基丙烯酸二甲氨基乙酯型离子液体(DEMA). 通过失重实验、 电化学分析、 原子力显微镜(AFM)、 接触角测试和量子化学计算等研究了DEMA在1 mol/L盐酸中对Q235钢的缓蚀性能, 并揭示了其在Q235钢表面的吸附行为和吸附机理. 失重实验结果表明, DEMA在盐酸中对Q235钢具有优异的缓蚀效果, 且在较高温度(60 ℃)下也能保持高效吸附; 电化学实验结果与失重测试结果一致; 接触角测试结果表明, DEMA可明显增强Q235钢表面的疏水性; 分析热力学参数可知, DEMA在Q235钢表面的吸附为自发、 放热过程, 符合Langmuir等温式, 且以化学吸附为主; 量子化学计算结果证实DEMA的结构中包含大量吸附活性位点.
中图分类号:
TrendMD:
董秋辰,张光华,张万斌,张雪,刘晶. 甲基丙烯酸二甲氨基乙酯类离子液体对Q235钢的缓蚀性能. 高等学校化学学报, 2019, 40(12): 2556.
Qiuchen DONG,Guanghua ZHANG,Wanbin ZHANG,Xue ZHANG,Jing LIU. Corrosion Inhibition of Q235 Steel by Ionic Liquid Based on the 2-(Dimethylamino)ethyl Methacrylate †. Chem. J. Chinese Universities, 2019, 40(12): 2556.
| Temp./℃ | c(DEMA)/ (mg·L-1) | v/(mg· cm-2·h-1) | θ | ηw(%) | Temp./℃ | c(DEMA)/ (mg·L-1) | v/(mg· cm-2·h-1) | θ | ηw(%) |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 0 | 2.7016 | — | — | 50 | 0 | 6.7581 | — | — |
| 20 | 0.0824 | 0.9695 | 96.95 | 20 | 0.3899 | 0.9423 | 94.23 | ||
| 40 | 0.0686 | 0.9746 | 97.46 | 40 | 0.3393 | 0.9498 | 94.98 | ||
| 60 | 0.0546 | 0.9798 | 97.98 | 60 | 0.2879 | 0.9574 | 95.74 | ||
| 80 | 0.0413 | 0.9847 | 98.47 | 80 | 0.2467 | 0.9635 | 96.35 | ||
| 100 | 0.0394 | 0.9854 | 98.54 | 100 | 0.2041 | 0.9698 | 96.98 | ||
| 40 | 0 | 4.3276 | — | — | 60 | 0 | 10.5565 | — | — |
| 20 | 0.1878 | 0.9566 | 95.66 | 20 | 0.6957 | 0.9341 | 93.41 | ||
| 40 | 0.1580 | 0.9635 | 96.35 | 40 | 0.5510 | 0.9478 | 94.78 | ||
| 60 | 0.1389 | 0.9679 | 96.79 | 60 | 0.5035 | 0.9523 | 95.23 | ||
| 80 | 0.1207 | 0.9721 | 97.21 | 80 | 0.4518 | 0.9572 | 95.72 | ||
| 100 | 0.1021 | 0.9764 | 97.64 | 100 | 0.4085 | 0.9613 | 96.13 |
Table 1 Corrosion rate of Q235 steel and inhibition efficiency of various concentrations of DEMA in 1 mol/L HCl at different temperatures obtained from mass loss measurements
| Temp./℃ | c(DEMA)/ (mg·L-1) | v/(mg· cm-2·h-1) | θ | ηw(%) | Temp./℃ | c(DEMA)/ (mg·L-1) | v/(mg· cm-2·h-1) | θ | ηw(%) |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 0 | 2.7016 | — | — | 50 | 0 | 6.7581 | — | — |
| 20 | 0.0824 | 0.9695 | 96.95 | 20 | 0.3899 | 0.9423 | 94.23 | ||
| 40 | 0.0686 | 0.9746 | 97.46 | 40 | 0.3393 | 0.9498 | 94.98 | ||
| 60 | 0.0546 | 0.9798 | 97.98 | 60 | 0.2879 | 0.9574 | 95.74 | ||
| 80 | 0.0413 | 0.9847 | 98.47 | 80 | 0.2467 | 0.9635 | 96.35 | ||
