

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (5): 988.doi: 10.7503/cjcu20180776
收稿日期:2018-11-19
出版日期:2019-05-06
发布日期:2019-01-09
作者简介:联系人简介: 唐亚文, 男, 博士, 教授, 博士生导师, 主要从事电化学与能源材料方面的研究. E-mail:
基金资助:
LI Jiahui, QIN Menghan, ZHANG Jie, DU Yi, SUN Dongmei, TANG Yawen*( )
)
Received:2018-11-19
Online:2019-05-06
Published:2019-01-09
Contact:
TANG Yawen
E-mail:tangyawen@njnu.edu.cn
Supported by:摘要:
以K2PdCl4/K2Ni(CN)4为前驱体制备了具有凝胶特性的氰胶(Cyanogels), 利用硼氢化钠还原氰胶得到三维多孔珊瑚状PdNi合金前驱体, 在此基础上通过原位Galvanic置换反应, 制备得到内核为PdNi合金、 表面具有不同厚度Au层的三维多孔PdNi@Au催化剂. X射线衍射(XRD)分析和透射电子显微镜(TEM)观测结果显示, 该三维网状结构由粒径约7 nm的纳米颗粒相互连接形成; 能量分散光谱(EDX)线性扫描和元素分布(Mapping)分析显示该催化剂具有典型的核壳结构. 电化学测试结果表明, 表面Au层的厚度影响PdNi@Au催化剂的性能, 当Au的含量(摩尔分数)为5.6%时, 催化剂显示出对甲酸最佳的电催化活性, 对甲酸电催化氧化的峰电流密度达到商业化铂黑催化剂的7.2倍.
中图分类号:
TrendMD:
李家辉, 秦梦寒, 张洁, 杜仪, 孙冬梅, 唐亚文. 三维多孔核壳结构PdNi@Au催化剂的制备及对甲酸的电催化氧化. 高等学校化学学报, 2019, 40(5): 988.
LI Jiahui,QIN Menghan,ZHANG Jie,DU Yi,SUN Dongmei,TANG Yawen. Fabrication of 3D Porous Core-shell PdNi@Au Nanocatalyst for Formic Acid Electro-oxidation†. Chem. J. Chinese Universities, 2019, 40(5): 988.
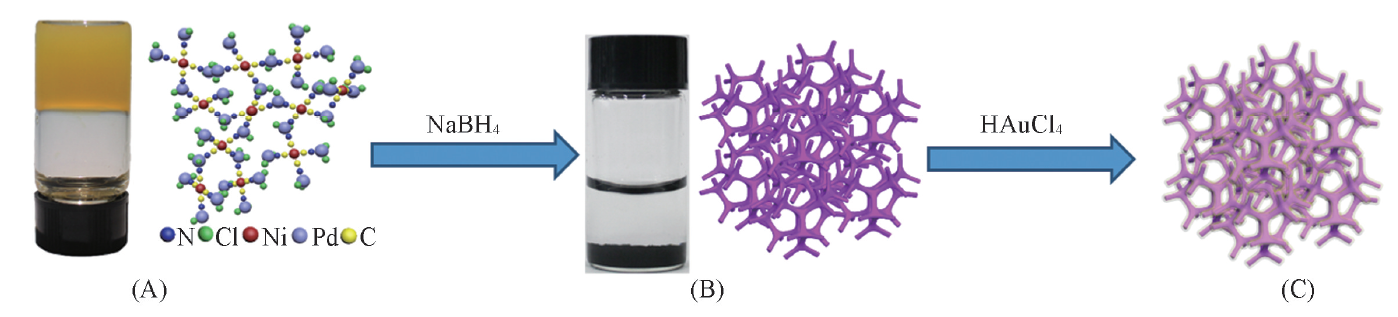
Scheme 1 Synthetic routes of PdNi@Au catalyst(A) Orange jelly-like PdNi cyanogel and its ball-stick model; (B) photograph of 3D porous PdNi alloy with network structure; (C) schematic diagram of PdNi@Au catalyst.
