

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (8): 1739.doi: 10.7503/cjcu20140260
收稿日期:2014-03-25
出版日期:2014-08-10
发布日期:2014-06-16
作者简介:联系人简介: 丁开宁, 男, 博士, 副教授, 主要从事固体表面吸附行为和光催化理论研究. E-mail:基金资助:
DING Kaining*( ), LI Yulu, CHENG Peisi, ZHANG Yongfan
), LI Yulu, CHENG Peisi, ZHANG Yongfan
Received:2014-03-25
Online:2014-08-10
Published:2014-06-16
Contact:
DING Kaining
E-mail:dknfzu@fzu.edu.cn
Supported by:摘要:
采用DMol3程序包中的GGA-PW91方法, 结合周期平板模型, 对CH3O和CO在Pd(111)表面的反应进行了系统研究. 计算结果表明, 吸附在Pd(111)表面顶位上的CO分子中C原子所带正电荷最多, 容易与亲核试剂反应, 化学吸附能稍低, 有利于在表面上移动发生亲电插入反应; CH3O 在Pd(111)表面fcc穴位吸附稳定, O原子上所带的负电荷较多, 易被亲电试剂进攻. 过渡态搜索表明, Pd(111)表面顶位上的CO与fcc穴位上CH3O反应生成CH3OOC的为放热反应, 反应能垒较低, 有利于偶联反应的进行.
中图分类号:
TrendMD:
丁开宁, 李玉璐, 程蓓斯, 章永凡. CH3O和CO在Pd(111)表面偶联反应机理的理论研究. 高等学校化学学报, 2014, 35(8): 1739.
DING Kaining, LI Yulu, CHENG Peisi, ZHANG Yongfan. Theoretical Studies on the Reaction Mechanisms of Methoxy Group and Carbon Monoxide over the Surfaces of Pd(111)†. Chem. J. Chinese Universities, 2014, 35(8): 1739.
| Layer | Δd1-2(%) | Δd2-3(%) | Δd3-4 (%) | Esurf /(J·m-2) |
|---|---|---|---|---|
| 3 | 0.33(0.44[ | -0.39(-0.32[ | 1.26(1.40[ | |
| 4 | 0.34 | -0.27 | 1.28 | |
| 5 | 0.31 | -0.31 | 1.30 | |
| 6 | 0.33 | -0.29 | 1.34 | |
| 7 | 0.41 | 0.30 | -0.33 | 1.32 |
Table 1 Calculated relaxation degree and surface energy of 3×3 Pd(111) surface with different layers
| Layer | Δd1-2(%) | Δd2-3(%) | Δd3-4 (%) | Esurf /(J·m-2) |
|---|---|---|---|---|
| 3 | 0.33(0.44[ | -0.39(-0.32[ | 1.26(1.40[ | |
| 4 | 0.34 | -0.27 | 1.28 | |
| 5 | 0.31 | -0.31 | 1.30 | |
| 6 | 0.33 | -0.29 | 1.34 | |
| 7 | 0.41 | 0.30 | -0.33 | 1.32 |
| Layer | Eads(CO)/(kJ·mol-1) | Eads(H3CO)/( kJ·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Top | Bridge | fcc | hcp | Top | Bridge | fcc | hcp | ||
| 3 | -159.41 | -144.87 | -204.18 | -201.67 | -180.33 | -211.71 | -218.82 | -197.48 | |
| 4 | -151.46 | -186.61 | -199.99 | -196.23 | -189.95 | -200.41 | -212.97 | -196.72 | |
| 5 | -161.08 | -173.22 | -187.02 | -144.78 | -196.14 | -210.04 | -215.06 | -196.42 | |
| 6 | -158.16 | -176.98 | -186.61 | -187.86 | -192.05 | -203.76 | -214.22 | -212.55 | |
| 7 | -155.64 | -187.02 | -196.23 | -192.88 | -195.68 | -205.43 | -216.31 | -210.87 | |
