

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (11): 2390.doi: 10.7503/cjcu20140513
• Physical Chemistry • Previous Articles Next Articles
LI Shaochen, YU Guangtao, CHEN Wei*( ), HUANG Xuri*(
), HUANG Xuri*( )
)
Received:2014-06-06
Online:2014-11-10
Published:2014-09-28
Contact:
CHEN Wei,HUANG Xuri
E-mail:w_chen@jlu.edu.cn;huangxr@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
LI Shaochen, YU Guangtao, CHEN Wei, HUANG Xuri. Investigation on Structures and Nonlinear Optical Properties of Super-short Carbon Nanotube Systems with Surface-adsorbing Lithium Atoms†[J]. Chem. J. Chinese Universities, 2014, 35(11): 2390.
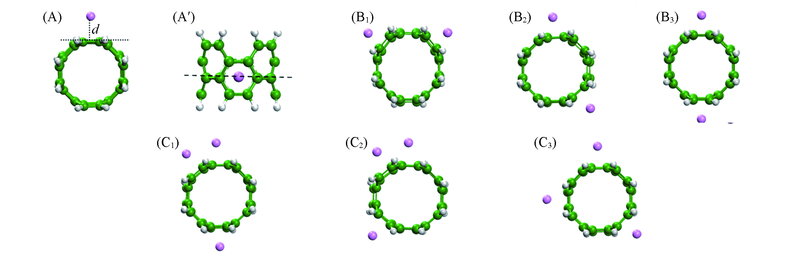
Fig.1 Optimized geometries of Li1@cyclophenace [the side(A) and top(A') views] and Lin@cyclophenace [n=2(B1—B3) and n=3(C1—C3)] (A) The “d” represents the vertical distance between the doped Li atom and super-short carbon nanotube. (B1) Li2a@cyclophenace; (B2) Li2b@cyclophenace; (B3) Li2c@cyclophenace; (C1) Li3a@cyclophenace; (C2) Li3b@cyclophenace; (C3) Li3c@cyclophenace.
| System | d/nm | Ead/(kJ·mol-1) | System | d/nm | Ead/(kJ·mol-1) |
|---|---|---|---|---|---|
| Li1@cyclophenace | 0.171 | 84.0 | Li3a@cyclophenace | 0.228* | 99.1 |
| Li2a@cyclophenace | 0.205 | 106.2 | Li3b@cyclophenace | 0.228* | 99.5 |
| Li2b@cyclophenace | 0.204 | 106.2 | Li3c@cyclophenace | 0.223* | 85.7 |
| Li2c@cyclophenace | 0.205 | 104.9 |
Table 1 Adsorption energies(Ead) of doped Li atoms in the Lin@cyclophenace series(n=1—3) and their corresponding vertical distances(d) between the Li atom and carbon nanotube
| System | d/nm | Ead/(kJ·mol-1) | System | d/nm | Ead/(kJ·mol-1) |
|---|---|---|---|---|---|
| Li1@cyclophenace | 0.171 | 84.0 | Li3a@cyclophenace | 0.228* | 99.1 |
| Li2a@cyclophenace | 0.205 | 106.2 | Li3b@cyclophenace | 0.228* | 99.5 |
| Li2b@cyclophenace | 0.204 | 106.2 | Li3c@cyclophenace | 0.223* | 85.7 |
| Li2c@cyclophenace | 0.205 | 104.9 |
| System | α/a.u. | β0/a.u. | f0/a.u. | ΔE/eV | Δμ/a.u. | (Δμ·f0)/ΔE3 | CT |
|---|---|---|---|---|---|---|---|
