

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (3): 513.doi: 10.7503/cjcu20150742
• Physical Chemistry • Previous Articles Next Articles
GAO Zhifang, WANG Weina, MA Qian, LIU Fengyi, WANG Wenliang*
Received:2015-09-22
Online:2016-03-10
Published:2016-01-24
Contact:
WANG Wenliang
Supported by:CLC Number:
TrendMD:
GAO Zhifang, WANG Weina, MA Qian, LIU Fengyi, WANG Wenliang. Theoretical Studies on the Reaction Mechanism of Criegee Intermediates CH3CHOO with OH Radicals†[J]. Chem. J. Chinese Universities, 2016, 37(3): 513.
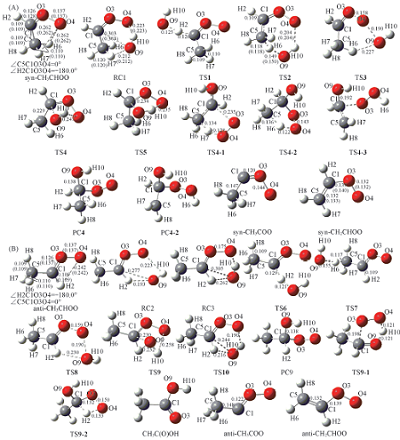
Fig.1 Optimized geometries of stationary point of syn-CH3CHOO+OH(A) and anti-CH3CHOO+OH(B) at the B3LYP/6-311+G(d,p) level The values in parentheses are at the CCSD(T)/6-311+G(d,p) level. The bond lengths are in nm.
| Species | ΔfH0—/(kJ·mol-1) | Species | ΔfH0—/(kJ·mol-1) |
|---|---|---|---|
| CH3CHO | -166.54(-170.70[ | RC3 | 91.01 |
| CH3C(O)OH | -430.54(-432.30[ | syn-CH3CHOO | 37.78 |
| CH2CHOH | -123.67(-128.00[ | anti-CH3CHOO | 52.69 |
| CH3CH(OH)(OO)PC4 | -216.36 | syn-CH2CHOO | 108.91 |
| CH2CH(OH)(OOH)PC4-2 | -142.69 | syn-CH3COO | 224.21 |
| CH3CH(OH)(OO)PC9 | -220.76 | anti-CH2CHOO | 104.74 |
| RC1 | 107.80 | anti-CH3COO | 302.44 |
| RC2 | 84.69 |
Table 1 Formation enthalpies ΔfH0— for some species at the G4 level
| Species | ΔfH0—/(kJ·mol-1) | Species | ΔfH0—/(kJ·mol-1) |
|---|---|---|---|
| CH3CHO | -166.54(-170.70[ | RC3 | 91.01 |
| CH3C(O)OH | -430.54(-432.30[ | syn-CH3CHOO | 37.78 |
| CH2CHOH | -123.67(-128.00[ | anti-CH3CHOO | 52.69 |
| CH3CH(OH)(OO)PC4 | -216.36 | syn-CH2CHOO | 108.91 |
| CH2CH(OH)(OOH)PC4-2 | -142.69 | syn-CH3COO | 224.21 |
| CH3CH(OH)(OO)PC9 | -220.76 | anti-CH2CHOO | 104.74 |
| RC1 | 107.80 | anti-CH3COO | 302.44 |
| RC2 | 84.69 |
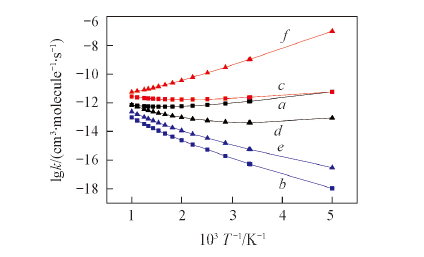
Fig.5 Fitted plots of the rate constants vs. the reciprocal of temperature for the favorable channels over the range of 200—1000 Ka. syn-β-Hab(R2); b. syn-Oxi(R3); c. syn-Add(R5); d. anti-α-Hab(R6); e. anti-Oxi(R8); f. anti-Add(R10).