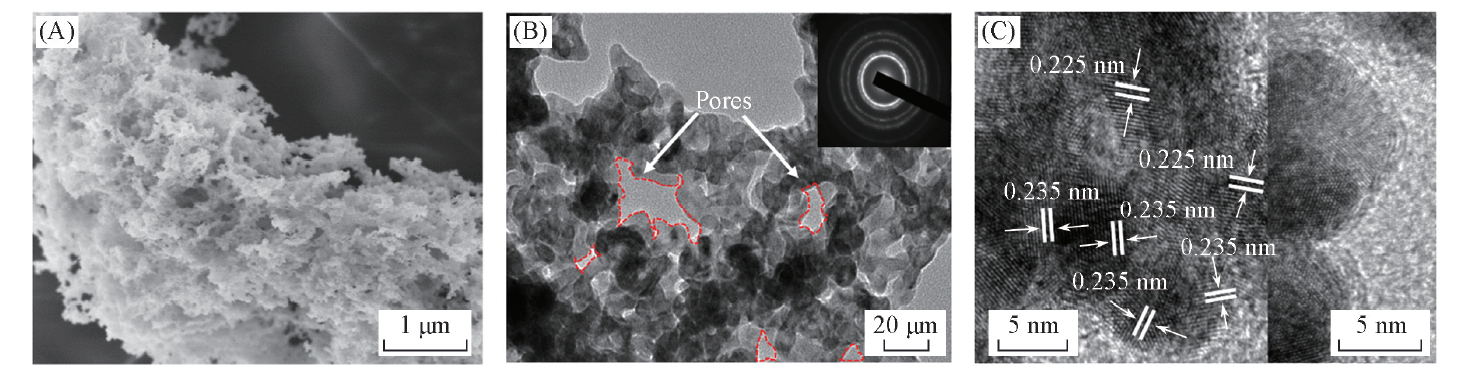
Fig.3 Typical SEM(A), TEM(B) and HRTEM(C) images of PdNi@Au-5.6 catalyst The insets in (B) and (C) are corresponding SAED pattern and the outer Au layer, respectively.
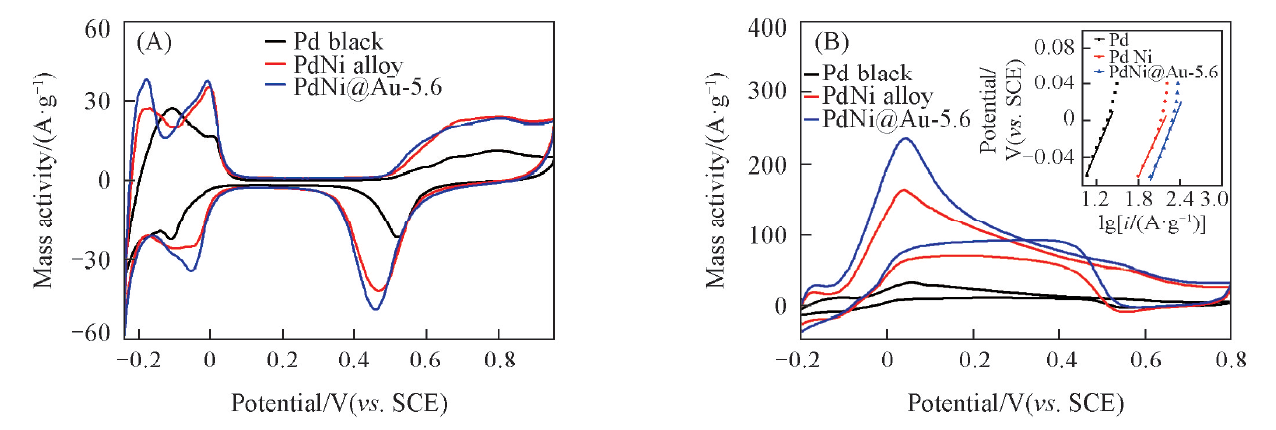
Fig.8 CV curves of different catalysts in N2-saturated 0.5 mol/L H2SO4(A) and in N2-saturated 0.5 mol/L H2SO4+0.1 mol/L HCOOH(B)Inset in (B) is corresponding Tafel plots.