| 100 | 0.0394 | 0.9854 | 98.54 | 100 | 0.2041 | 0.9698 | 96.98 | ||
| 40 | 0 | 4.3276 | — | — | 60 | 0 | 10.5565 | — | — |
| 20 | 0.1878 | 0.9566 | 95.66 | 20 | 0.6957 | 0.9341 | 93.41 | ||
| 40 | 0.1580 | 0.9635 | 96.35 | 40 | 0.5510 | 0.9478 | 94.78 | ||
| 60 | 0.1389 | 0.9679 | 96.79 | 60 | 0.5035 | 0.9523 | 95.23 | ||
| 80 | 0.1207 | 0.9721 | 97.21 | 80 | 0.4518 | 0.9572 | 95.72 | ||
| 100 | 0.1021 | 0.9764 | 97.64 | 100 | 0.4085 | 0.9613 | 96.13 |
| c(DEMA)/(mg·L-1) | Rs/(Ω·cm2) | Rct/(Ω·cm2) | CPE | ηe(%) | |
|---|---|---|---|---|---|
| Cdl/(μF·cm-2) | n | ||||
| 0 | 0.89 | 17.3 | 343.1 | 0.90 | |
| 20 | 1.13 | 245.7 | 168.7 | 0.84 | 92.96 |
| 40 | 1.02 | 256.7 | 166.4 | 0.86 | 93.26 |
| 60 | 0.95 | 275.0 | 159.2 | 0.83 | 93.71 |
| 80 | 1.17 | 300.9 | 150.2 | 0.91 | 94.25 |
| 100 | 0.88 | 364.2 | 136.7 | 0.84 | 95.25 |
Table 2 Impedance parameters of Q235 steel with various mass concentrations of DEMA
| c(DEMA)/(mg·L-1) | Rs/(Ω·cm2) | Rct/(Ω·cm2) | CPE | ηe(%) | |
|---|---|---|---|---|---|
| Cdl/(μF·cm-2) | n | ||||
| 0 | 0.89 | 17.3 | 343.1 | 0.90 | |
| 20 | 1.13 | 245.7 | 168.7 | 0.84 | 92.96 |
| 40 | 1.02 | 256.7 | 166.4 | 0.86 | 93.26 |
| 60 | 0.95 | 275.0 | 159.2 | 0.83 | 93.71 |
| 80 | 1.17 | 300.9 | 150.2 | 0.91 | 94.25 |
| 100 | 0.88 | 364.2 | 136.7 | 0.84 | 95.25 |
| c(DEMA)/(mg·L-1) | Ecorr/mV(vs. SCE) | icorr/(μA·cm-2) | βa/(mV·dec-1) | βc/(mV·dec-1) | ηp(%) |
|---|---|---|---|---|---|
| 0 | -483.4 | 52.2 | 85.4 | -131.0 | |
| 20 | -445.5 | 3.8 | 86.2 | -132.8 | 92.72 |
| 40 | -437.8 | 3.3 | 75.6 | -126.9 | 93.68 |
| 60 | -432.8 | 2.3 | 79.8 | -126.2 | 95.59 |
| 80 | -430.6 | 2.0 | 82.6 | -127.4 | 96.17 |
| 100 | -426.4 | 1.6 | 88.3 | -122.3 | 96.93 |
Table 3 Electrochemical parameters for the corrosion of Q235 steel in 1 mol/L HCl containing different concentrations of DEMA at 30 ℃
| c(DEMA)/(mg·L-1) | Ecorr/mV(vs. SCE) | icorr/(μA·cm-2) | βa/(mV·dec-1) | βc/(mV·dec-1) | ηp(%) |
|---|---|---|---|---|---|
| 0 | -483.4 | 52.2 | 85.4 | -131.0 | |
| 20 | -445.5 | 3.8 | 86.2 | -132.8 | 92.72 |
| 40 | -437.8 | 3.3 | 75.6 | -126.9 | 93.68 |
| 60 | -432.8 | 2.3 | 79.8 | -126.2 | 95.59 |
| 80 | -430.6 | 2.0 | 82.6 | -127.4 | 96.17 |
| 100 | -426.4 | 1.6 | 88.3 | -122.3 | 96.93 |
| Temperature/℃ | Kads/(L·mol-1) | Δ | Δ | Δ |