Table 2 Adsorption energy of CO and CH3O over 3×3 Pd(111) surface with different layers
| Layer | Eads(CO)/(kJ·mol-1) | Eads(H3CO)/( kJ·mol-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Top | Bridge | fcc | hcp | Top | Bridge | fcc | hcp | ||
| 3 | -159.41 | -144.87 | -204.18 | -201.67 | -180.33 | -211.71 | -218.82 | -197.48 | |
| 4 | -151.46 | -186.61 | -199.99 | -196.23 | -189.95 | -200.41 | -212.97 | -196.72 | |
| 5 | -161.08 | -173.22 | -187.02 | -144.78 | -196.14 | -210.04 | -215.06 | -196.42 | |
| 6 | -158.16 | -176.98 | -186.61 | -187.86 | -192.05 | -203.76 | -214.22 | -212.55 | |
| 7 | -155.64 | -187.02 | -196.23 | -192.88 | -195.68 | -205.43 | -216.31 | -210.87 | |
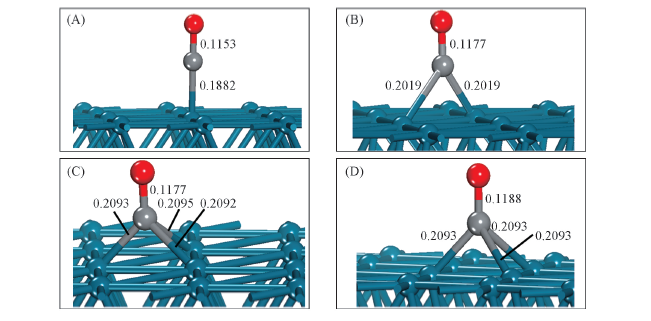
Fig.2 Lateral views for CO adsorbed on Pd(111) surface with four balanced geometrical configurations (A) Top site; (B) bridge site; (C) fcc site; (D) hcp site.
| Species | Eads/(kJ·mol-1) | Species | Eads/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nt | Nb | Nf | Nh | Nt | Nb | Nf | Nh | ||
| 1×1 | -181.17 | -171.96 | -74.89 | -84.94 | Calcd.[ | -112.13 | -141.84 | -167.36 | |
| 2×2 | -178.66 | -177.82 | -79.08 | -86.19 | Calcd.[ | -131.38 | -174.47 | -194.14 | -191.21 |
| 3×3 | -159.41 | -144.87 | -204.18 | -201.67 | 4×4 | -181.60 | -158.16 | -184.51 | -182.00 |
| Calcd.[ | -161.92 | -181.59 | -185.35 | -186.19 | |||||
Table 3 Calculated adsorption energy of CO on 1×1, 2×2, 3×3, 4×4 Pd(111) surface
| Species | Eads/(kJ·mol-1) | Species | Eads/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nt | Nb | Nf | Nh | Nt | Nb | Nf | Nh | ||
| 1×1 | -181.17 | -171.96 | -74.89 | -84.94 | Calcd.[ | -112.13 | -141.84 | -167.36 | |
| 2×2 | -178.66 | -177.82 | -79.08 | -86.19 | Calcd.[ | -131.38 | -174.47 | -194.14 | -191.21 |
| 3×3 | -159.41 | -144.87 | -204.18 | -201.67 | 4×4 | -181.60 | -158.16 | -184.51 | -182.00 |
| Calcd.[ | -161.92 | -181.59 | -185.35 | -186.19 | |||||
| Parameter | Nt | Nb | Nf | Nh | Free CO |
|---|---|---|---|---|---|
| dC—O/nm(This work) | 0.1153 | 0.1177 | 0.1187 | 0.1188 | 0.1141 |
| dC—O/nm(Calcd.[ | 0.115 | 0.116 | 0.117 | 0.117 | 0.1128 |
| dC—O/nm(Calcd.[ | 0.1152 | 0.1171 | 0.1183 | ||
| dC—O/nm(Calcd.[ | 0.1152 | 0.1178 | 0.1189 | 0.1188 | 0.1146 |
| dC—O/nm(Expt.[ | 0.1131 | ||||