| Cyclophenace | 312 | 0 | 0.1483 | 4.260 | 0 | 0 | |
| Li1@cyclophenace | 374 | 8.29×103 | 0.0368 | 1.778 | 3.174 | 418 | H→L+3 H→L+4 |
| Li2a@cyclophenace | 378 | 6.94×103 | 0.1190 | 2.236 | 1.747 | 374 | H→L+4 H→L+8 |
| Li2b@cyclophenace | 409 | 4.46×103 | 0.0973 | 2.166 | 1.437 | 277 | H→L+4 H→L+5 |
| Li2c@cyclophenace | 421 | 3.42×103 | 0.0908 | 2.055 | 0.684 | 144 | H→L+4 H-1→L |
| Li3a@cyclophenace | 529 | 8.44×103 | 0.0933 | 1.446 | 0.800 | 497 | H→L+5 H→L+6 |
| Li3b@cyclophenace | 520 | 1.11×104 | 0.0914 | 1.431 | 1.194 | 749 | H→L+4 H→L+5 |
| Li3c@cyclophenace | 2814 | 2.59×106 | 0.0868 | 1.247 | 2.411 | 2172 | H→L+9 H→L+10 |
Table 2 Polarizability(α), the first hyperpolarizability(β0), the oscillator strengh(f0), the transition energy(ΔE), the difference of dipole moments(Δμ) between the crucial excited state and ground state, the estimated β0 values under the two-level approach, and the main compositions of the crucial transition(CT) state for the Lin@cyclophenace(n=1—3) series*
| System | α/a.u. | β0/a.u. | f0/a.u. | ΔE/eV | Δμ/a.u. | (Δμ·f0)/ΔE3 | CT |
|---|---|---|---|---|---|---|---|
| Cyclophenace | 312 | 0 | 0.1483 | 4.260 | 0 | 0 | |
| Li1@cyclophenace | 374 | 8.29×103 | 0.0368 | 1.778 | 3.174 | 418 | H→L+3 H→L+4 |
| Li2a@cyclophenace | 378 | 6.94×103 | 0.1190 | 2.236 | 1.747 | 374 | H→L+4 H→L+8 |
| Li2b@cyclophenace | 409 | 4.46×103 | 0.0973 | 2.166 | 1.437 | 277 | H→L+4 H→L+5 |
| Li2c@cyclophenace | 421 | 3.42×103 | 0.0908 | 2.055 | 0.684 | 144 | H→L+4 H-1→L |
| Li3a@cyclophenace | 529 | 8.44×103 | 0.0933 | 1.446 | 0.800 | 497 | H→L+5 H→L+6 |
| Li3b@cyclophenace | 520 | 1.11×104 | 0.0914 | 1.431 | 1.194 | 749 | H→L+4 H→L+5 |
| Li3c@cyclophenace | 2814 | 2.59×106 | 0.0868 | 1.247 | 2.411 | 2172 | H→L+9 H→L+10 |
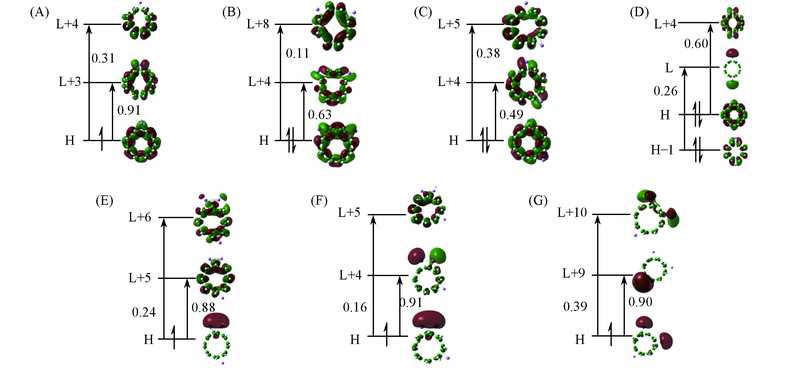
Fig.2 Crucial transition states of the Lin@cyclophenace(n =1—3) systems (A) Li1@cyclophenace; (B)Li2a@cyclophenace; (C) Li2b@cyclophenace; (D) Li2c@cyclophenace; (E) Li3a@cyclophenace; (F) Li3b@cyclophenace; (G) Li3c@cyclophenace. The relatively large component coefficients are marked.