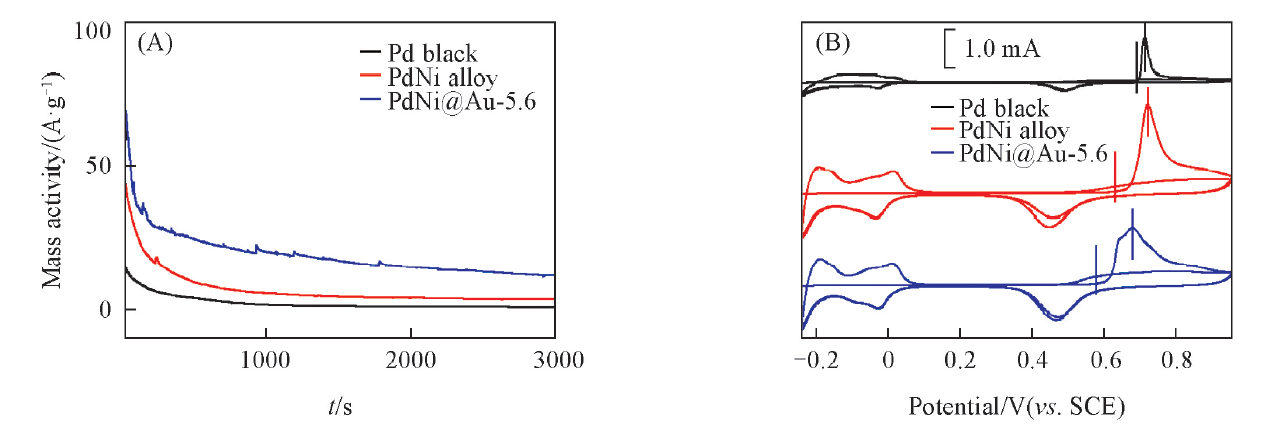
Fig.9 Chronoamperometry curves at 0 V for 3000 s in N2-saturated 0.5 mol/L H2SO4+0.1 mol/L HCOOH(A) and CO-stripping voltammograms in N2-saturated 0.5 mol/L H2SO4(B) of different catalysts
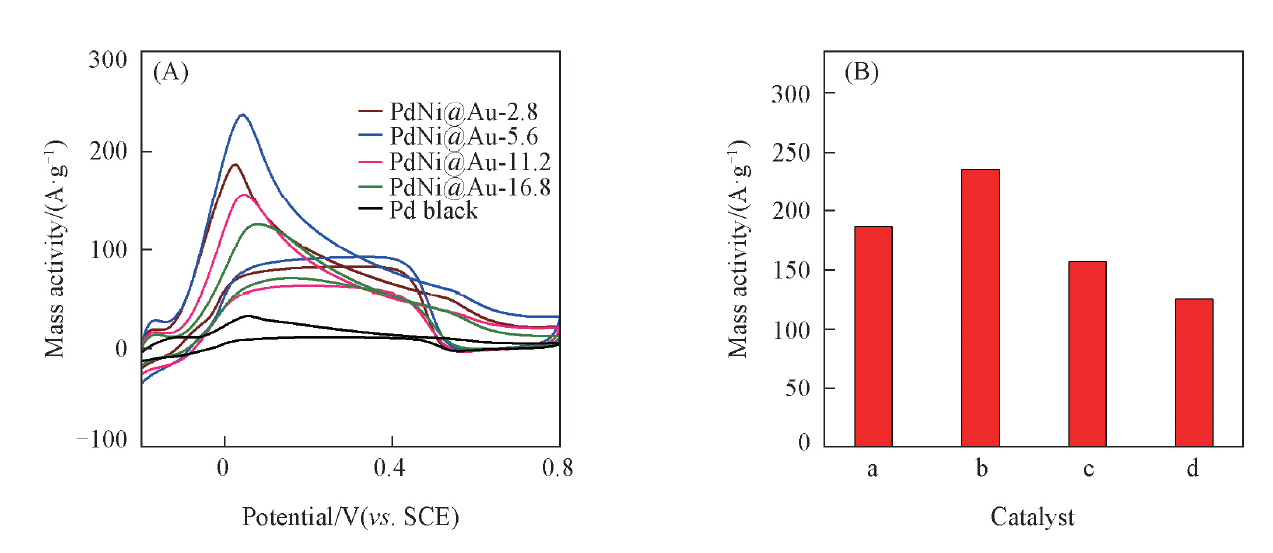
Fig.10 CV curves of different catalysts in N2-saturated 0.5 mol/L H2SO4+ 0.1 mol/L HCOOH solution(A) and histogram of mass-normalized peak current for different catalysts(B)a. PdNi@Au-2.8; b. PdNi@Au-5.6; c. PdNi@Au-11.2; d. PdNi@Au-16.8.
| [1] | Au L., Chen Y., Zhou F., Camargo P. H. C., Lim B., Li Z. Y., Ginger D. S., Xia Y. N., Nano Res., 2008, 1(6), 441—449 |
| [2] | Jiang T. T., Song J. L. Q., Zhang W. T., Wang H., Li X. D., Xia R. X., Zhu L. X., Xu X. L., ACS Appl. Mater. Interfaces,2015, 7(39), 21985—21994 |
| [3] | Liu Z. Y., Fu G. T., Tang Y. W., Sun D. M., Chen Y., Lu T. H., Cryst. Eng. Comm.,2014, 16(36), 8576—8581 |
| [4] | Geetha B. R., Muthoosamy K., Zhou M. F., Ashokkumar M., Huang N. M., Manickam S., Biosens. Bioelectron.,2017, 87, 622—629 |
| [5] | Wu H. X., Wang P., He H. L., Jin Y. D., Nano Res., 2012, 5(2), 135—144 |
| [6] | Liu X. L., Liang S., Nan F., Yang Z. J., Yu X. F., Zhou L., Hao Z. H., Wang Q. Q., Nanoscale,2013, 5(12), 5368—5374 |
| [7] | Lang X.Y., Yuan H. T., Iwasa Y., Chen M. W.,Scr. Mater., 2011, 64(9), 923—926 |
| [8] | Jiang X., Qiu X. Y., Fu G. T., Sun J. Z., Huang Z. N., Sun D. M., Xu L., Zhou J. C., Tang Y. W., J. Mater. Chem. A,2018, 6(36), 17682—17687 |
