

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (5): 949.doi: 10.7503/cjcu20170720
Previous Articles Next Articles
LU Lilin1,2,*, SHU Hongfei2, RUAN Zhuhua2, NI Jiaqi2, ZHANG Haijun1
Received:2017-11-10
Online:2018-04-10
Published:2018-04-10
Contact:
LU Lilin
Supported by:CLC Number:
TrendMD:
LU Lilin,SHU Hongfei,RUAN Zhuhua,NI Jiaqi,ZHANG Haijun. Preparation of Graphene-supported Pt-Pd Catalyst and Its Catalytic Activity and Mechanism for Hydrogen Generation Reaction†[J]. Chem. J. Chinese Universities, 2018, 39(5): 949.
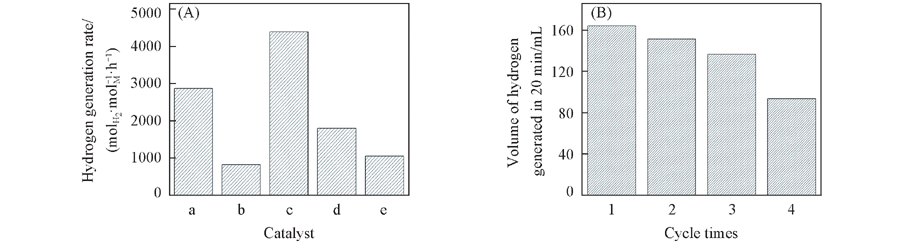
Fig.1 Catalytic activities of graphene-supported metallic catalysts for hydrolysis reaction of KBH4(A) and the durability of graphene-supported Pt-Pd[n(Pt)/n(Pd)=1∶1] bimetallic catalyst(B) Note:(A) Catalyst: a. Pt; b. n(Pt)/n(Pd)=4∶1; c. n(Pt)/n(Pd)=1∶1; d. n(Pt)/n(Pd)=1∶4; e. Pd.
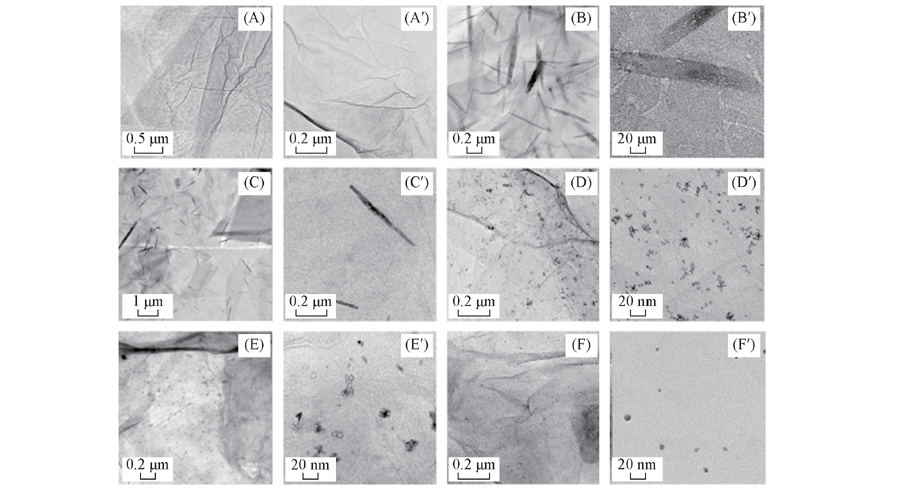
Fig.4 TEM images of graphene(A,A'), graphene-supported Pd catalyst(B,B'), graphene-supported Pt-Pd catalysts with Pt/Pd molar ratios of 1∶4(C,C'), 1∶1(D,D'), 4∶1(E,E') and graphene-supported Pt catalyst(F, F') with different magnifications
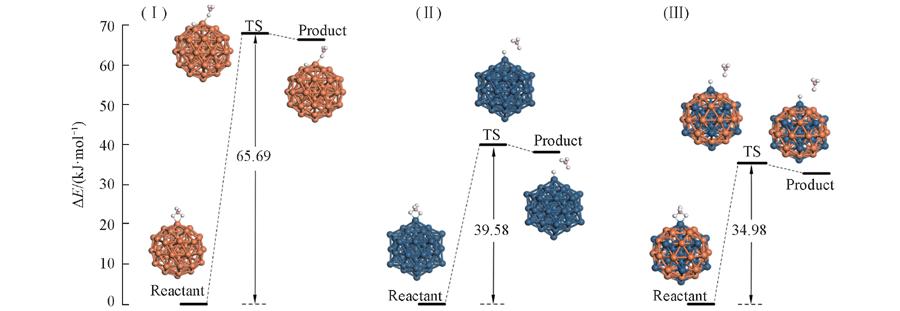
Fig.6 BLYP/DNP calculated energetic profiles for the rate-determining step in the borohydride hydrolysis catalyzed by Pd55(Ⅰ), Pt55(Ⅱ) and Pt25Pd30(Ⅲ)
| [1] | Marrero-Alfonso E.Y., Beaird A. M., Davis T. A., Matthews M. A., Ind. Eng. Chem. Res., 2009, 48(8), 3703—3712 |
| [2] | Liu J.X., Song Y. A., Huang Y. D., Chem. [J]. Chinese Universities, 2016, 37(7), 1372—1379 |
| (刘佳欣, 宋义安, 黄玉东. 高等学校化学学报, 2016, 37(7), 1372—1379) | |
| [3] | Tian Y., Li Y.X., Peng S. Q., Chem. [J]. Chinese Universities, 2017, 38(10), 1841—1849 |
| (田毅, 李越湘, 彭绍琴. 高等学校化学学报, 2017, 38(10), 1841—1849) | |
| [4] | Schlesinger H.I., Brown H. C., Finholt A. B., Gilbreath J. R., Hockstra H. R., Hydo E. K., [J]. Am. Chem. Soc., 1953, 75(1), 215—219 |
| [5] | Gu R., Zhang M., Wang C.Y., Huang W. J., Liu D. M., Chem. [J]. Chinese Universities, 2016, 37(4), 688—692 |
| (顾润, 张明, 王春阳, 黄维军, 柳东明. 高等学校化学学报, 2016, 37(4), 688—692) | |
| [6] | Yadav M., Xu Q., Energy Environ.Sci., 2012, 5(12), 9698—9725 |
| [7] | Demirci U.B., Miele P., Energy Environ. Sci., 2009, 2(6), 627—637 |
| [8] | Tu G.P., He J. L., Xiao X. Z., Chen L. X., Ren Q. J., Du X. Y., Luo M. E., Chem. [J]. Chinese Universities, 2016, 37(10), 1757—1762 |
