

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (6): 1286.doi: 10.7503/cjcu20140003
• Physical Chemistry • Previous Articles Next Articles
ZHU Suiyi, XU Dongfang, FANG Shuai, GENG Zhi*( ), YANG Xia
), YANG Xia
Received:2014-01-02
Online:2014-06-10
Published:2014-05-12
Contact:
GENG Zhi
E-mail:aaavbackkom@163.com
Supported by:CLC Number:
TrendMD:
ZHU Suiyi, XU Dongfang, FANG Shuai, GENG Zhi, YANG Xia. Sunlight-Responsive Ag2S/Ag3PO4 Composite Preparation and Degradation of Salicylic Acid†[J]. Chem. J. Chinese Universities, 2014, 35(6): 1286.
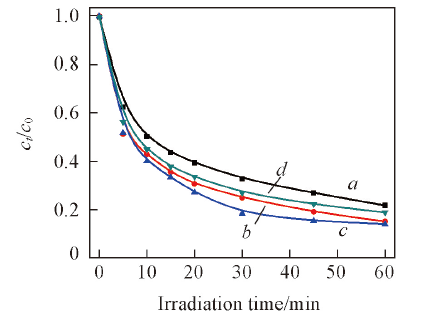
Fig.6 Influence of Ag2S doping in Ag2S/Ag3PO4 with hydrothermal treated at 120 ℃ for 4 h on degradation of salicylic acida. Ag3PO4; b. 0.5%Ag2S/Ag3PO4; c. 1%Ag2S/Ag3PO4; d. 2%Ag2S/Ag3PO4.
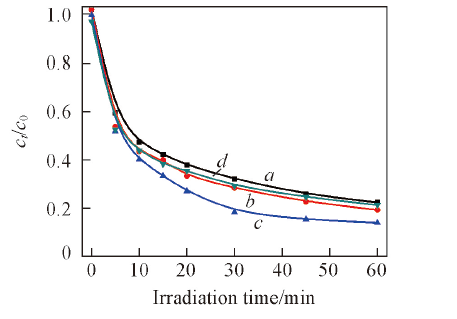
Fig.7 Influence of hydrothermal temperature of 1%Ag2S/Ag3PO4 with hydrothermal treated for 4 h on degradation of salicycic acida. 40 ℃; b. 80 ℃; c. 120 ℃; d. 160 ℃.
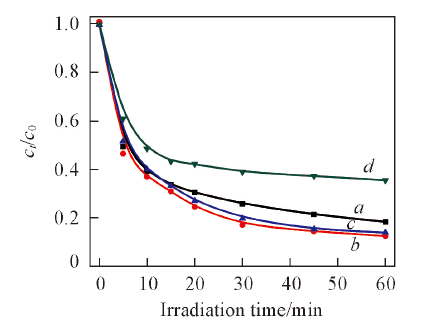
Fig.8 Influence of hydrothermal time of 1%Ag2S/Ag3PO4 with hydrothermal treated at 120 ℃ on degradation of salicylic aeidTime/h: a. 2; b. 4; c. 8; d. 16.
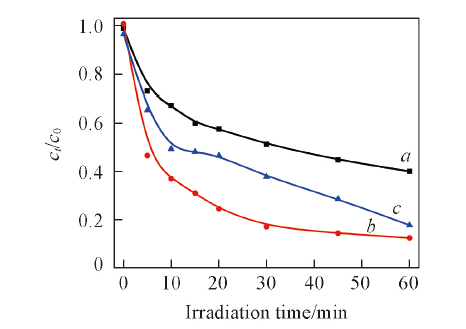
Fig.9 Influence of different light source current intensity on degradation of salicylic acid by 1%Ag2S/Ag3PO4pH value of salicylic acid is 6.0; c0=20 mg/L; amount of catalysts is 0.15 g. I/A: a. 10; b. 15; c. 20.
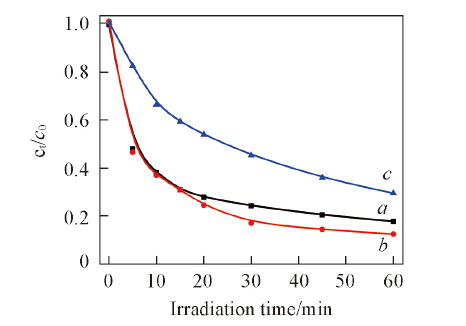
Fig.10 Influence of initial pH value in solution on degradation of salicylic acid by 1%Ag2S/Ag3PO4Light source current intensity is 15 A; the amount of catalysts is 0.15 g; c0=20 mg/L. a. pH=4.0; b. pH=6.0; c. pH=8.0.
