

Chem. J. Chinese Universities ›› 2020, Vol. 41 ›› Issue (2): 253.doi: 10.7503/cjcu20190493
• Articles:Inorganic Chemistry • Previous Articles Next Articles
LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia( )
)
Received:2019-09-16
Online:2020-02-10
Published:2019-10-29
Contact:
Xia LI
E-mail:xiali@cnu.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Dongmei,SU Yajing,LI Shanshan,XU Qiwei,LI Xia. Transition Metal Coordination Polymers Constructed by 4-(4-Carboxyphenoxy)isophthalic Acid: Synthesis, Crystal Structure, Fluorescence Sensing and Photocatalysis †[J]. Chem. J. Chinese Universities, 2020, 41(2): 253.
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C25H18N2O8Zn | C27H18N2O8Zn | C27H18MnN2O8 | C54H36Co2N4O16 |
| Formula weight | 539.78 | 563.80 | 553.37 | 1114.73 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P | P | P21/c | P |
| a/nm | 0.97529(7) | 0.99103(5) | 1.42226(4) | 0.97396(3) |
| b/nm | 1.01915(5) | 1.00828(5) | 1.09657(3) | 1.00174(3) |
| c/nm | 1.34860(6) | 1.40052(6) | 1.53718(5) | 1.38980(4) |
| α/(°) | 69.525(4) | 96.263(2) | 90 | 97.159(2) |
| β/(°) | 89.020(5) | 106.8670(10) | 96.0640(10) | 107.019 |
| γ/(°) | 68.440(6) | 110.7200(10) | 90 | 107.595 |
| Volume/nm3 | 1.1583(12) | 1.2171(10) | 2.3840(12) | 1.2017(7) |
| Z | 2 | 2 | 4 | 1 |
| Dcalcd/(g·cm-3) | 1.548 | 1.538 | 1.542 | 1.540 |
| F(000) | 552.0 | 576.0 | 1132.0 | 570 |
| Crystal size/mm3 | 0.25×0.23×0.21 | 0.21×0.25×0.23 | 0.32×0.25×0.14 | 0.15×0.1×0.05 |
| 2θ range for data collection/(°) | 9.832—133.2 | 4.632—52.744 | 4.572—55.014 | 9.526—133.192 |
| Index range | -11≤h≤11, | -11≤h≤11, | -16≤h≤18, | -6≤h≤11 |
| -12≤k≤11, | -11≤k≤11, | -14≤k≤14, | -12≤k≤11, | |
| -15≤l≤16 | -14≤l≤16 | -19≤l≤19 | -16≤l≤17 | |
| Reflections collected | 9868 | 11800 | 27932 | 13362 |
| Independent reflections | 3999[Rint=0.0914] | 4166[Rint=0.0197] | 5473[Rint=0.0480] | 4700[Rint=0.0619] |
| Data/restraints/parameters | 3999/0/327 | 4166/7/345 | 5473/1/345 | 4700/0/345 |
| Goodness-of-fit on F2 | 1.113 | 1.063 | 1.084 | 1.113 |
| Final R indexes[I≥2σ(I)] | R1=0.0851, | R1=0.0334, | R1=0.0619, | R1=0.0547, |
| wR2=0.2576 | wR2=0.0847 | wR2=0.1583 | wR2=0.1582 | |
| Final R indexes(all data) | R1=0.0952, | R1=0.0386, | R1=0.0963, | R1=0.0560, |
