

Chem. J. Chinese Universities ›› 2022, Vol. 43 ›› Issue (2): 20210546.doi: 10.7503/cjcu20210546
• Physical Chemistry • Previous Articles Next Articles
LI Xiaohui1,3, WEI Aijia1,2,3( ), MU Jinping1,2,3, HE Rui1,3, ZHANG Lihui1,3, WANG Jun3, LIU Zhenfa1,3(
), MU Jinping1,2,3, HE Rui1,3, ZHANG Lihui1,3, WANG Jun3, LIU Zhenfa1,3( )
)
Received:2021-08-02
Online:2022-02-10
Published:2021-12-04
Contact:
LIU Zhenfa
E-mail:weiaijia2012@126.com;lzf63@sohu.com
Supported by:CLC Number:
TrendMD:
LI Xiaohui, WEI Aijia, MU Jinping, HE Rui, ZHANG Lihui, WANG Jun, LIU Zhenfa. Effects of SmPO4 Coatingon Electrochemical Performance of High-voltage LiNi0.5Mn1.5O4 Cathode Materials[J]. Chem. J. Chinese Universities, 2022, 43(2): 20210546.
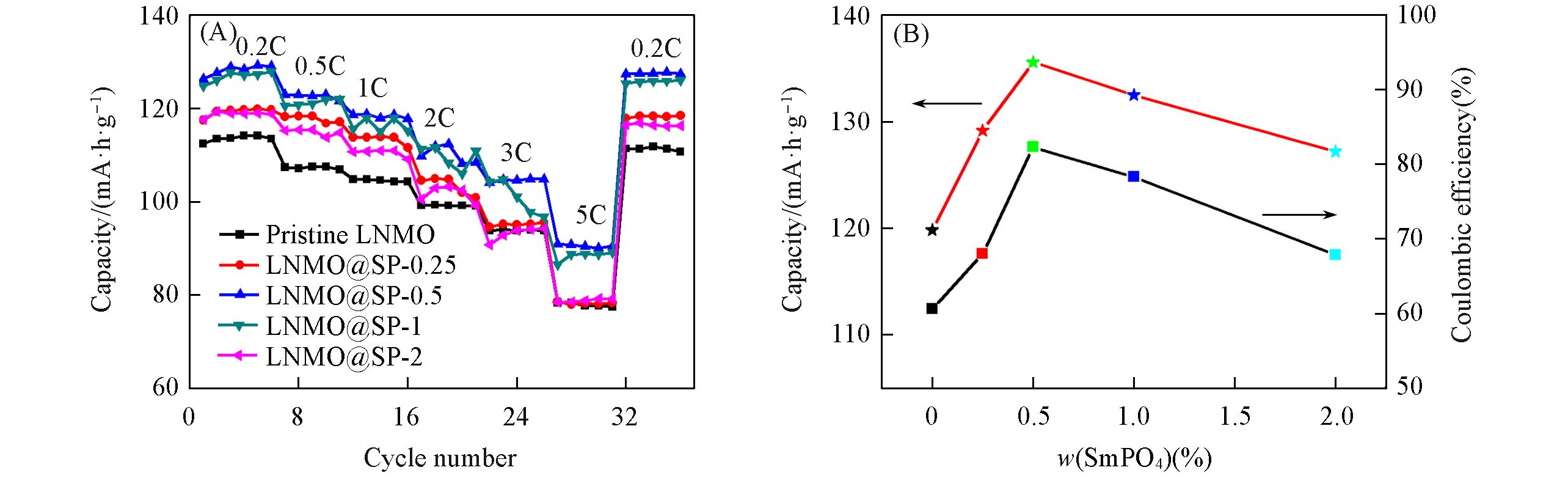
Fig.7 Rate capability curves(A) and initial discharge capacities and coulombic efficiencies(B) of Pristine LNMO, LNMO@SP?0.25, LNMO@SP?0.5, LNMO@SP?1 and LNMO@SP?2 samples
| Sample | Discharge capacity/(mA·h·g-1) | |||||
|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 3C | 5C | |
| Pristine LNMO | 114.2 | 107.5 | 104.6 | 99.2 | 93.8 | 77.7 |
| LNMO@SP?0.25 | 119.7 | 118.3 | 113.8 | 104.8 | 95.2 | 78.2 |
| LNMO@SP?0.5 | 129.2 | 122.9 | 118.8 | 112.3 | 104.9 | 90.9 |
| LNMO@SP?1 | 127.9 | 122.0 | 118.0 | 111.6 | 101.0 | 88.8 |