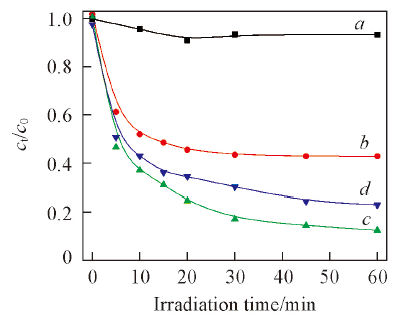
Fig.11 Photolysis of salicylic acid(a) and influence of catalyst amount(c—d) on degradation of salicylic acid by 1%Ag2S/Ag3PO4Light source current intensity is 15 A; V=100 mL, pH=6.0; c0=20 mg/L. m(1%Ag2S/Ag3PO4)/g: a. 0; b. 0.05; c. 0.15; d. 0.25.
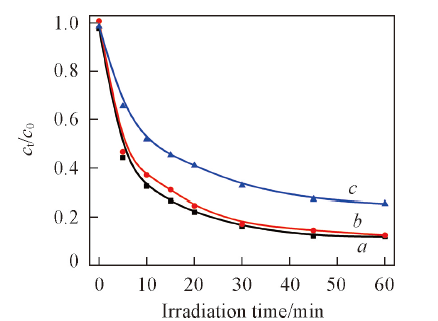
Fig.12 Influence of initial concentration of salicylic acid on degradation of salicylic acid by 1%Ag2S/Ag3PO4Light source current intensity is 15 A; pH value is 6.0; the amount of catalysts is 0.15 g. csalicylic acid/(mg·L-1): a. 10; b. 20; c. 30.
| [1] | Tong H., Ouyang S. X., Bi Y. P., Umezawa N., Oshikiri M., Ye J. H., Adv. Mater., 2011, 24(2), 229—251 |
| [2] | Liu W. M., Chem. Res. Chinese Universities, 2013, 29(2), 314—318 |
| [3] | Yi Z. G., Ye J., Kikugawa N., Kako T., Ouyang S., Williams H., Yang H., Cao J., Luo W., Li Z., Liu Y., Withers R., Nat. Mater., 2010, 9, 559—564 |
| [4] | Liu Y., Fang L., Lu H., Li Y., Hu C., Yu H., Appl. Catal. B-Environ., 2012, 115, 45—51 |
| [5] | Cao J., Luo B., Lin H., Xu B., Chen S., J. Hazard Mater., 2012, 217/218, 107—115 |
| [6] | Yu C. L., Zhou W. Q., Yu J. M., Yang J. G., Fan Q. Z., Chem. Res. Chinese Universities, 2012, 28(1), 124—128 |
| [7] | Ratanatawanate C., Tao Y., Balkus K. J., J. Phys. Chem. C, 2009, 113, 10755—10760 |
| [8] | Li X., Li J. H., Li S. J., Fang X., Fang F., Chu X. Y., Wang X. H., Hu J. X., Chem. Res. Chinese Universities, 2013, 29(6), 1032—1035 |
| [9] | Collado S., Garrido L., Laca A., Diaz M., Environ. Sci. Technol., 2010, 44, 8629—8635 |
| [10] | Tian M., Adams B., Wen J., Asmussen R. M., Chen A., Electrochim Acta., 2009, 54, 3799—3805 |
| [11] | Zhang L.Y., Liu Z. X., Yu X. L., Lü Z. F., Cao Y., J. Funct. Mater., 2010, 41, 2169—2173 |
| (张理元, 刘钟馨, 于晓龙, 吕作凤, 曹阳. 功能材料, 2010, 41, 2169—2173) | |
| [12] | Teng W., Li X. Y., Zhao Q. D., Zhao J. J., Zhang D. K., Appl. Catal. B-Environ., 2012, 125, 538—545 |
| [13] | Pan Y., Guan S., Guo Y. P., Chem. J. Chinese Universities, 2013, 34(9), 2068—2076 |
| (潘琰, 关爽, 郭玉鹏.高等学校化学学报, 2013,34(9), 2068—2076) | |
| [14] | Liu Y. P., Fang L., Lu H. D., Li Y. W., Hua C. Z., Yu H. G., Appl. Catal. B-Environ., 2012, 115/116, 245—252 |
| [15] | Yi H. H., Yu Q. F., Tang X. L., Ning P., Yang L. P., Ye Z. Q., J. Ind. Eng. Chem. Res., 2011, 50, 3960—3965 |
| [16] | Wang Y. F., Wang Y. W., Ding G. Y., Fan C. M., J. Synth. Cryst., 2012, 41, 1286—1297 |
| (王韵芳, 王雅文, 丁光月, 樊彩梅. 人工晶体学报, 2012, 41, 1286—1297) | |
| [17] | Bi Y. P., Ouyang S. X., Umezawa N. J., Cao J. Y., Ye J. H., J. Am. Chem. Soc., 2011, 33, 6490—6496 |
| [18] | Buddee S., Wongnawa S., Sirimahachai U., Mater. Chem. Phys., 2011, 126, 167—177 |
| [19] | Liu X. H., He X. B., Fu Y. B., Acta. Chimica. Sinica., 2008, 66, 1725—1730 |
| (刘秀华, 何小波, 傅依备. 化学学报, 2008, 66, 1725—1730) | |
| [20] | Gorska P., Zaleska A., Kowalska E., Klimczuk T., Sobczak W. J., Skwarek E., Janusz W., Hupka J., Appl. Catal. B-Environ., 2008, 84, 440—447 |