| dPd—C/nm(This work) | 0.1882 | 0.2018 | 0.209 | 0.209 | |
| dPd—C/nm(Calcd.[ | 0.168 | 0.190 | 0.200 | 0.200 | |
| dPd—C/nm(Calcd.[ | 0.1884 | 0.205 | 0.210 | ||
| νC—O/cm-1(This work) | 2031 | 1850 | 1770 | 1769 | 2120 |
| νC—O/cm-1(Calcd.[ | 1987 | 1876 | 1803 | ||
| νC—O/cm-1(Calcd.[ | 2014 | 1848 | 1779 | 1781 | 2087 |
| νC—O/cm-1(Expt.[ | 2138 |
Table 4 Geometry parameters and stretching vibration frequency of C—O for CO adsorbed on Pd(111) surface with four balance configurations
| Parameter | Nt | Nb | Nf | Nh | Free CO |
|---|---|---|---|---|---|
| dC—O/nm(This work) | 0.1153 | 0.1177 | 0.1187 | 0.1188 | 0.1141 |
| dC—O/nm(Calcd.[ | 0.115 | 0.116 | 0.117 | 0.117 | 0.1128 |
| dC—O/nm(Calcd.[ | 0.1152 | 0.1171 | 0.1183 | ||
| dC—O/nm(Calcd.[ | 0.1152 | 0.1178 | 0.1189 | 0.1188 | 0.1146 |
| dC—O/nm(Expt.[ | 0.1131 | ||||
| dPd—C/nm(This work) | 0.1882 | 0.2018 | 0.209 | 0.209 | |
| dPd—C/nm(Calcd.[ | 0.168 | 0.190 | 0.200 | 0.200 | |
| dPd—C/nm(Calcd.[ | 0.1884 | 0.205 | 0.210 | ||
| νC—O/cm-1(This work) | 2031 | 1850 | 1770 | 1769 | 2120 |
| νC—O/cm-1(Calcd.[ | 1987 | 1876 | 1803 | ||
| νC—O/cm-1(Calcd.[ | 2014 | 1848 | 1779 | 1781 | 2087 |
| νC—O/cm-1(Expt.[ | 2138 |
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CO | Pd | |
| Free | 0.101(0) | -0.101(0) | 0.000(0) | -0.261(0) |
| Nh | 0.332(0.231) | -0.167(-0.066) | 0.165(0.165) | -0.453(-0.192) |
| Nf | 0.341(0.240) | -0.123(-0.022) | 0.218(0.218) | -0.550(-0.289) |
| Nb | 0.362(0.261) | -0.206(-0.105) | 0.156(0.156) | -0.414(-0.153) |
| Nt | 0.414(0.313) | -0.267(-0.166) | 0.147(0.147) | -0.407(-0.146) |
Table 5 Mulliken charge distribution for the adsorption system of CO/Pd(111)
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CO | Pd | |
| Free | 0.101(0) | -0.101(0) | 0.000(0) | -0.261(0) |
| Nh | 0.332(0.231) | -0.167(-0.066) | 0.165(0.165) | -0.453(-0.192) |
| Nf | 0.341(0.240) | -0.123(-0.022) | 0.218(0.218) | -0.550(-0.289) |
| Nb | 0.362(0.261) | -0.206(-0.105) | 0.156(0.156) | -0.414(-0.153) |
| Nt | 0.414(0.313) | -0.267(-0.166) | 0.147(0.147) | -0.407(-0.146) |
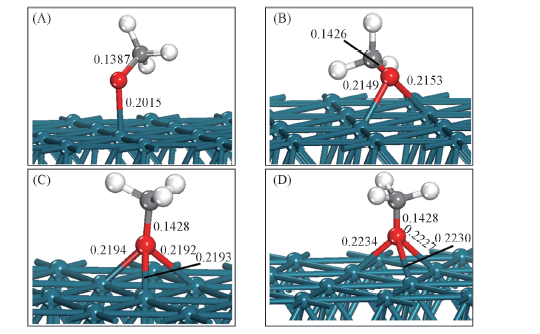
Fig.3 Lateral views for CH3O adsorbed on Pd(111) surface with balanced geometrical configurations (A) Top site; (B) bridge site; (C) fcc site; (D) hcp site.