| LNMO@SP?2 | 119.3 | 115.4 | 110.9 | 103.2 | 94.2 | 79.2 |
Table 1 Discharge capacity of Pristine LNMO, LNMO@SP-0.25, LNMO@SP-0.5, LNMO@SP-1 and LNMO@SP-2 samples at different rates
| Sample | Discharge capacity/(mA·h·g-1) | |||||
|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 3C | 5C | |
| Pristine LNMO | 114.2 | 107.5 | 104.6 | 99.2 | 93.8 | 77.7 |
| LNMO@SP?0.25 | 119.7 | 118.3 | 113.8 | 104.8 | 95.2 | 78.2 |
| LNMO@SP?0.5 | 129.2 | 122.9 | 118.8 | 112.3 | 104.9 | 90.9 |
| LNMO@SP?1 | 127.9 | 122.0 | 118.0 | 111.6 | 101.0 | 88.8 |
| LNMO@SP?2 | 119.3 | 115.4 | 110.9 | 103.2 | 94.2 | 79.2 |
| Sample | Discharge capacity/(mA·h·g-1) | Capacity retention(%) | |
|---|---|---|---|
| 1st cycle | 200th cycle | ||
| Pristine LNMO | 96.4 | 83.5 | 86.6 |
| LNMO@SP?0.25 | 105.9 | 95.1 | 89.8 |
| LNMO@SP?0.5 | 113.2 | 105.7 | 93.4 |
| LNMO@SP?1 | 109.9 | 99.0 | 90.1 |
| LNMO@SP?2 | 106.0 | 94.5 | 89.2 |
Table 2 Discharge capacity and capacity retention of Pristine LNMO, LNMO@SP-0.25, LNMO@SP-0.5, LNMO@SP-1 and LNMO@SP-2 samples at 1C
| Sample | Discharge capacity/(mA·h·g-1) | Capacity retention(%) | |
|---|---|---|---|
| 1st cycle | 200th cycle | ||
| Pristine LNMO | 96.4 | 83.5 | 86.6 |
| LNMO@SP?0.25 | 105.9 | 95.1 | 89.8 |
| LNMO@SP?0.5 | 113.2 | 105.7 | 93.4 |
| LNMO@SP?1 | 109.9 | 99.0 | 90.1 |
