

高等学校化学学报 ›› 2016, Vol. 37 ›› Issue (9): 1596.doi: 10.7503/cjcu20160253
收稿日期:2016-04-18
出版日期:2016-09-10
发布日期:2016-08-26
作者简介:联系人简介: 杨水金, 男, 博士, 教授, 主要从事多酸化学和无机功能材料研究. E-mail:基金资助:
GONG Wenpeng, TIAN Chaoqiang, DU Xiaogang, YANG Shuijin*( )
)
Received:2016-04-18
Online:2016-09-10
Published:2016-08-26
Contact:
YANG Shuijin
E-mail:yangshuijin@163.com
Supported by:摘要:
在溶剂热条件下, 以H6P2Mo18O62、 对苯二甲酸(H2BDC)、 4,4'-联吡啶(Bipy)和硝酸锌为原料构筑了1个三维金属有机骨架复合材料H6P2Mo18O62/Zn(BDC)(Bipy)0.5, 并采用红外光谱(IR)、 X射线衍射(XRD)、 扫描电子显微镜(SEM)、 热重分析(TG)、 N2吸附-脱附等手段对产物进行了表征. 同时, 研究了产物对水溶液中亚甲基蓝(MB)的吸附性能, 并探讨了MB初始pH值、 初始浓度和温度对吸附量的影响. 结果表明, 产物的等温吸附模型符合Langmuir等温吸附模型, 动力学符合拟二级动力学. H6P2Mo18O62/Zn(BDC)(Bipy)0.5对亚甲基蓝的吸附是自发和放热的, 此外, 产物对甲基紫、 罗丹明B、 孔雀石绿等阳离子染料也具有良好的吸附性能.
中图分类号:
TrendMD:
龚文朋, 田超强, 杜晓刚, 杨水金. H6P2Mo18O62/Zn(BDC)(Bipy)0.5复合材料的合成、 表征及对亚甲基蓝的吸附. 高等学校化学学报, 2016, 37(9): 1596.
GONG Wenpeng, TIAN Chaoqiang, DU Xiaogang, YANG Shuijin. Synthesis and Characterization of Composite H6P2Mo18O62/Zn(BDC)(Bipy)0.5 and Its Adsorption Activity for Methylene Blue†. Chem. J. Chinese Universities, 2016, 37(9): 1596.
| Sample | Element | w(%) | n(Mo):n(P) |
|---|---|---|---|
| H6P2Mo18O62 | P | 2.13 | 9.1 |
| Mo | 60.02 | ||
| Zn | 0 | ||
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | P | 1.74 | 9.0 |
| Mo | 48.59 | ||
| Zn | 20.61 |
Table 1 Elemental analysis of Zn(BDC)(Bipy)0.5 and H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| Sample | Element | w(%) | n(Mo):n(P) |
|---|---|---|---|
| H6P2Mo18O62 | P | 2.13 | 9.1 |
| Mo | 60.02 | ||
| Zn | 0 | ||
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | P | 1.74 | 9.0 |
| Mo | 48.59 | ||
| Zn | 20.61 |
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | D/nm |
|---|---|---|---|
| Zn(BDC)(Bipy)0.5 | 710.6 | 0.32 | 1.79 |
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | 249.9 | 0.12 | 1.92 |
Table 2 Structural properties of Zn(BDC)(Bipy)0.5 and H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| Sample | SBET/(m2·g-1) | Vtotal/(cm3·g-1) | D/nm |
|---|---|---|---|
| Zn(BDC)(Bipy)0.5 | 710.6 | 0.32 | 1.79 |
| H6P2Mo18O62/Zn(BDC)(Bipy)0.5 | 249.9 | 0.12 | 1.92 |
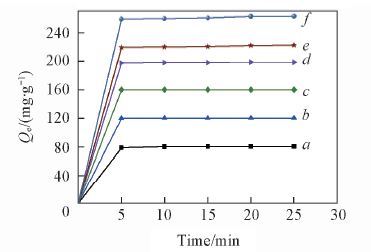
Fig.7 Effect of initial mass concentration on adsorption capacities for MB Initial mass concentration of MB/(mg·mL-1): a. 40;b. 60; c. 80; d. 100; e. 120; f. 140.