| Parameter | dC—O/ nm | dC—H/ nm | ∠H—C—H/ (°) | ∠O—C—H/ (°) | dPd—O/ nm | νs(C—O)/ cm-1 | νs(C—H)/ cm-1 | νas(C—H)/ cm-1 |
|---|---|---|---|---|---|---|---|---|
| Lt | 0.1387 | 0.1113 | 106.4 | 113.5 | 0.2015 | 1007 | 2577 | 2787 |
| Lb | 0.1426 | 0.1108 | 108.8 | 109.3 | 0.2151 | 989 | 2857 | 2975 |
| Lf | 0.1428 | 0.1097 | 109.3 | 109.6 | 0.2193 | 986 | 2969 | 3046 |
| Lh | 0.1425 | 0.1098 | 108.7 | 109.9 | 0.2230 | 995 | 2966 | 3040 |
| This work | 0.1356 | 0.1107 | 105.0 | 114.4 | 1101 | 2837 | 2912 | |
| Calcd.[ | 0.1405 | 0.1112 | 107.6 | 111.3 |
Table 6 Geometry parameters and stretching vibration frequency for CH3O adsorbed on Pd(111) surface with four balance configurations
| Parameter | dC—O/ nm | dC—H/ nm | ∠H—C—H/ (°) | ∠O—C—H/ (°) | dPd—O/ nm | νs(C—O)/ cm-1 | νs(C—H)/ cm-1 | νas(C—H)/ cm-1 |
|---|---|---|---|---|---|---|---|---|
| Lt | 0.1387 | 0.1113 | 106.4 | 113.5 | 0.2015 | 1007 | 2577 | 2787 |
| Lb | 0.1426 | 0.1108 | 108.8 | 109.3 | 0.2151 | 989 | 2857 | 2975 |
| Lf | 0.1428 | 0.1097 | 109.3 | 109.6 | 0.2193 | 986 | 2969 | 3046 |
| Lh | 0.1425 | 0.1098 | 108.7 | 109.9 | 0.2230 | 995 | 2966 | 3040 |
| This work | 0.1356 | 0.1107 | 105.0 | 114.4 | 1101 | 2837 | 2912 | |
| Calcd.[ | 0.1405 | 0.1112 | 107.6 | 111.3 |
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CH3O | Pd | |
| Free | 0.159(0) | -0.336(0) | 0.000(0) | -0.261(0) |
| Lt | 0.050(-0.109) | -0.462(-0.126) | -0.156(-0.156) | -0.041(0.220) |
| Lb | 0.055(-0.104) | -0.518(-0.182) | -0.199(-0.199) | 0.045(0.306) |
| Lf | 0.077(-0.082) | -0.556(-0.220) | -0.223(-0.223) | 0.080(0.341) |
| Lh | 0.079(-0.080) | -0.554(-0.218) | -0.204(-0.204) | 0.078(0.339) |
Table 7 Mulliken charge distribution for the adsorption system of CH3O/Pd(111) (translated from Pd to CH3O radical /e-)
| Species | Charge/e(Δe) | |||
|---|---|---|---|---|
| C | O | CH3O | Pd | |
| Free | 0.159(0) | -0.336(0) | 0.000(0) | -0.261(0) |
| Lt | 0.050(-0.109) | -0.462(-0.126) | -0.156(-0.156) | -0.041(0.220) |
| Lb | 0.055(-0.104) | -0.518(-0.182) | -0.199(-0.199) | 0.045(0.306) |
| Lf | 0.077(-0.082) | -0.556(-0.220) | -0.223(-0.223) | 0.080(0.341) |
| Lh | 0.079(-0.080) | -0.554(-0.218) | -0.204(-0.204) | 0.078(0.339) |
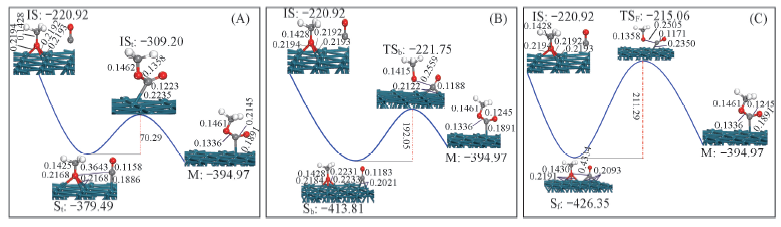
Fig.4 Schematic diagram of adsorbed CH3OOC formed by CH3O adsorbed on fcc site and CO on top site(A), bridge site(B)and fcc site(C) of Pd(111) surface Eabs is in kJ/mol, distances are in nm.