| [9] | Zhu W. L., Zhang Y. J., Zhang H. Y., Lv H. F., Li Q., Michalsky R., Peterson A. A., Sun S. H., J. Am. Chem. Soc.,2014, 136(46), 16132—16135 |
| [10] | Ye X. C., Gao Y. Z., Chen J., Reifsnyder D. C., Zheng C., Murray C. B., Nano Lett., 2013, 13(5), 2163—2171 |
| [11] | Garg N., Scholl C., Mohanty A., Jin R. C., Langmuir,2010, 26(12), 10271—10276 |
| [12] | Li Y. Q., Zhu Q., Xiao Z. L., Lv C. Z., Feng Z. M., Yin Y. L., Cao Z., Chem. J. Chinese Universities,2018, 39(4), 636—644 |
| (李雨晴, 朱钦, 肖忠良, 吕超志, 冯泽猛, 印遇龙, 曹忠. 高等学校化学学报,2018, 39(4), 636—644) | |
| [13] | Zhao N. N., Wei Y., Sun N. J., Chen Q., Bai J. W., Zhou L. P., Qin Y., Li M. X., Qi L. M., Langmuir,2008, 24(3), 991—998 |
| [14] | Zhang R. Y., Hummelgard M., Olin H., PLoS One,2012, 7(1), e30469 |
| [15] | Hu J., Gan Q. M., Zhang Y. L., Ren B., Li Y. J., Mater. Chem. Phys.,2015, 163, 529—536 |
| [16] | Xie J. P., Zhang Q. B., Lee J. Y., Wang D. I. C., ACS Nano,2008, 2(12), 2473—2480 |
| [17] | Li S. N., Zhang L. Y., Wang T. T., Li L., Wang C. G., Su Z. M., Chem. Commun.,2015, 51(76), 14338—14341 |
| [18] | Lee K. Y., Kim M., Noh J. S., Choi H. C., Lee W., J. Mater. Chem. A,2013, 1(44), 13890—13895 |
| [19] | Wang L., Imura M., Yamauchi Y., Cryst. Eng. Comm.,2012, 14(22), 7594—7599 |
| [20] | Ke X., Li Z. H., Gan L., Zhao J., Cui G. F., Kellogg W., Matera D., Higgins D., Wu G., Electrochim. Acta,2015, 170, 337—342 |
| [21] | Ke X., Xu Y. T., Yu C. C., Zhao J., Cui G. F., Higgins D., Chen Z. W., Li Q., Xu H., Wu G., J. Mater. Chem. A,2014, 2(39), 16474—16479 |