| LNMO@SP?2 | 106.0 | 94.5 | 89.2 |
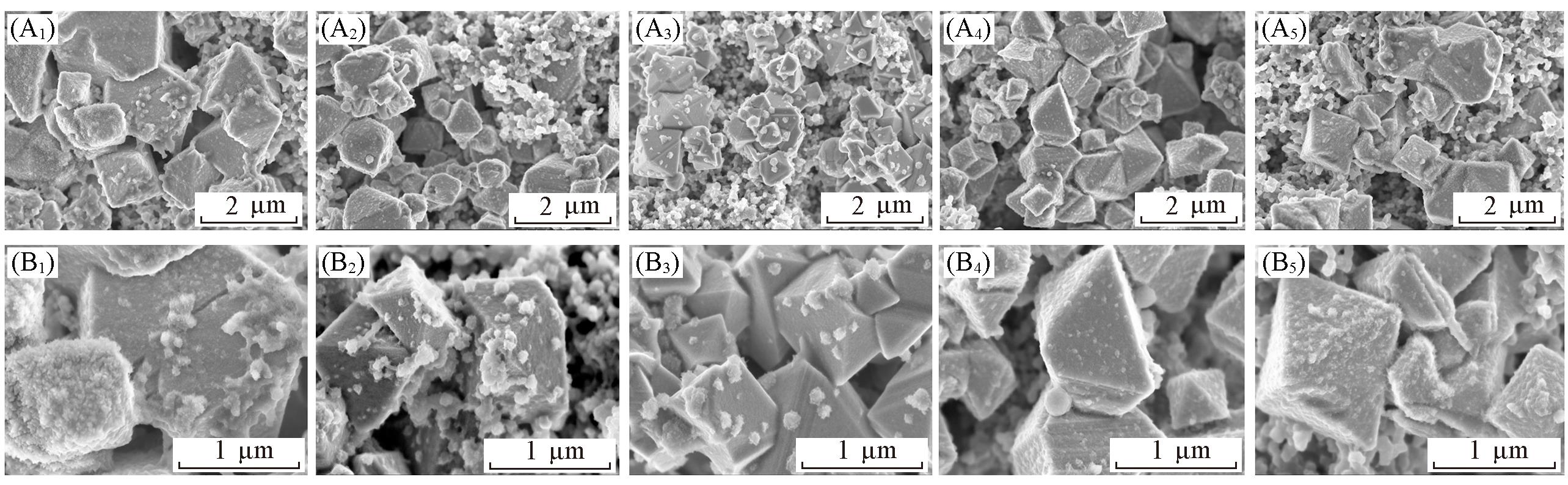
Fig.10 SEM images of Pristine LNMO(A1, B1), LNMO@SP?0.25(A2, B2), LNMO@SP?0.5(A3, B3), LNMO@SP?1(A4, B4) and LNMO@SP?2(A5, B5) electrodes before(A1—A5) and after(B1—B5) 200 cycles at 1C
| Sample | Φpa/V | Φpc/V | ?V/V |
|---|---|---|---|
| Pristine LNMO | 4.821 | 4.582 | 0.239 |
| LNMO@SP?0.25 | 4.830 | 4.611 | 0.219 |
| LNMO@SP?0.5 | 4.834 | 4.622 | 0.212 |
| LNMO@SP?1 | 4.828 | 4.611 | 0.217 |
| LNMO@SP?2 | 4.832 | 4.608 | 0.224 |
Table 3 Potential difference between anode and cathode peaks of Pristine LNMO, LNMO@SP-0.25, LNMO@SP-0.5, LNMO@SP-1 and LNMO@SP-2 samples*
| Sample | Φpa/V | Φpc/V | ?V/V |
|---|---|---|---|
| Pristine LNMO | 4.821 | 4.582 | 0.239 |
| LNMO@SP?0.25 | 4.830 | 4.611 | 0.219 |
| LNMO@SP?0.5 | 4.834 | 4.622 | 0.212 |
| LNMO@SP?1 | 4.828 | 4.611 | 0.217 |
| LNMO@SP?2 | 4.832 | 4.608 | 0.224 |
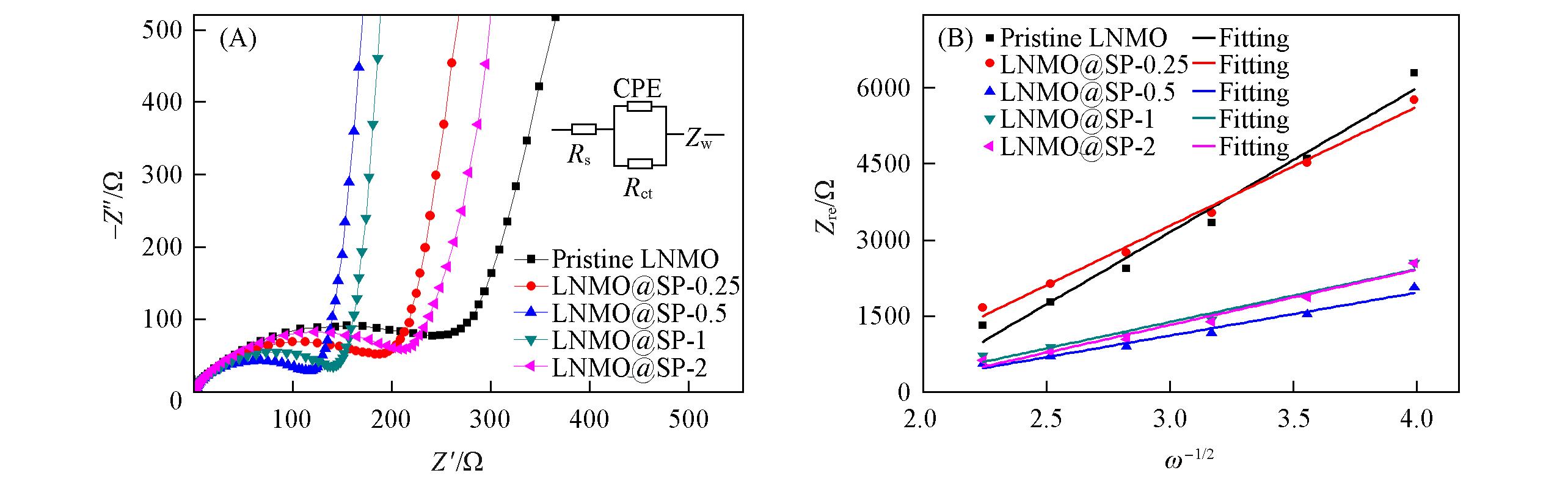
Fig.12 EIS spectra(A) and graph(B) of Zre plotted against ω-1/2 at low?frequency region of Pristine LNMO, LNMO@SP?0.25, LNMO@SP?0.5, LNMO@SP?1 and LNMO@SP?2 samplesInset of (A): equivalent circuit diagram. Rs: The solution resistance of the cell; CPE: constant phase element, representing the double layer capacitance of the interface; Zw: Warburg impedance.