| [1] | Ma X. B., Xu G. H., Chen J. W., Chen H. F., Chinese J. Chem. Eng., 1995, 46(1), 50—56 |
| (马新宾, 许根慧, 陈锦文, 陈洪钫.化工学报, 1995, 46(1), 50—56) | |
| [2] | Lin X., Ji Y., Tan J. Q., Xiao W. D., Chinese J. Catal., 2008, 29(4), 325—329 |
| (林茜, 计扬, 谭俊清. 催化学报, 2008, 29(4), 325—329) | |
| [3] | Song Y., Li Z. H., Gao Z. H., He F., Xu G. H., Liu C. H., Zhu Q. M., Chinese J. Catal., 2000, 21(6), 537—541 |
| (宋瑛, 李振花, 高正虹, 何菲, 许根慧, 刘崇微, 朱起明. 催化学报, 2000, 21(6), 537—541) | |
| [4] | Ji Y., Liu G., Li W., Xiao W., J. Mol. Catal. A: Chem., 2009, 314(1/2), 63—70 |
| [5] | Chen G. K., Yan H. M., Xue B., Nat. Gas Chem. Indus., 1995, 20(4), 5—9 |
| (陈庚申, 严慧敏. 天然气化工, 1995, 20(4), 5—9) | |
| [6] | Xu Z. N., Sun J., Lin C. S., Jiang X. M., Chen Q. S., Peng S. Y., Wang M. S., Guo G. C., ACS Catalysis, 2013, 3(2), 118—122 |
| [7] | Delley B., J. Chem. Phys., 2000, 113(18), 7756—7764 |
| [8] | Perdew J. P., Wang Y., Phys. Rev. B, 1992, 45(23), 13244—13249 |
| [9] | Delley B., Phys. Rev. B, 2002, 66(15), 155125—155131 |
| [10] | Rose M. K., Mitsui T., Dunphy J., Borg A., Ogletree D. F., Surf. Sci., 2002, 512, 48—60 |
| [11] | Gravil P. A., Toulhoat H., Surf. Sci., 1999, 430, 176—191 |
| [12] | Loffreda D., Simon D., Sautet P., Surf. Sci., 1999, 425(1), 68—80 |
| [13] | Radilla J., Boronat M., Corma A., Illas F., Theor. Chem. Acc., 2010, 126(3/4), 223—229 |
| [14] | Ilya V. Y., Riadh S., Konstantin M. N., Notker R., J. Phys. Chem. B, 2003, 107, 255—264 |
| [15] | Zhang J., Wang Z., Wang Z. X., Surf. Interface Anal., 2011, 43(7), 1038—1045 |
| [16] | Herzberg G., Crawford B. L. Jr., J. Phys. Chem., 1946, 50(3), 288 |
| [17] | Lide D.R., CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 2000, 9—22 |
| [18] | Jackels C. F., J. Chem. Phys., 1982, 76(1), 505—515 |
| [19] | Yamamoto Y., Catalysis Surveys from Asia, 2010, 14(3/4), 103—110 |
| [20] | Song K., Ji Y., Xiao W. D., Guangdong Chemical Industry, 2007, 34(6), 12—14 |
| (宋轲, 计扬, 肖文德. 广东化工, 2007, 34(6), 12—14) | |
| [21] | Li Z. H., He C. Y., Xiang T. L., Wang B. W., Ma X. B., Xu G. H., Chemical Reaction Engineering and Technology, 2004, 20(3), 280—283(李振花, 何翠英, 项铁丽, 王保伟, 马新宾, 许根慧. 化学反应工程与工艺, 2004, 20(3), 280—283) |