| [1] | Eaton D. F., Science, 1991, 253, 281—287 |
| [2] | Kanis D. R., Ratner M. A., Marks T. J., Chem. Rev., 1994, 94, 195—242 |
| [3] | Xiao D., Bulat F. A., Yang W. T., Beratan D. N., Nano Lett., 2008, 8, 2814—2818 |
| [4] | Ma F., Zhou Z. J., Li Z. R., Wu D., Li Y., Li Z. S., Chem. Phys. Lett., 2010, 488, 182—186 |
| [5] | Yu G. T., Zhao X. G., Niu M., Huang X. R., Zhang H., Chen W., J. Mater. Chem. C,2013, 1, 3833—3841 |
| [6] | Tu C. Y., Yu G. T., Yang G. H., Zhao X. G., Chen W., Li S. C., Huang X. R., Phys. Chem. Chem. Phys., 2014, 16, 1597—1606 |
| [7] | Chen L. W., Yu G. T., Chen W., Tu C. Y., Zhao X. G., Huang X. R., Phys. Chem. Chem. Phys., 2014, 16, 10933—10942 |
| [8] | Zhou Z. J., Yu G. T., Ma F., Huang X. R., Wu Z. J., J. Mater. Chem. C,2014, 2, 306—311 |
| [9] | Liu H. B., Qiu Y. Q., Yang G. C., Liu C. G., Sun S. L., Chem. Res. Chinese Universities,2012, 28(2), 308—312 |
| [10] | Xu H. L., Zhong R. L., Sun S. L., Su Z. M., J. Phys. Chem. C,2011, 115, 16340—16346 |
| [11] | Xu H. L., Li Z. R., Wu D., Ma F., Li Z. J., Gu F. L., J. Phys. Chem. C,2009, 113, 4984—4986 |
| [12] | Xu H. L., Wang F. F., Li Z. R., Wang B. Q., Wu D., Chen W., Yu G. T., Gu F. L., Aoki Y., J. Comput. Chem., 2009, 30, 1128—1134 |
| [13] | Sun S. L., Hu Y. Y., Xu H. L., Su Z. M., Hao L. Z., J. Mol. Model., 2012, 18, 3219—3225 |
| [14] | Shi Z. M., Chen W., Wan S. Q., Li H., Huang X. R., Chem. J. Chinese Universities,2013, 34(2), 441—446 |
| (石芝铭, 陈巍, 万素琴, 李辉, 黄旭日. 高等学校化学学报,2013, 34(2), 441—446) | |
| [15] | Niu M., Yu G. T., Yang G. H., Chen W., Zhao X. G., Huang X. R., Inorg. Chem., 2014, 53, 349—358 |
| [16] | Zhou Z. J., Li H., Huang X. R., Wu Z. J., Ma F., Li Z. R., Comput. Theor. Chem., 2013, 1023, 99—103 |
| [17] | Chen W., Li Z. R., Wu D., Li Y., Sun C. C., Gu F. L., J. Am. Chem. Soc., 2005, 127, 10977—10981 |
| [18] | Muhammad S., Xu H. L., Liao Y., Kan Y. H., Su Z. M., J. Am. Chem. Soc., 2009, 131, 11833—11840 |
| [19] | Zhao X. G., Yu G. T., Huang X. R., Chen W., Niu M., J. Mol. Model., 2013, 19, 5601—5610 |
| [20] | Yu G. T., Huang X. R., Chen W., Sun C. C., J. Comput. Chem., 2011, 32, 2005—2011 |
| [21] | Chen W., Yu G. T., Jin P., Li Z. R., Huang X. R., J. Comput. Theor. Nanosci., 2011, 8, 2482—2487 |
| [22] | Xu H. L., Li Z. R., Su Z. M., Muhammad S., Gu F. L., Harigaya K., J. Phys. Chem. C,2009, 113, 15380—15383 |
| [23] | Wang F. F., Li Z. R., Wu D., Wang B. Q., Li Y., Li Z. J., J. Phys. Chem. B,2008, 112, 1090—1094 |
| [24] | Xu H. L., Sun S. L., Muhammad S., Su Z. M., Theor. Chem. Acc., 2011, 128, 241—248 |
| [25] | Wei W., Bai F. Q., Xia B. H., Chen H. B., Zhang H. X., Chem. Res. Chinese Universities,2013, 29(5), 962—968 |
| [26] | Zhang S. S., Shi L. L., Su Z. M., Ceng Y., Zhao L., Chem. Res. Chinese Universities,2013, 29(2), 361—365 |
| [27] | Chen J., Wang J., Bai F. Q., Zheng Q. C., Zhang H. X., Chem. Res. Chinese Universities,2012, 28(4), 696—702 |
| [28] | Wang X. L., Zhang K., Chem. Res. Chinese Universities,2012, 28(4), 703—706 |
| [29] | Maroulis G., Struct. Bond., 2012, 149, 95—130 |
| [30] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A. Jr., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A., Gaussian 03, Revision C.02, Gaussian Inc., Wallingford CT, 2012 |
| [31] | Oudar J. L., J. Chem. Phys., 1977, 67, 446—457 |
| [1] | ZHENG Xuelian, YANG Cuicui, TIAN Weiquan. The Second Order Nonlinear Optical Properties of Azulene-defect Graphene Nanosheets with Full Armchair Edge [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210806. |