| Sample | Rs/Ω | Rct/Ω |
|---|---|---|
| Pristine LNMO | 1.141 | 240.6 |
| LNMO@SP?0.25 | 1.084 | 188.4 |
| LNMO@SP?0.5 | 1.005 | 118.2 |
| LNMO@SP?1 | 1.070 | 141.3 |
| LNMO@SP?2 | 1.075 | 211.7 |
Table 4 Alternating current(AC) impedance parameters of Pristine LNMO, LNMO@SP-0.25, LNMO@SP-0.5, LNMO@SP-1 and LNMO@SP-2 samples
| Sample | Rs/Ω | Rct/Ω |
|---|---|---|
| Pristine LNMO | 1.141 | 240.6 |
| LNMO@SP?0.25 | 1.084 | 188.4 |
| LNMO@SP?0.5 | 1.005 | 118.2 |
| LNMO@SP?1 | 1.070 | 141.3 |
| LNMO@SP?2 | 1.075 | 211.7 |
| 21 | Wang J., Lin W., Wu B., Zhao J., Electrochim. Acta, 2014, 145, 245—253 |
| 22 | Luo Y., Lu T. L., Zhang Y. X., Yan L. Q., Mao S. S., Xie J. Y., J. Alloys Compd., 2017, 703, 289—297 |
| 23 | Shiu J. J., Pang W. K., Wu S. H., J. Power Sources, 2013, 224, 35—42 |
| 24 | Sun H. Y., Kong X., Wang B. S., Luo T. B., Liu G. Y., Ceram. Int., 2018, 44, 4603—4610 |
| 25 | Qiao Q. Q., Zhang H. Z., Li G. R., Ye S. H., Wang C. W., Gao X. P., J. Mater. Chem., 2013, A1, 5262—5268 |
| 26 | Wu C., Fang X., Guo X., Mao Y., Ma J., Zhao C., Wang Z., Chen L., J. Power Sources, 2013, 231, 44—49 |
| 27 | Li Y., Zhang Q., Xu T., Wang D. D., Pan D., Zhao H. L., Bai Y., Ceram. Int., 2018, 44(4), 4058—4066 |
| 28 | Kim J. H., Myung S. T., Yoon C. S., Kang S. G., Sun Y. K., Chem. Mater., 2004, 16, 906—914 |
| 29 | Deng Y., He L., Ren J., Zheng Q. J., Xu C. G., Lin D. M., Mater. Res. Bull., 2018, 100, 333—344 |
| 30 | Deng Y. F., Zhao S. X., Xu Y. H., Nan C. W., J. Power Sources, 2015, 296, 261—267 |
| 31 | Kunduraci M., Amatucci G. G., J. Electrochem. Soc., 2006, 153, 1345—1352 |
| 32 | Park J. S., Roh K. C., Lee J. W., Song K., Kim Y. I., Kang Y. M., J. Power Sources, 2013, 230, 138—142 |
| 33 | Gu Y. J., Li Y., Chen Y. B., Liu H. Q., Electrochim. Acta, 2016, 213, 368—374 |
| 34 | He L., Xu J. M., Han T., Han H., Wang Y. J., Yang J., Wang J. R., Zhu W. K., Zhang C. J., Zhang Y. H., Ceram. Int., 2017, 43(6), 5267―5273 |
| 35 | Liu W. J., Shi Q., Qu Q. T., Gao T., Zhu G. B., Shao J., Zheng H. H., J. Mater. Chem., 2017, A5(1), 145—154 |
| 36 | Mou J., Deng Y., Song Z., Zheng Q., Lam K. H., Lin D., Dalton T., 2018, 47(20), 7020—7028 |
| 37 | Huang B., Li X. H., Wang Z. X., Guo H. J., Xiong X. H., Wang J. H., J. Alloy. Compd., 2014, 583, 313—319 |
| 38 | Gao X. W., Deng Y. F., Wexler D., J. Mater. Chem. A, 2015, 3, 404—411 |
| 39 | Jia G. L., Jiao C. M., Xue W. J., Zheng S. H., Wang J., Solid State Ionics, 2016, 292, 15—21 |
| 40 | Zhang Q., Chen J. J., Wang X. Y., Yang C., Zheng M. S., Dong Q. F., Phys. Chem. Chem. Phys., 2015, 17, 10353—10357 |
| 41 | Yang L., Ravdel B., Lucht Brett L., Electrochem. Solid St., 2010, 13, A95—A97 |