| Kinetics model | Initial concentration of MB/ (mg·L-1) | Qe,exp/ (mg·g-1) | Qe,cal/ (mg·g-1) | K1/min-1 or K2/ (g·mg-1·min-1) | R2 |
|---|---|---|---|---|---|
| Pseudo-first-order model | 90 | 179.43 | 1.1693 | 0.09343 | 0.9661 |
| 100 | 198.64 | 1.7124 | 0.10918 | 0.9669 | |
| Pseudo-second-order model | 90 | 179.43 | 179.53 | 0.000021 | 1 |
| 100 | 198.64 | 198.81 | 0.0000157 | 1 |
Table 3 Parameters of pseudo-first-order and pseudo-second-order adsorption kinetics model in different initial concentrations
| Kinetics model | Initial concentration of MB/ (mg·L-1) | Qe,exp/ (mg·g-1) | Qe,cal/ (mg·g-1) | K1/min-1 or K2/ (g·mg-1·min-1) | R2 |
|---|---|---|---|---|---|
| Pseudo-first-order model | 90 | 179.43 | 1.1693 | 0.09343 | 0.9661 |
| 100 | 198.64 | 1.7124 | 0.10918 | 0.9669 | |
| Pseudo-second-order model | 90 | 179.43 | 179.53 | 0.000021 | 1 |
| 100 | 198.64 | 198.81 | 0.0000157 | 1 |
| Temperature/K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(mg·g-1) | n | R2 | |
| 293 | 277.78 | 0.00319 | 0.7337 | 0.9986 | 244.84 | 13.18 | 0.9606 |
| 303 | 275.48 | 1.9621 | 0.00459 | 0.9893 | 194.54 | 7.76 | 0.9137 |
| 313 | 273.22 | 0.7205 | 0.01242 | 0.9695 | 157.66 | 5.76 | 0.7916 |
Table 4 Isotherm parameters of the adsorption of methylene blue by H6P2Mo18O62/Zn(BDC)(Bipy)0.5 at different temperature
| Temperature/K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mg-1) | RL | R2 | KF/(mg·g-1) | n | R2 | |
| 293 | 277.78 | 0.00319 | 0.7337 | 0.9986 | 244.84 | 13.18 | 0.9606 |
| 303 | 275.48 | 1.9621 | 0.00459 | 0.9893 | 194.54 | 7.76 | 0.9137 |
| 313 | 273.22 | 0.7205 | 0.01242 | 0.9695 | 157.66 | 5.76 | 0.7916 |
| ΔGo/(kJ·mol-1) | ΔHo/(kJ·mol-1) | ΔSo/(J·mol-1·K-1) | ||
|---|---|---|---|---|
| 293 K | 303 K | 313 K | ||
| -14.87 | -11.33 | -9.5 | -93.81 | -270.31 |
Table 5 Thermodynamic parameters of adsorption methylene blue onto H6P2Mo18O62/Zn(BDC)(Bipy)0.5
| ΔGo/(kJ·mol-1) | ΔHo/(kJ·mol-1) | ΔSo/(J·mol-1·K-1) | ||
|---|---|---|---|---|
| 293 K | 303 K | 313 K | ||
| -14.87 | -11.33 | -9.5 | -93.81 | -270.31 |
| [1] | Rebane R., Leito I., Yurchenko S., J. Chromatogr. A,2010, 1217, 747—757 |
| [2] | Lin H., Yang S., Ni Y., J. Wood. Chem. Technol., 2010, 30, 118—128 |
| [3] | Gupta V. K., Shilpi A., Saleh T. A., Instrum. Sci. Technol., 2007, 202(4—7), 915—919 |
| [4] | Carneiro P. A., Umbuzeiro G. A., Oliveira D. P., J. Hazard. Mater., 2010, 174, 694—699 |