| wR2=0.2929 | wR2=0.0875 | wR2=0.1747 | wR2=0.1593 | |
| CCDC No. | 1943993 | 1943994 | 1943992 | 1943991 |
| Complex | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C25H18N2O8Zn | C27H18N2O8Zn | C27H18MnN2O8 | C54H36Co2N4O16 |
| Formula weight | 539.78 | 563.80 | 553.37 | 1114.73 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P | P | P21/c | P |
| a/nm | 0.97529(7) | 0.99103(5) | 1.42226(4) | 0.97396(3) |
| b/nm | 1.01915(5) | 1.00828(5) | 1.09657(3) | 1.00174(3) |
| c/nm | 1.34860(6) | 1.40052(6) | 1.53718(5) | 1.38980(4) |
| α/(°) | 69.525(4) | 96.263(2) | 90 | 97.159(2) |
| β/(°) | 89.020(5) | 106.8670(10) | 96.0640(10) | 107.019 |
| γ/(°) | 68.440(6) | 110.7200(10) | 90 | 107.595 |
| Volume/nm3 | 1.1583(12) | 1.2171(10) | 2.3840(12) | 1.2017(7) |
| Z | 2 | 2 | 4 | 1 |
| Dcalcd/(g·cm-3) | 1.548 | 1.538 | 1.542 | 1.540 |
| F(000) | 552.0 | 576.0 | 1132.0 | 570 |
| Crystal size/mm3 | 0.25×0.23×0.21 | 0.21×0.25×0.23 | 0.32×0.25×0.14 | 0.15×0.1×0.05 |
| 2θ range for data collection/(°) | 9.832—133.2 | 4.632—52.744 | 4.572—55.014 | 9.526—133.192 |
| Index range | -11≤h≤11, | -11≤h≤11, | -16≤h≤18, | -6≤h≤11 |
| -12≤k≤11, | -11≤k≤11, | -14≤k≤14, | -12≤k≤11, | |
| -15≤l≤16 | -14≤l≤16 | -19≤l≤19 | -16≤l≤17 | |
| Reflections collected | 9868 | 11800 | 27932 | 13362 |
| Independent reflections | 3999[Rint=0.0914] | 4166[Rint=0.0197] | 5473[Rint=0.0480] | 4700[Rint=0.0619] |
| Data/restraints/parameters | 3999/0/327 | 4166/7/345 | 5473/1/345 | 4700/0/345 |
| Goodness-of-fit on F2 | 1.113 | 1.063 | 1.084 | 1.113 |
| Final R indexes[I≥2σ(I)] | R1=0.0851, | R1=0.0334, | R1=0.0619, | R1=0.0547, |
| wR2=0.2576 | wR2=0.0847 | wR2=0.1583 | wR2=0.1582 | |
| Final R indexes(all data) | R1=0.0952, | R1=0.0386, | R1=0.0963, | R1=0.0560, |
| wR2=0.2929 | wR2=0.0875 | wR2=0.1747 | wR2=0.1593 | |
| CCDC No. | 1943993 | 1943994 | 1943992 | 1943991 |
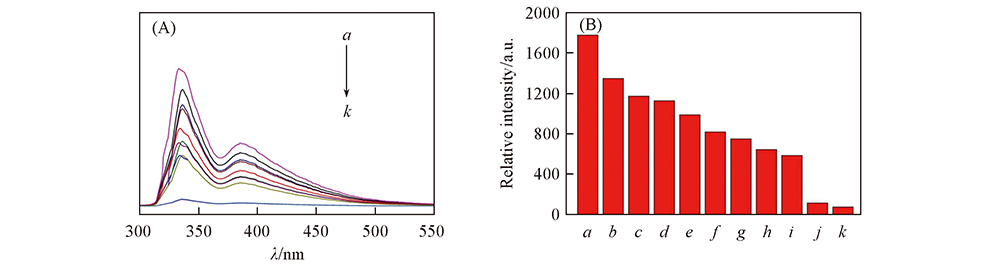
Fig.5 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in aqueous solution containing different antibiotics a. Thiamphenicol; b. H2O; c. amoxicillin; d. sulfathiazole; e. sulfadiazine; f. roxithromycin; g. azithromycin; h. penicillin; i. sulfadimidine; j. ornidazole; k. metronidazole.