| [21] | Egerton T. A., King C. J., J. Oil. Col. Chem. Assoc., 1979, 62, 386—391 |
| [22] | Augugliaro V., Marci G., Palmisano L., Pramauro E., Bianco P. A., Bull. Chem. Soc. Jpn., 1993, 19, 839—851 |
| [23] | Han Y. Y., Wang W., Song M. X., Chem. J. Chinese Universities, 2012, 33(3), 604—607 |
| (韩燕燕, 王威, 宋明昕.高等学校化学学报, 2012,33(3), 604—607) | |
| [24] | Curco D., Gimenez J., Addardak A., Catal. Today, 2002, 76, 177—188 |
| [25] | Xu X. Q., Yu X. F., Tang Y., Wu C. D., J. Chin. Ceramic. Soc., 2012, 40, 1796—1801 |
| (徐秀泉, 于小凤, 唐燕, 吴春笃. 硅酸盐学报, 2012, 40, 1796—1801) |
| [1] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [2] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [3] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [4] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [5] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [6] | QIU Liqi, YAO Xiangyang, HE Liangnian. Visible-light-driven Selective Reduction of Carbon Dioxide Catalyzed by Earth-abundant Metalloporphyrin Complexes [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220064. |
| [7] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| [8] | TAO Yu, OU Honghui, LEI Yongpeng, XIONG Yu. Research Progress of Single-atom Catalysts in Photocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220143. |
| [9] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [10] | MENG Xiangyu, ZHAN Qi, WU Yanan, MA Xiaoshuang, JIANG Jingyi, SUN Yueming, DAI Yunqian. Photothermal Enhanced Photocatalytic Hydrogenation Performance of Au/RGO/Na2Ti3O7 [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210655. |
| [11] | GUO Biao, ZHAO Chencan, LIU Xinxin, YU Zhou, ZHOU Lijing, YUAN Hongming, ZHAO Zhen. Effects of Surface Hydrothermal Carbon Layer on the Photocatalytic Activity of Magnetic NiFe2O4 Octahedron [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220472. |
| [12] | LI Chenchen, NA Yong. g-C3N4/CdS/Ni Composite as a Bifunctional Photocatalyst for H2 Generation and 5-Hydroxymethylfurfural Oxidation [J]. Chem. J. Chinese Universities, 2021, 42(9): 2896. |
| [13] | LI Yishan, GUO Liang, PENG Sifan, ZHANG Qingmao, ZHANG Yuhao, XU Shiqi. Cobalt Substitutions in Lanthanum Manganate Photocatalyst: First-principles and Visible-light Photocatalytic Ability Investigation [J]. Chem. J. Chinese Universities, 2021, 42(6): 1881. |
| [14] | WANG Peng, YANG Min, TANG Sengpei, CHEN Feitai, LI Youji. Preparation of Cellular C3N4/CoSe2/GA Composite Photocatalyst and Its CO2 Reduction Activity [J]. Chem. J. Chinese Universities, 2021, 42(6): 1924. |
| [15] | YANG Sixian, ZHONG Wenyu, LI Chaoxian, SU Qiuyao, XU Bingjia, HE Guping, SUN Fengqiang. Photochemical Fabrication and Performance of Polyaniline Nanowire/SnO2 Composite Photocatalyst [J]. Chem. J. Chinese Universities, 2021, 42(6): 1942. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||