| [5] | Marin M. O., Prete V. D., Moruno E. G., Food Control., 2009, 20, 298—303 |
| [6] | Farahani M., Rozaimah S. A. S., Soraya H., Procedia. Environ. Sci., 2011, 10, 203—208 |
| [7] | Crini G., Bioresour. Technol., 2006, 97, 1061—1085 |
| [8] | Gupta V. K., Saleh T. A., Environ. Sci. Pollut. Res., 2013, 20(5), 2828—2843 |
| [9] | Mittal A., Kaur D., Malviya A., Mittal J., Gupta V. K., J. Colloid Interface Sci., 2009, 337, 345—354 |
| [10] | Gupta V. K., Agarwal S., Saleh T. A., J. Hazard. Mater., 2011, 185, 17—23 |
| [11] | Zhang W. J., Zhou C. J., Zhou W. C., Bull. Environ. Contam. Toxicol., 2011, 87, 86—90 |
| [12] | Wang H. L., Zhao Y., Ma L. K., Fan P. H., Xu C. B., Jiao C. L., Lin A. J., Chem. J. Chinese Universities,2016, 37(2), 335—341 |
| (王会丽, 赵越, 马乐宽, 范鹏浩, 徐从斌, 焦春磊, 林爱军. 高等学校化学学报, 2016, 37(2), 335—341) | |
| [13] | Hasan Z., Jhung S. H., J. Hazard Mater., 2015, 283, 329—339 |
| [14] | Tong M., Liu D., Yang Q., Devautour V. S., Maurin G., Zhong C., J. Mater. Chem. A,2013, 1, 8534—8537 |
| [15] | Haque E., Lo V., Minett A. I., J. Mater. Chem. A,2014, 2, 193—203 |
| [16] | Dai M., Su X. R., Wang X., Wu B., Ren Z. G., Zhou X., Lang J. P., Cryst. Growth & Des., 2014, 14, 240—248 |
| [17] | Chen B. L., Liang C. D., Yang J., Contreras D. S., Clancy Y. L., Lobkousky E. B., Yaghi O. M., Dai S., Angew. Chem. Int. Ed., 2006, 45, 1390—1393 |
| [18] | Liang D. D., Liu S. X., Ma F. J., Adv. Synth. Catal., 2011, 353, 733—742 |
| [19] | Ma F. J., Liu S. X., Liang D. D., Eur. J. Inorg. Chem., 2010, 3756—3761 |
| [20] | Sha J. Q., Peng J., Lan Y. Q., Inorg. Chem., 2008, 47, 5145—5153 |
| [21] | Yan A., Yao X. S., Wang E. B., Chem. Eur. J., 2014, 20, 6927—6933 |
| [22] | Yi F. Y., Zhu W., Sun Z. M., Chem. Commun., 2015, 51, 3336—3339 |
| [23] | Fu H., Li Y. G., Lu Y., Chen W. L., Wu Q., Meng J. X., Wang X. L., Zhang Z. M., Wang E. B., Crystal. Growth & Des., 2011, 11, 458—465 |
| [24] | Sun C. Y., Liu S. X., Liang D. D., J. Am. Chem. Soc., 2009, 131, 1883—1888 |
| [25] | Park D. R., Song J. H., Lee S. H., Applied Catalysis A: General,2008, 349, 222—228 |
| [26] | Wang E. B., Gao L.H., Liu J. F., Acta Chim. Sinica,1988, 46, 757—762 |
| (王恩波, 高丽华, 刘景福. 化学学报, 1988, 46, 757—762) | |
| [27] | Liu Y.W., Liu S. M., Liu S. X., J. Am. Chem. Soc., 2015, 137, 12697—12703 |