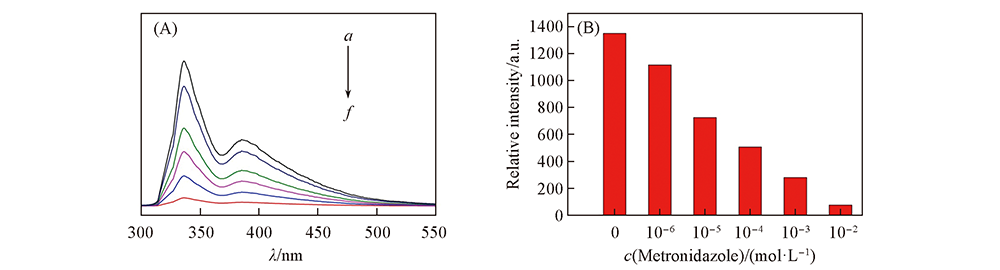
Fig.6 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in different concentrations of metronidazole solution c(Metronidazole)/(mol·L-1): a. 0; b. 10-6; c. 10-5; d. 10-4; e. 10-3; f. 10-2.
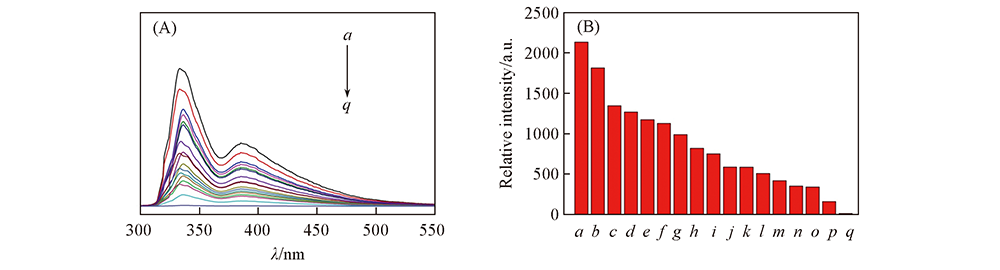
Fig.7 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in solution containing different metal ions a. Pb2+; b. Al3+; c. Cu2+; d. Na+; e. Ba2+; f. Ni2+; g. K+; h. Ag+; i. Mg2+; j. H2O; k. Li2+; l. Cd2+; m. Co2+; n. Ca2+; o. Zn2+; p. Cr3+; q. Fe3+.
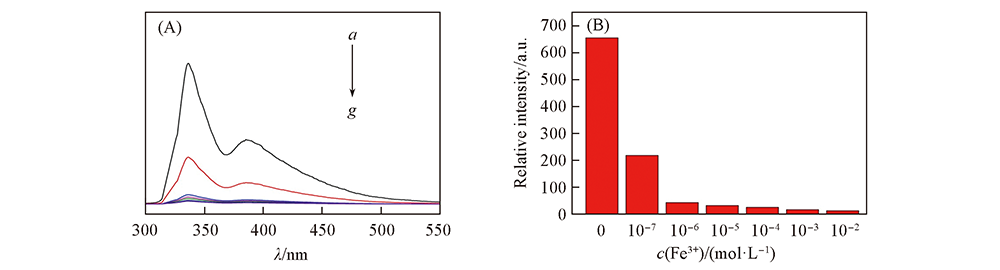
Fig.8 Emission spectra(A) and histogram of fluorescence intensity(B) of complex 1 in different concentrations of Fe3+ ions solution. c(Fe3+)/(mol·L-1): a. 0; b. 10-7; c. 10-6; d. 10-5; e. 10-4; f. 10-3; g. 10-2.