| (屠国平, 何剑灵, 肖学章, 陈立新, 任钱江, 杜锡勇, 骆明儿. 高等学校化学学报, 2016, 37(10), 1757—1762) | |
| [9] | Zhang Q., Mohring R.M., Ind. Eng. Chem. Res., 2009, 48(3), 1603—1607 |
| [10] | Audrieux J., Demirci U.B., Hannauer J., Gervais C., Goutaudier C., Miele P., Int. [J]. Hydrogen Energ., 2011, 36(1), 224—233 |
| [11] | Liu B.H., Li Z. P., [J]. Power Source, 2009, 187(2), 527—534 |
| [12] | Li C., Peng P., Zhou D.W., Wan L., Int. [J]. Hydrogen Energ., 2011, 36(22), 14512—14526 |
| [13] | Keceli E., Ozkar S., J. Mol. Catal. A: Chem., 2008, 286(1/2), 87—91 |
| [14] | Lu L.L., Zhang H. J., Zhang S. W., Li F. L., Angew. Chem., Int. Ed., 2015, 54(32), 9328—9332 |
| [15] | Amendola S.C., Sharp-Goldman S. L., Janjua M. S., Kelly M. T., Petillo P. J., Binder M., [J]. Power Source, 2000, 85(2), 186—189 |
| [16] | Brown H.C., Brown C. A., [J]. Am. Chem. Soc., 1962, 84(8), 1493—1494 |
| [17] | Patel N., Patton B., Zanchetta C., Fernandes R., Guella G., Kale A., Miotello A., Int. J.Hydrogen Energ., 2008, 33(1), 287—292 |
| [18] | Hung T.F., Kuo H. C., Tsai C. W., Chen H. M., Liu R. S., Weng B. J., Lee J. F., [J]. Mater. Chem., 2011, 21(32), 11754—11759 |
| [19] | Zhang H., Lu L., Kawashima K., Okumura M., Haruta M., Toshima N., Adv. Mater., 2015, 27(8), 1383—1388 |
| [20] | Zhang H., Lu L., Cao Y., Du S., Cheng Z., Zhang S., Materials Research Bulletin, 2014, 49(1), 393—398 |
| [21] | Liu C.H., Chen B. H., Hsueh C. L., Ku J. R., Jeng M. S., Tsau F., Int. [J]. Hydrogen Energ., 2009, 34(5), 2153—2163 |
| [22] | Kong D.C., Gu Y. J., Xiang S., Wang P., Cheng J., Zhang H. J., Zhang S. W., Chem. J. Chinese Universities, 2013, 34(10), 2377—2382(孔德成, 古亚军, 向胜, 王鹏, 成君, 张海军, 张少伟. 高等学校化学学报, 2013, 34(10), 2377—2382) |
| [23] | Zhao W.G., Su L., Zhou Z. N., Zhang H. J., Lu L. L., Zhang S. W., Acta Phys. Chim. Sin., 2015, 31(1), 145—152 |
| (赵万国, 苏丽, 周振宁, 张海军, 鲁礼林, 张少伟. 物理化学学报, 2015, 31(1), 145—152) | |
| [24] | Jiao C., Huang Z., Wang X., Zhang H., Lu L., Zhang S., RSC Adv., 2015, 5(43), 34364—34371 |
| [25] | Li W., Zhao W., Zhou X., Su L., Zhang H., Lu L., Zhang S., Chem. J. Chinese Universities, 2014, 35(10), 2164—2169 |
| (李文绮, 赵万国, 周兴赟, 苏丽, 张海军, 鲁礼林, 张少伟. 高等学校化学学报, 2014, 35(10), 2164—2169) | |
| [26] | Hummers W., Offeman R., [J]. Am. Chem. Soc., 1958, 80(6), 1339—1339 |
| [27] | Guella G., Patton B., Miotello A., J. Phys. Chem. C, 2007, 111(50), 18744—18750 |
| [28] | Shang Y., Chen R., Energy Fuels, 2006, 20(5), 2142—2148 |