| [1] | Paulson S. E., Chung M. Y., Hasson A. S., J. Phys. Chem. A, 1999, 103(41), 8125—8138 |
| [2] | Jacobson M., Atmospheric Environment, 1997, 31(3), 511—512 |
| [3] | Johnson D., Marston G., Chem. Soc. Rev., 2008, 37(4), 699—716 |
| [4] | Kumar M., Busch D. H., Subramaniam B., Thompson W. H., J. Phys. Chem. A, 2014, 118(10), 1887—1894 |
| [5] | Ryzhkov A. B., Ariya P. A., Phys. Chem. Chem. Phys., 2004, 6(21), 5042—5050 |
| [6] | Qi B., Chao Y. T., Acta Chim. Sinica, 2007, 65(19),2117—2123 |
| (齐斌, 晁余涛. 化学学报, 2007,65(19), 2117—2123) | |
| [7] | Jiang L., Xu Y.S., Ding A. Z.,J. Phys. Chem. A, 2010, 114(47), 12452—12461 |
| [8] | Bo L., Tan X. F., Long Z. W., Wang Y. B., Ren D. S., Zhang W. J., J. Phys. Chem. A, 2013, 3(8), 1693—1699 |
| [9] | Mansergas A., Anglada J. M., J. Phys. Chem. A, 2006, 110(11), 4001—4011 |
| [10] | Sadezky A., Winterhalter R., Kanawati B., Rompp A., Spengler B., Mellouki A., Bras G. L., Chaimbault P., Moortgat G. K., Atmos. Chem. Phys., 2008, 8(10), 2667—2699 |
| [11] | Kumar M., Busch D. H., Subramaniam B., Thompson W. H., Phys. Chem. Chem. Phys., 2014, 16, 22968—22973 |
| [12] | Nguyen T. N., Putikam R., Lin M. C., J. Chem. Phys., 2015, 142(12), 124312 |
| [13] | Lee S., Kamens R. M., Atmos. Environ., 2005, 39(36), 6822—6832 |
| [14] | Heard D. E., Carpenter L. J., Creasey D. J., Hopkins J. R., Lee J. D., Lewis A. C., Pilling M. J., Seakins P. W., Carslaw N., Emmerson K. M., Geo. Res. Lett., 2004, 31(18), 355—366 |
| [15] | Li H.W., Fang Y., Kidwell N. M., Beames J. M., Lester M. I., J. Phys. Chem. A, 2015, 119(30), 8328—8337 |
| [16] | Kettner M., Karton A., McKinley A. J., Wild D. A., Chemical Physics Letters, 2015, 621, 193—198 |
| [17] | Frisch M.J., T. G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A.G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 09, Gaussian Inc., Wallingford CT, 2009 |
| [18] | Curtiss L. A., Raghavachari K., Redfern P. C., Pople J. A., J. Chem. Phys., 1997, 106, 1063—1079 |
| [19] | Curtiss L. A., Redfern P. C., Raghavachari K., J. Chem. Phys., 2007, 127(12), 66—70 |
| [20] | Zhang S.W., Truong N. T., VKLab Version 1.0, University of Utah,Salt Lake City, 2001 |
| [21] | Kuwata K. T., Hermes M. R., Carlson M. J., Zogg C. K., J. Phys. Chem. A, 2010, 114(34), 9192—9204 |
| [22] | Nakajima M., Yue Q., Endo Y., Journal of Molecular Spectroscopy, 2015, 310, 109—112 |
| [23] | Smith M. C., Ting W. L., Chang C. H., Takahashi K., Boering K. A., Lin J. J. M., J. Chem. Phys., 2014, 141(7), 074302 |
| [24] | Nakajima M., Endo Y., J. Chem. Phys., 2014, 140(1), 011101 |
| [25] | Lin H. Y., Huang Y. H., Wang X., Bowman J. M., Nishimura Y., Witek H. A., Lee Y. P., Nat. Commun., 2015, 6, 7012 |
| [26] | NIST Chemistry Webbook( |
| [27] | Li D. M., Wang Y., Han K. L., Coord. Chem. Rev., 2012, 256, 1137—1150 |
| [28] | Balucani N., Capozza G., Leonori F., Segoloni E., Casavecchia P., Int. Rev. Phys. Chem., 2006, 25, 109—163 |
| [29] | Guo S., Wang W. N., Jin L. X., Wang S., Wang W. L., Chem. J. Chinese Universities, 2014, 36(8), 1300—1306 |
| (郭莎, 王渭娜, 靳玲侠, 王帅, 王文亮. 高等学校化学学报, 2014, 36(8), 1300—1306) | |