| [2] | CHENG Xiao, K BORA Debajeet, GLANS Per⁃Anders, GUO Jinghua, LUO Yi. An In-depth Theoretical Study of Ligand Field and Charge Transfer Effects on Co2+2pL2,3-edges X-ray Absorption Spectra [J]. Chem. J. Chinese Universities, 2021, 42(7): 2197. |
| [3] | CHANG Hui, YAO Shuangquan, HAN Wenjia, KANG Xiena, ZHANG Li, LI Xinping, ZHANG Zhao. Highly Solvatochromic Terpyridine Compounds for Identification of Butanol Isomers [J]. Chem. J. Chinese Universities, 2021, 42(3): 902. |
| [4] | WANG Linshuo, LI Kunjie, LIU Yumin, ZHAO Ruihong, LI Qing, QIAN Xin, ZHANG Fan, XUE Zhiwei. Theoretical Studies of Triphenyl-s-triazine Groups Regulating Photoelectric Properties of Sensitizing Dyes† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1653. |
| [5] | KANG Huimin,WANG Hongqiang,WANG Huiying,WU Lixin,QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of a Porphyrin-o-Carborane-Boron-dipyrromethene Triad Compound† [J]. Chem. J. Chinese Universities, 2019, 40(5): 965. |
| [6] | WU Juan, WANG Hongqiang, LIU Xiaoyun, SHI Zhiyuan, QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of D-A-D(D') o-Carborane Triads† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1490. |
| [7] | LIU Chong, LIU Lilai, NIE Jiahui. Synthesis of Carbon Ball Modified g-C3N4 for Improved Photocatalytic Activity† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1511. |
| [8] | YAN Lingling,LIU Chunguang,JIANG Mengxu. Theoretical Study on Cation Detection Ability of Pyridine-substituted Lindqvist-type Ployoxometalates Based on Linear and Nonlinear Optical Properties† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1034. |
| [9] | CHEN Deli, YANG Pengyong, WU Shengnan, HE Sihui, WANG Fangfang. Ab initio Molecular Dynamics Simulations on the Structures and Stabilities of Pd Clusters Encapsulated UiO-66 Materials† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1210. |
| [10] | CHEN Jiuju. Theoretical Studies on the of Ambipolar Charge Transport in Terazulene Single Crystal† [J]. Chem. J. Chinese Universities, 2016, 37(1): 121. |
| [11] | LI Shaochen, YU Guangtao, CHEN Wei, ZHOU Zhongjun, HUANG Xuri. Investigation on Structures and Nonlinear Optical Properties of PPV and Its Derivatives Systems with Adsorbing Alkali Metal Atom† [J]. Chem. J. Chinese Universities, 2015, 36(6): 1146. |
| [12] | PANG Ran, JIN Xi, ZHAO Liubin, DING Songyuan, WU Deyin, TIAN Zhongqun. Quantum Chemistry Study of Electrochemical Surface-enhanced Raman Spectroscopy† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2087. |
| [13] | ZHANG Xueying, YU Guangtao, CHEN Wei, HUANG Xuri. Theoretical Investigation on Nonlinear Optical Properties of the Donor/Acceptor-decorated Zigzag Graphene Nanoribbons with the 5-9 Defect† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2204. |
| [14] | HOU Na, LI Ying, WU Di, LI Zhiru. Theoretical Studies on Structures and Nonlinear Optical Properties of Superalkali-based Electrides Li3@calix[4]pyrrole and Li3O@calix[4]pyrrole† [J]. Chem. J. Chinese Universities, 2014, 35(4): 798. |
| [15] | JIANG Xin, QIN Xiaoyu, GONG Mengdi, LI Xiuling, LI Guangzhi, YANG Libin, ZHAO Bing. Improvement of Surface-enhanced Raman Scattering Properties of TiO2 Nanoparticles by Metal Ni Doping† [J]. Chem. J. Chinese Universities, 2014, 35(3): 488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||