| 42 | Liu M. H., Huang H. T., Lin C. M., Chen J. M., Liao S. C., Electrochim. Acta, 2014, 120, 133—139 |
| 1 | Diouf B., Pode R., Renew. Energ., 2015, 76, 375—380 |
| 2 | Ren G., Ma G., Cong N., Renew. Sust. Energ. Rev., 2015, 41, 225—236 |
| 3 | Lu J., Lee K. S., Mater. Technol., 2016, 31(11), 628—641 |
| 4 | Jafta Charl J., Mathe Mkhulu K., Manyala N., ACS Appl. Mater. Inter., 2013, 5, 7592—7598 |
| 5 | Bai G. Y., Wang Y., Materials Reports, 2015, 29(6), 15—18(白钢印, 王英. 材料导报, 2015, 29(6), 15—18) |
| 6 | Kim J. W., Kim D. H., Oh D. Y., Lee H., Kim J. H., Lee J. H., Jung Y. S., J. Power Sources, 2015, 274, 1254—1262 |
| 7 | Wang G., Wen W. C., Chen S. H., Yu R. Z., Wang X. Y., Yang X. K., Electrochim. Acta, 2016, 212, 791—799 |
| 8 | Yi T. F., Han X., Chen B., Zhu Y. R., Xie Y., J. Alloy. Compd., 2017, 703, 103—113 |
| 9 | Tron A., Mun J., J. Solid State Chem., 2021, 302(15), 122411 |
| 10 | Wu Q., Yin Y. F., Sun S. W., Zhang X. P., Wan N., Bai Y., Electrochim. Acta, 2015, 158, 73—80 |
| 11 | Yu C. Y., Dong L., Zhang Y. X., Du K., Gao M. M., Zhao H. L., Bai Y., Solid State Ionics, 2020, 357, 115464 |
| 12 | Liu D., Bai Y., Zhao S., Zhang W., J. Power Sources, 2012, 219, 333—338 |
| 13 | Xu T. H., Li Y. P., Wang D. D., Wu M. Y., Pan D., Zhao H. L., Bai Y., ACS Sustainable Chem. Eng., 2018, 6, 5818—5825 |
| 14 | Liu Y. L., Lu Z. P., Deng C. F., Ding J. J., Xu Y., Lu X. J., Yang G., J. Mater. Chem., 2017, A5, 996—1004 |
| 15 | Hwang T., Lee J. K., Mun J., Choi W., J. Power Sources, 2016, 322, 40—48 |
| 16 | Zheng X., Liu W., Qu Q., Shi Q., Zheng H., Huang Y., Appl. Surf. Sci., 2018, 455, 349—356 |
| 17 | Cho J. H., Park J. H., Lee M. H., Song H. K., Lee S. Y., Energy Environ. Sci., 2012, 5, 7124—7131 |
| 18 | Mou J. R., Deng Y. L., He L. H., Zheng Q. J., Jiang N., Lin D. M., Electrochim. Acta, 2018, 260, 101—111 |
| 19 | Deng H., Nie P., Luo H., Zhang Y., Wang J., Zhang X., J. Mater. Chem., 2014, A2(43), 18256—18262 |
| 20 | Deng Y., Mou J., Wu H., Jiang N., Zheng Q., Lam K. H., Xu C. G., Lin D. M., Electrochim. Acta, 2017, 235, 19—31 |
| [1] | RONG Hua, WANG Chungang, ZHOU Ming. Synthesis and Electrochemical Performance of FeS2 Microspheres as an Anode for Li-ion Batteries † [J]. Chem. J. Chinese Universities, 2020, 41(3): 447. |
| [2] | HUANG He, LI Chunguang, SHI Zhan, FENG Shouhua. Microwave-assisted Hydrothermal Synthesis of Carbon Dots Based on Tyrosine and Their Application in Ion Detection and Bioimaging [J]. Chem. J. Chinese Universities, 2019, 40(8): 1579. |
| [3] | DONG Xiangyang,NIU Xiaoqing,WEI Jishi,XIONG Huanming. One-step Hydrothermal Synthesis of Copper Doped Carbon Dots and Their Application in White Light Devices† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1288. |