| [28] | Liu H., Zhao Y. G., Zhang Z. J., Nijem N., Chabal Y. J., Adv. Funct. Mater., 2011, 21, 4754—4762 |
| [29] | Canioni R., Marchal R. C., Secheresse F., Horcajada P., Serre C., J. Mater. Chem., 2011, 21, 1226—1233 |
| [30] | Lin S., Song Z., Che G., Microporous Mesoporous Mater., 2014, 193(2), 27—34 |
| [31] | Luo H. M., Zhao X., Chen N. L., Ion Exchange And Adsorption,2011, 27(2), 152—159 |
| [32] | Eftekhari S., Habibi Y. A., Sohrabnezhand S., Hazard. Mater., 2010, 78, 349—355 |
| [33] | Li R., Ren X. Q., Zhao J. S., J. Mater. Chem. A, 2014, 2, 2168—2173 |
| [34] | Liu X. X., Gong W. P., Luo J., Appl. Surf. Sci., 2016, 362, 517—524 |
| [35] | Chen S. H., Zhang J., Zhang C. L., Desalination,2010, 252, 149—156 |
| [36] | Langmuir I., J. Am. Chem. Soc., 1918, 40, 1361—1403 |
| [37] | Hameed B. H., J. Hazard. Mater., 2008, 154, 204—212 |
| [1] | 姜宏斌, 代文臣, 张娆, 徐晓晨, 陈捷, 杨光, 杨凤林. Co3O4/UiO-66@α-Al2O3陶瓷膜对VOCs废气的分离催化性能[J]. 高等学校化学学报, 2022, 43(6): 20220025. |
| [2] | 戴卫, 侯华, 王宝山. 七氟异丁腈负离子结构与反应活性的理论研究[J]. 高等学校化学学报, 2022, 43(6): 20220044. |
| [3] | 郝宏蕾, 孟繁雨, 李若钰, 李迎秋, 贾明君, 张文祥, 袁晓玲. 生物质基氮掺杂多孔炭材料的制备及对水中亚甲基蓝的吸附性能[J]. 高等学校化学学报, 2022, 43(6): 20220055. |
| [4] | 王红宁, 黄丽, 清江, 马腾洲, 蒋伟, 黄维秋, 陈若愚. 香蒲基生物炭的活化及对VOCs吸附的应用[J]. 高等学校化学学报, 2022, 43(4): 20210824. |
| [5] | 孟祥龙, 杨歌, 郭海玲, 刘晨光, 柴永明, 王纯正, 郭永梅. 纳米分子筛的合成及硫化氢吸附性能[J]. 高等学校化学学报, 2022, 43(3): 20210687. |
| [6] | 陈潇禄, 袁珍闫, 仲迎春, 任浩. 机械球磨制备三苯胺基PAF-106s及C2烃吸附性质[J]. 高等学校化学学报, 2022, 43(3): 20210771. |
| [7] | 靳科研, 白璞, 李小龙, 张佳楠, 闫文付. 新型Mg-Al吸附剂去除压水堆核电厂废水中高浓度硼[J]. 高等学校化学学报, 2022, 43(2): 20210516. |
| [8] | 王寿柏, 吴修明, 束辰, 钟敏, 黄卫, 颜德岳. 含叔丁基聚酰亚胺均质膜的气体分离性能[J]. 高等学校化学学报, 2022, 43(11): 20220357. |
| [9] | 谭乐见, 仲宣树, 王锦, 刘宗建, 张爱英, 叶霖, 冯增国. β-环糊精的低临界溶解温度现象及其在有序纳米孔道片晶制备中的应用[J]. 高等学校化学学报, 2022, 43(11): 20220405. |
| [10] | 郑美琪, 毛方琪, 孔祥贵, 段雪. 类水滑石材料在核废水处理领域的应用[J]. 高等学校化学学报, 2022, 43(10): 20220456. |
| [11] | 田晓康, 张青松, 杨舒淋, 白洁, 陈冰洁, 潘杰, 陈莉, 危岩. 微生物发酵诱导多孔材料: 制备方法和应用[J]. 高等学校化学学报, 2022, 43(10): 20220216. |
| [12] | 唐元晖, 李春玉, 林亚凯, 张春晖, 刘泽, 余立新, 王海辉, 王晓琳. 链段刚性对非溶剂致相分离成膜过程影响的耗散粒子动力学模拟[J]. 高等学校化学学报, 2022, 43(10): 20220169. |
| [13] | 柳雪广, 杨晓珊, 马菁菁, 刘伟生. 铕基金属有机框架材料从混合染料中选择性分离亚甲基蓝[J]. 高等学校化学学报, 2022, 43(1): 20210715. |
| [14] | 马鉴新, 刘晓东, 徐娜, 刘国成, 王秀丽. 一种具有发光传感、 安培传感和染料吸附性能的多功能Zn(II)配位聚合物[J]. 高等学校化学学报, 2022, 43(1): 20210585. |
| [15] | 张弛, 孙福兴, 朱广山. 双金属同构金属-有机框架材料CAU-21-Al/M的合成、 氮气吸附及复合膜性能[J]. 高等学校化学学报, 2022, 43(1): 20210578. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||