| [1] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [2] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [3] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [4] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [5] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [6] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [7] | LI Xinyi, LIU Yongjun. Theoretical Insights into the Cleavage of β-Hydroxy Ketone Catalyzed by Artificial Retro-aldolase RA95.5-8F [J]. Chem. J. Chinese Universities, 2021, 42(7): 2306. |
| [8] | REN Ying, LI Changhua, WANG Tao, XUE Shanshan, ZHANG Tingting, JIA Jianfeng, WU Haishun. Theoretical Studies on Pd-catalyzed Oxidative N─H Carbonylation to Synthesis of 1,3,4-Oxadiazole-2(3H)-one Heterocyclic Compounds [J]. Chem. J. Chinese Universities, 2021, 42(6): 1793. |
| [9] | LI Yiwei, SHENTU Jiangtao, WANG Jingbo, LI Xiangyuan. Combustion Mechanism Construction Based on Minimized Reaction Network: C1⁃Oxygen Combustion [J]. Chem. J. Chinese Universities, 2021, 42(6): 1871. |
| [10] | TIAN Shengqiao, WEI Meiju. Reaction Mechanism for Rh(Ⅱ)-catalyzed [3+3] Cyclization of Indole Derivatives and Propertis of Product [J]. Chem. J. Chinese Universities, 2021, 42(6): 1899. |
| [11] | QI Guodong, YE Xiaodong, XU Jun, DENG Feng. Progress in NMR Studies of Carbohydrates Conversion on Zeolites [J]. Chem. J. Chinese Universities, 2021, 42(1): 148. |
| [12] | LI Xiangyuan, SHENTU Jiangtao, LI Yiwei, LI Juanqin, WANG Jingbo. Combustion Mechanism Construction Based on Minimized Reaction Network: Hydrogen-Oxygen Combustion † [J]. Chem. J. Chinese Universities, 2020, 41(4): 772. |
| [13] | LI Xiangyuan,YAO Xiaoxia,SHENTU Jiangtao,SUN Xiaohui,LI Juanqin,LIU Mingxia,XU Shimin. Combustion Reaction Mechanism Construction by Two-parameter Rate Constant Method † [J]. Chem. J. Chinese Universities, 2020, 41(3): 512. |
| [14] | ZHANG Lin, ZHANG Wei, YUE Xin, LI Pengjie, YANG Zuoyin, PU Min, LEI Ming. Theoretical Study on Mechanism of CO2 Hydrogenation to Formic Acid Catalyzed by Manganese Complex † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1911. |
| [15] | ZHANG Xiaoying,DU Guifang,ZHU Bo,GUAN Wei. Theoretical Mechanistic Study on Nickel-Catalyzed Cycloaddition of Azetidinone with Butadiene† [J]. Chem. J. Chinese Universities, 2019, 40(4): 770. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||