| [4] | JIA Hongliang,ZHAO Jianwei,QIN Lirong,ZHAO Min. Uric Acid Biosensor Based on Ni Wire Modified with NiO Nanosheets† [J]. Chem. J. Chinese Universities, 2019, 40(2): 240. |
| [5] | SUN Dawei,LI Yuejun,CAO Tieping,ZHAO Yanhui,YANG Diankai. Preparation of Dy 3+-doped YVO4/TiO2 Composite Nanofibers with Three-dimensional Net-like Structure and Enhanced Photocatalytic Activity for Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2348. |
| [6] | PAN Shuai, HU Xiaobing, SONG Runmin, XIE Lili, ZHU Zhigang, ZHENG Liaoying. Ionic Liquid Assisted Synthesis of α-Fe2O3 Nanospheres Based on Potassium Acetate Solution and Their Gas-sensing Properties† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1631. |
| [7] | KANG Yuanyuan, GUO Zeqing, ZHOU Jianping. Hydrothermal Preparation and Adsorption Property of MoS2/Na2Fe2Ti6O16† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1364. |
| [8] | LI Juan, ZHU Linfang, ZHAO Anting, LEI Guoming, GAO Li, XIA Wen, WANG Li. Catalytic Activity of Cucurbit[6]uril Modified Copper Flower Clusters† [J]. Chem. J. Chinese Universities, 2018, 39(3): 422. |
| [9] | ZHANG Yiqing, LIU Jiaxiang. Hydrothermal Preparation of Cubic ITO Powder and Its Photoelectric Performance† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1110. |
| [10] | SUN Lianzhi, ZHAO Shengzhe, GAO Zhiling, CHENG Zhiqiang. Controllable Synthesis of Ag Decorated ZnO Nanofibers for Enhanced Photocatalysis [J]. Chem. J. Chinese Universities, 2017, 38(6): 907. |
| [11] | HAN Yanmei, GAO Zhihua, HUANG Wei. Effects of AlOOH Structure on the Reaction of Methanol and Carbon Monoxide† [J]. Chem. J. Chinese Universities, 2017, 38(5): 823. |
| [12] | HUANG Haiping, YUE Yafeng, XU Liang, LÜ Lianlian, HU Yongmei. Glucose Biosensor Based on Dy2(MoO4)3-AuNPs Composite Nanomaterial† [J]. Chem. J. Chinese Universities, 2017, 38(4): 554. |
| [13] | SHI Yanlong, FENG Xiaojuan, WANG Suiqian, FENG Chunchun. Fabrication of Superhydrophobic Film of Co(OH)2CO3 Nanowires and Its Anticorrosion† [J]. Chem. J. Chinese Universities, 2017, 38(3): 456. |
| [14] | ZHU Jielian, XIA Xiaofeng, ZHU Shanshan, LIU Xiang, LI Hexing. Synthesis and Photocatalytic Activity of Cr Doped TiO2 Nanowires/Reduced Graphene Oxide Composites† [J]. Chem. J. Chinese Universities, 2016, 37(10): 1833. |
| [15] | LU Yonghong, WU Pingxiao, HUANG Junyi, TRAN Lytuong, ZHU Nengwu, DANG Zhi. Alkaline-assisted Hydrothermal Fabrication of CdZnS with Enhanced Visible-light Photocatalytic Performance† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1563. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||