| [22] | Zhong S. L., Zhuang J. Y., Yang D. P., Tang D. P., Biosens. Bioelectron., 2017, 96, 26—32 |
| [23] | Ke X., Xu Y. T., Yu C. C., Zhao J., Cui G. F., Higgins D., Li Q., Wu G., J. Power Sources,2014, 269, 461—465 |
| [24] | El-Said W. A., Lee J. H., Oh B. K., Choi J. W., Electrochem. Commun., 2010, 12(12), 1756—1759 |
| [25] | Xu C. X., Su J. X., Xu X. H., Liu P. P., Zhao H. J., Tian F., Ding Y., J. Am. Chem. Soc., 2007, 129(1), 42—43 |
| [26] | Liu R., Liu J. F., Zhou X. X., Sun M. T., Jiang G. B., Anal. Chem.,2011, 83(23), 9131—9137 |
| [27] | Kafi A. K. M., Ahmadalinezhad A., Wang J. P., Thomas D. F., Chen A. C., Biosens-Bioelectron., 2010, 25(11), 2458—2463 |
| [28] | Zhang G. J., Wang Y. N., Wang X., Chen Y., Zhou Y. M., Tang Y. W., Lu L. D., Bao J. C., Lu T. H., Appl. Catal. B: Environ., 2011, 102(3), 614—619 |
| [29] | Szumełda T., Drelinkiewicz A., Lalik E., Kosydar R., Duraczyńska D., Gurgul J., Appl. Catal. B: Environ.,2018, 221, 393—405 |
| [30] | Yuan T., Chen H. Y., Ma X. H., Feng J. J., Yuan P. X., Wang A. J., J. Colloid Interface Sci.,2018, 513, 324—330 |
| [31] | Huang T., Moon S. K., Lee J. M., Sustainable Energy Fuels,2017, 1(3), 450—457 |
| [32] | Sun D. D., Si L., Fu G. T., Liu C., Sun D. M., Chen Y., Tang Y. W., Lu T. H., J. Power Sources,2015, 280, 141—146 |
| [33] | Wang Y. X., Xiong Z., Xia Y. Z., RSC Adv., 2017, 7(64), 40462—40469 |
| [34] | Hu S. Z., Scudiero L., Ha S., Electrochem. Commun., 2014, 38, 107—109 |
| [35] | Maiyalagan T., Wang X., Manthiram A., RSC Adv., 2014, 4(8), 4028—4033 |