| [22] | Chen Z. X., Neyman K. M., Lim K. H., Rösch N., Langmuir, 2004, 20(19), 8068—8077 |
| [23] | Wang G. C., Zhou Y. H., Nakamura J., J. Chem. Phys., 2005, 122(4), 044707-1—044707-8 |
| [24] | Ren R., Niu C., Bu S., Zhou Y., Lv Y., Wang G., Journal of Natural Gas Chemistry, 2011, 20(1), 90—98 |
| [25] | Gates J., Kesmodel L., J. Catal., 1983, 83(2), 437—445 |
| [26] | Yang H., Whitten J. L., Langmuir, 1995, 11(3), 853—859 |
| [27] | Huberty J., Madix R., Surf. Sci., 1996, 360(1), 144—156 |
| [28] | Guang Z. R., Zheng H. Y., Wang B. J., Li Z., Chem. J. Chinese Universities, 2010, 31(6),1246—1251(章日光, 郑华艳, 王宝俊, 李忠. 高等学校化学学报, 2010, 31(6), 1246—1251) |
| (Ed.: Y, Z) |
| [1] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [2] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [3] | 王丽君, 李欣, 洪崧, 詹新雨, 王迪, 郝磊端, 孙振宇. 调节氧化镉-炭黑界面高效电催化CO2还原生成CO[J]. 高等学校化学学报, 2022, 43(7): 20220317. |
| [4] | 黄汉浩, 卢湫阳, 孙明子, 黄勃龙. 石墨炔原子催化剂的崭新道路:基于自验证机器学习方法的筛选策略[J]. 高等学校化学学报, 2022, 43(5): 20220042. |
| [5] | 刘洋, 李旺昌, 张竹霞, 王芳, 杨文静, 郭臻, 崔鹏. Sc3C2@C80与[12]CPP纳米环之间非共价相互作用的理论研究[J]. 高等学校化学学报, 2022, 43(11): 20220457. |
| [6] | 王园月, 安梭梭, 郑旭明, 赵彦英. 5-巯基-1, 3, 4-噻二唑-2-硫酮微溶剂团簇的光谱和理论计算研究[J]. 高等学校化学学报, 2022, 43(10): 20220354. |
| [7] | 程媛媛, 郗碧莹. ·OH自由基引发CH3SSC |
| [8] | 周成思, 赵远进, 韩美晨, 杨霞, 刘晨光, 贺爱华. 硅烷类外给电子体对丙烯-丁烯序贯聚合的调控作用[J]. 高等学校化学学报, 2022, 43(10): 20220290. |
| [9] | 黄罗仪, 翁约约, 黄旭慧, 王朝杰. 车前草中黄酮类成分结构和性质的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2752. |
| [10] | 钟声广, 夏文生, 张庆红, 万惠霖. 电中性团簇MCu2Ox(M=Cu2+, Ce4+, Zr4+)上甲烷和二氧化碳直接合成乙酸的理论研究[J]. 高等学校化学学报, 2021, 42(9): 2878. |
| [11] | 高中楠, 郭丽红, 赵东越, 李新刚. A位缺陷对La-Sr-Co-O钙钛矿结构和催化氧化性能的影响[J]. 高等学校化学学报, 2021, 42(9): 2869. |
| [12] | 马丽娟, 高升启, 荣祎斐, 贾建峰, 武海顺. Sc, Ti, V修饰B/N掺杂单缺陷石墨烯的储氢研究[J]. 高等学校化学学报, 2021, 42(9): 2842. |
| [13] | 郑若昕, 张颖, 徐昕. 低标度XYG3双杂化密度泛函的开发与测评[J]. 高等学校化学学报, 2021, 42(7): 2210. |
| [14] | 刘昌辉, 梁国俊, 李妍璐, 程秀凤, 赵显. NH3在硼纳米管表面吸附的密度泛函理论研究[J]. 高等学校化学学报, 2021, 42(7): 2263. |
| [15] | 柳扬, 李清波, 孙杰, 赵显. Ga对在AlN衬底上直接生长石墨烯的远程催化[J]. 高等学校化学学报, 2021, 42(7): 2271. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||