|---|---|---|---|---|
| 30 | 558659 | -43.45 | -17.27 | 86.4 |
| 40 | 452489 | -44.34 | -17.27 | 86.5 |
| 50 | 358423 | -45.13 | -17.27 | 86.3 |
| 60 | 302115 | -46.05 | -17.27 | 86.4 |
Table 4 Adsorption parameters obtained from mass loss tests for DEMA in 1 mol/L HCl at different temperatures
| Temperature/℃ | Kads/(L·mol-1) | Δ | Δ | Δ |
|---|---|---|---|---|
| 30 | 558659 | -43.45 | -17.27 | 86.4 |
| 40 | 452489 | -44.34 | -17.27 | 86.5 |
| 50 | 358423 | -45.13 | -17.27 | 86.3 |
| 60 | 302115 | -46.05 | -17.27 | 86.4 |
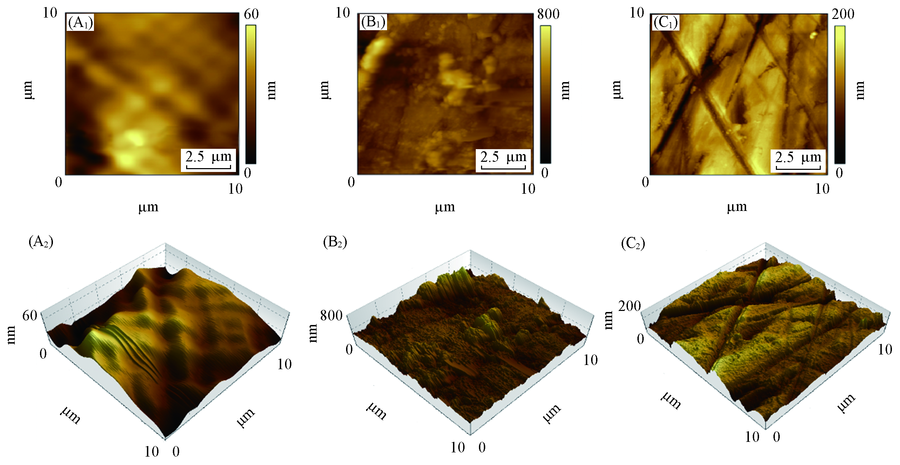
Fig.10 AFM images of Q235 steel surface after immersion in 1 mol/L HCl at 30 ℃ for 1 h (A1, A2) Before immersion; (B1, B2) without DEMA; (C1, C2) with 80 mg/L DEMA. (A1—C1) Plane graphs; (A2—C2) three-dimensional images.
| EHOMO/eV | ELUMO/eV | ELUMO-EHOMO,Fe/eV | ELUMO,Fe-EHOMO/eV | ΔE/eV | ||
|---|---|---|---|---|---|---|
| DEMA | Fe | DEMA | Fe | |||
| -8.022 | -7.810[ | -4.622 | -0.250[ | 3.188 | 7.772 | 3.40 |
Table 5 Frontier orbital energies of DEMA
| EHOMO/eV | ELUMO/eV | ELUMO-EHOMO,Fe/eV | ELUMO,Fe-EHOMO/eV | ΔE/eV | ||
|---|---|---|---|---|---|---|
| DEMA | Fe | DEMA | Fe | |||
| -8.022 | -7.810[ | -4.622 | -0.250[ | 3.188 | 7.772 | 3.40 |
| [1] |
Zou C., Qin Y., Yan X., Zhou L., Luo P ., Ind. Eng. Chem. Res., 2014,53(33), 12901— 12910
doi: 10.1021/ie501569d URL |
| [2] |
Barmatov E., Hughes T., Nagl M ., Corros. Sci., 2015,92, 85— 94
doi: 10.1016/j.corsci.2014.11.038 URL |
| [3] | Xiong D. Z., Zhang G. Y ., Oilfield Chem., 1991,8(4), 310— 313 |
| ( 熊德珍, 张功亚 . 油田化学, 1991,8(4), 310— 313) | |
| [4] |
Finšgar M., Jackson J ., Corros. Sci., 2014,86(3), 17— 41
doi: 10.1016/j.corsci.2014.04.044 URL |
| [5] |
Abdallah M., Helal E. A., Fouda A. S ., Corros. Sci., 2006,48(7), 1639— 1654
doi: 10.1016/j.corsci.2005.06.020 URL |
| [6] |
Abdallah M., Meghed H. E., Sobhi M ., Mater. Chem. Phys., 2009,118(1), 111— 117
doi: 10.1016/j.matchemphys.2009.07.013 URL |
| [7] |
Dehri İ., Özcan M ., Mater. Chem. Phys., 2006,98(2), 316— 323
doi: 10.1016/j.matchemphys.2005.09.020 URL |
| [8] |
Verma C., Ebenso E. E., Quraishi M. A ., J. Mol. Liq., 2017,233, 403— 414
doi: 10.1016/j.molliq.2017.02.111 URL |
| [9] |
Likhanova N. V., Domínguez-Aguilar M. A., Olivares-Xometl O., Nava-Entzana N., Arce E., Dorantes H ., Corros. Sci., 2010,52(6), 2088— 2097