| [30] | Taatjes C. A., Welz O., Eskola A. J., Savee J. D., Scheer A. M., Shallcross D. E., Rotavera B., Lee E. P. F., Dyke J. M., Mok D. K. W., Osborn D. L., Percival C. J., Science,2013, 340(6129), 177—180 |
| [1] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [2] | YANG Dan, LIU Xu, DAI Yihu, ZHU Yan, YANG Yanhui. Research Progress in Electrocatalytic CO2 Reduction Reaction over Gold Clusters [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220198. |
| [3] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [4] | CHENG Yuanyuan, XI Biying. Theoretical Study on the Fragmentation Mechanism of CH3SSCH3 Radical Cation Initiated by OH Radical [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220271. |
| [5] | MENG Fanwei, GAO Qi, YE Qing, LI Chenxi. Potassium Poisoning Mechanism of Cu-SAPO-18 Catalyst for Selective Catalytic Reduction of NOx by Ammonia [J]. Chem. J. Chinese Universities, 2021, 42(9): 2832. |
| [6] | YANG Yiying, ZHU Rongxiu, ZHANG Dongju, LIU Chengbu. Theoretical Study on Gold-catalyzed Cyclization of Alkynyl Benzodioxin to 8-Hydroxy-isocoumarin [J]. Chem. J. Chinese Universities, 2021, 42(7): 2299. |
| [7] | WANG Jian, ZHANG Hongxing. Theoretical Study on the Structural-photophysical Relationships of Tetra-Pt Phosphorescent Emitters [J]. Chem. J. Chinese Universities, 2021, 42(7): 2245. |
| [8] | LI Xinyi, LIU Yongjun. Theoretical Insights into the Cleavage of β-Hydroxy Ketone Catalyzed by Artificial Retro-aldolase RA95.5-8F [J]. Chem. J. Chinese Universities, 2021, 42(7): 2306. |
| [9] | REN Ying, LI Changhua, WANG Tao, XUE Shanshan, ZHANG Tingting, JIA Jianfeng, WU Haishun. Theoretical Studies on Pd-catalyzed Oxidative N─H Carbonylation to Synthesis of 1,3,4-Oxadiazole-2(3H)-one Heterocyclic Compounds [J]. Chem. J. Chinese Universities, 2021, 42(6): 1793. |
| [10] | LI Yiwei, SHENTU Jiangtao, WANG Jingbo, LI Xiangyuan. Combustion Mechanism Construction Based on Minimized Reaction Network: C1⁃Oxygen Combustion [J]. Chem. J. Chinese Universities, 2021, 42(6): 1871. |
| [11] | TIAN Shengqiao, WEI Meiju. Reaction Mechanism for Rh(Ⅱ)-catalyzed [3+3] Cyclization of Indole Derivatives and Propertis of Product [J]. Chem. J. Chinese Universities, 2021, 42(6): 1899. |
| [12] | WANG Jianyu, ZHANG Qiang, YAN Wenfu, YU Jihong. Roles of Hydroxyl Radicals in Zeolite Synthesis [J]. Chem. J. Chinese Universities, 2021, 42(1): 11. |
| [13] | QI Guodong, YE Xiaodong, XU Jun, DENG Feng. Progress in NMR Studies of Carbohydrates Conversion on Zeolites [J]. Chem. J. Chinese Universities, 2021, 42(1): 148. |
| [14] | LI Xiangyuan, SHENTU Jiangtao, LI Yiwei, LI Juanqin, WANG Jingbo. Combustion Mechanism Construction Based on Minimized Reaction Network: Hydrogen-Oxygen Combustion † [J]. Chem. J. Chinese Universities, 2020, 41(4): 772. |
| [15] | LI Xiangyuan,YAO Xiaoxia,SHENTU Jiangtao,SUN Xiaohui,LI Juanqin,LIU Mingxia,XU Shimin. Combustion Reaction Mechanism Construction by Two-parameter Rate Constant Method † [J]. Chem. J. Chinese Universities, 2020, 41(3): 512. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||