| [1] | 孙竹梅 傅杰 李鑫 王海芳 卢静 童天星 朱明飞 王云燕. 电吸附除氯过程电化学阻抗谱及动力学研究[J]. 高等学校化学学报, 0, (): 20220528. |
| [2] | 陈佳琪 程晚亭 温秋慧 韩静茹 马福秋 颜永得 薛云. 活性炭电极的改性及其对Co2+、Mn2+、Ni2+的电吸附性能研究[J]. 高等学校化学学报, 0, (): 20220598. |
| [3] | 刘杰, 李金晟, 柏景森, 金钊, 葛君杰, 刘长鹏, 邢巍. 降低直接甲醇燃料电池浓差极化的含磺化碳管阻水夹层的构建[J]. 高等学校化学学报, 2022, 43(11): 20220420. |
| [4] | 刘坤, 尹远, 耿文强, 夏昊天, 李华. 不同组分过渡金属氧化物催化剂对介质阻挡放电固氮的影响机制[J]. 高等学校化学学报, 2022, 43(11): 20220278. |
| [5] | 丁钦, 张梓轩, 徐培程, 李晓宇, 段莉梅, 王寅, 刘景海. Cu, Ni, Co掺杂对Fe碳纳米管的结构及电催化性能的影响[J]. 高等学校化学学报, 2022, 43(11): 20220421. |
| [6] | 何宇婧, 李佳乐, 王东洋, 王福玲, 肖作旭, 陈艳丽. 锌活化Fe/Co/N掺杂的生物质碳基高效氧还原催化剂[J]. 高等学校化学学报, 2022, 43(11): 20220475. |
| [7] | 胡平澳 张琪 张会茹. 锂硫电池中硒缺陷WSe2催化性能理论预测[J]. 高等学校化学学报, 0, (): 20220595. |
| [8] | 吴钰洁 黄文治 潘俊达 石凯祥 刘全兵. “蛋黄-蛋壳”结构纳米反应器的设计、调控及其在锂硫电池正极中的应用研究[J]. 高等学校化学学报, 0, (): 20220619. |
| [9] | 张玲玲 董欢欢 何祥喜 李丽 李林 吴星樵 侴术雷. 中空碳材料用于钠离子电池负极的研究进展[J]. 高等学校化学学报, 0, (): 20220620. |
| [10] | 侯从聪, 王惠颖, 李婷婷, 张志明, 常春蕊, 安立宝. N-CNTs/NiCo-LDH复合材料的制备及电化学性能[J]. 高等学校化学学报, 2022, 43(10): 20220351. |
| [11] | 姜宝正, 黄文婷, 刘文宝, 郭荣胜, 徐成俊, 康飞宇. 纳米铜修饰三维锌网电极的制备及锌离子电池负极的电化学性能[J]. 高等学校化学学报, 2022, 43(10): 20220257. |
| [12] | 刘坤, 左杰, 李华, 项红甫, 冉从福, 杨明昊, 耿文强. 电子能量对沿面介质阻挡放电等离子体化学产物的影响[J]. 高等学校化学学报, 2022, 43(10): 20220249. |
| [13] | 王鹏飞, 富文豪, 孙少妮, 曹学飞, 袁同琦. 纤维素纳米晶模板法制备多级孔炭材料及其电化学性能[J]. 高等学校化学学报, 0, (): 20220497. |
| [14] | 胡诗颖 沈佳艳 韩峻山 郝婷婷 李星. CoO纳米颗粒/石墨烯纳米纤维复合材料的制备及其电化学性能[J]. 高等学校化学学报, 0, (): 20220462. |
| [15] | 王茹玥, 魏呵呵, 黄凯, 伍晖. 单原子材料的冷冻合成[J]. 高等学校化学学报, 2022, 43(9): 20220428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||