doi: 10.1016/j.corsci.2010.02.030 URL |
| [10] | Luo Y. J., Zhang X. S., Wang Z. L ., Speciality Petrochem., 2004,2, 58— 60 |
| ( 罗娅君, 张新申, 王照丽 . 精细石油化工, 2004,2, 58— 60) | |
| [11] | Zhang G. H., Dong Q. C., Zhang W. B., Wang S ., Chem. J Chinese Universities, 2019,40(1), 130— 137 |
| ( 张光华, 董秋辰, 张万斌, 王爽 . 高等学校化学学报, 2019,40(1), 130— 137) | |
| [12] | Dong Q. C., Zhang G. H., Zhang W. B., Liu Y ., Fine Chem., 2019,36(5), 1005— 1011 |
| ( 董秋辰, 张光华, 张万斌, 刘瑜 . 精细化工, 2019,36(5), 1005— 1011) | |
| [13] |
Hanza A. P., Naderi R., Kowsari E., Sayebani M ., Corros. Sci., 2016,107, 96— 106
doi: 10.1016/j.corsci.2016.02.023 URL |
| [14] |
Sığırcık G., Yildirim D., Tüken T ., Corros. Sci., 2017,120, 184— 193
doi: 10.1016/j.corsci.2017.03.003 URL |
| [15] | Wang T. Y., Zou C. J., Li D. X., Chen Z. L., Liu Y., Li X. K., Li M ., Acta Phys. Chim. Sin., 2015,31(12), 2294— 2302 |
| [16] |
Wang X., Yang H., Wang F ., Corros. Sci., 2010,52(4), 1268— 1276
doi: 10.1016/j.corsci.2009.12.018 URL |
| [17] |
Qiang Y., Zhang S., Tan B., Chen S ., Corros. Sci., 2018,133, 6— 16
doi: 10.1016/j.corsci.2018.01.008 URL |
| [18] | Guo R., Li Y. P., Tu R. X., Song B., Guo Y ., Chem. J Chinese Universities, 2018,39(5), 1018— 1025 |
| ( 郭睿, 李云鹏, 土瑞香, 宋博, 郭煜 . 高等学校化学学报, 2018,39(5), 1018— 1025) | |
| [19] |
Cao S., Liu D., Ding H., Wang J., Lu H., Gui J ., Corros. Sci., 2019,153, 301— 313
doi: 10.1016/j.corsci.2019.03.035 URL |
| [20] |
Khaled K. F., Hackerman N ., Electrochim. Acta, 2003,48(19), 2715— 2723
doi: 10.1016/S0013-4686(03)00318-9 URL |
| [21] | Fan B. M., Hao H., Yang B., Ma Z., Feng Y. H ., Surf. Technol., 2018,47(10), 22— 29 |
| ( 樊保民, 郝华, 杨彪, 马震, 冯云皓 . 表面技术, 2018,47(10), 22— 29) | |
| [22] |
Ahamad I., Prasad R., Quraishi M. A ., Corros. Sci., 2010,52(4), 1472— 1481
doi: 10.1016/j.corsci.2010.01.015 URL |
| [23] |
Tian H., Li W., Liu A., Gao X., Han P., Ding R., Yang C., Wang D ., Corros. Sci., 2018,131, 1— 16
doi: 10.1016/j.corsci.2017.11.010 URL |
| [24] | Liu Z., Li B. R., Pan Y. X., Shi K., Wang W. C ., Chem. J Chinese Universities, 2017,38(4), 669— 677 |
| ( 刘志, 李炳睿, 潘艳雄, 石凯, 王伟财 . 高等学校化学学报, 2017,38(4), 669— 677) | |
| [25] |
Zarrouk A., Zarrok H., Ramli Y., Bouachrine M., Hammouti B., Sahibed-Dine A ., J. Mol. Liq., 2016,222, 239— 252
doi: 10.1016/j.molliq.2016.07.046 URL |
| [26] |
Abdallah M ., Corros. Sci., 2002,44(4), 717— 728
doi: 10.1016/S0010-938X(01)00100-7 URL |
| [27] | Hu S. Q., Hu J. C., Gao Y. J., Jia X. L., Guo W. Y ., CIESC. J., 2011,62(1), 147— 155 |
| ( 胡松青, 胡建春, 高元军, 贾晓林, 郭文跃 . 化工学报, 2011,62(1), 147— 155) | |
| [28] |
Khalil N ., Electrochim. Acta, 2003,48(18), 2635— 2640
doi: 10.1016/S0013-4686(03)00307-4 URL |
| [29] | Hu S. Q., Hu J. C., Fan C. C., Mi S. Q., Zhang J., Guo W. Y ., Acta Phys. Chim. Sin., 2010,26(8), 2163— 2170 |
| [30] |
Ansari K. R., Quraishi M. A ., J. Ind. Eng. Chem., 2014,20(5), 2819— 2829
doi: 10.1016/j.jiec.2013.11.014 URL |
| [31] |
Yurt A., Balaban A., Ustün Kandemir S., Bereket G., Erk B ., Mater. Chem. Phys., 2004,85(2), 420— 426
doi: 10.1016/j.matchemphys.2004.01.033 URL |
| [32] | Singh A., Gajra A., Cardiovasc. Hematol . Agents Med. Chem., 2011,9(1), 7— 13 |
| [1] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [2] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [3] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [4] | 戴卫, 侯华, 王宝山. 七氟异丁腈负离子结构与反应活性的理论研究[J]. 高等学校化学学报, 2022, 43(6): 20220044. |
| [5] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [6] | 季双琦, 靳钊, 观文娜, 潘翔宇, 关彤. 双阳离子型离子液体和十八烷基修饰的混合模式硅胶固定相的制备及色谱性能[J]. 高等学校化学学报, 2022, 43(6): 20220008. |
| [7] | 王红宁, 黄丽, 清江, 马腾洲, 蒋伟, 黄维秋, 陈若愚. 香蒲基生物炭的活化及对VOCs吸附的应用[J]. 高等学校化学学报, 2022, 43(4): 20210824. |
| [8] | 陈潇禄, 袁珍闫, 仲迎春, 任浩. 机械球磨制备三苯胺基PAF-106s及C2烃吸附性质[J]. 高等学校化学学报, 2022, 43(3): 20210771. |
| [9] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [10] | 靳科研, 白璞, 李小龙, 张佳楠, 闫文付. 新型Mg-Al吸附剂去除压水堆核电厂废水中高浓度硼[J]. 高等学校化学学报, 2022, 43(2): 20210516. |
| [11] | 常斯惠, 陈涛, 赵黎明, 邱勇隽. 离子液体增塑生物基聚丁内酰胺的热分解机理[J]. 高等学校化学学报, 2022, 43(11): 20220353. |
| [12] | 谭乐见, 仲宣树, 王锦, 刘宗建, 张爱英, 叶霖, 冯增国. β-环糊精的低临界溶解温度现象及其在有序纳米孔道片晶制备中的应用[J]. 高等学校化学学报, 2022, 43(11): 20220405. |
| [13] | 郑美琪, 毛方琪, 孔祥贵, 段雪. 类水滑石材料在核废水处理领域的应用[J]. 高等学校化学学报, 2022, 43(10): 20220456. |
| [14] | 田晓康, 张青松, 杨舒淋, 白洁, 陈冰洁, 潘杰, 陈莉, 危岩. 微生物发酵诱导多孔材料: 制备方法和应用[J]. 高等学校化学学报, 2022, 43(10): 20220216. |
| [15] | 马鉴新, 刘晓东, 徐娜, 刘国成, 王秀丽. 一种具有发光传感、 安培传感和染料吸附性能的多功能Zn(II)配位聚合物[J]. 高等学校化学学报, 2022, 43(1): 20210585. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||