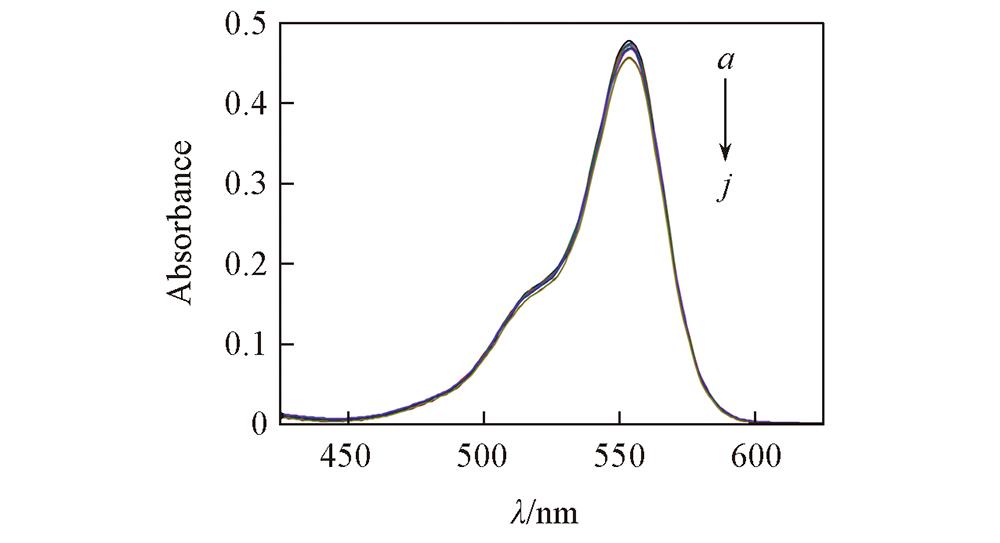
Fig.9 Time-dependent UV-Vis absorption spectra for degradation of RhB using complex 4 t/min: a. 0; b. 10; c. 20; d. 30; e. 40; f. 50; g. 60; h. 70; i. 80; j. 90.
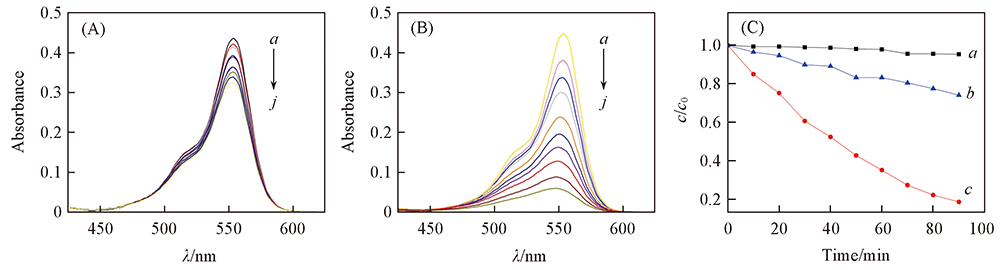
Fig.10 Time-dependent UV-Vis absorption spectra for degradation of RhB with H2O2(A), RhB with complex 4+H2O2(B) and the degradation rate of RhB under different conditions(C) (A), (B) t/min: a. 0; b. 10; c. 20; d. 30; e. 40; f. 50; g. 60; h. 70; i. 80; j. 90. (C) a. Complex 4+RhB; b. H2O2+RhB; c. complex 4+H2O2+RhB.
| [1] |
Zhou H. C., Long J. R., Yaghi O. M., Chem. Rev., 2012,112(2), 673— 674
doi: 10.1021/cr300014x URL |
| [2] |
Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M., Science, 2013,341(6149), 1230444
doi: 10.1126/science.1230444 URL |
| [3] |
Kurmoo M., Chem. Soc. Rev., 2009,38(5), 1353— 1379
doi: 10.1039/b804757j URL |
| [4] |
Pal A., Chand S., Boquera J. C., Lloret F., Lin J. B., Pal S. C., Das M. C., Inorg. Chem., 2019,58(9), 6246— 6256
doi: 10.1021/acs.inorgchem.9b00471 URL |
| [5] |
Zhang Y. M., Yuan S., Day G., Wang X., Yang X. Y., Zhou H. C., Coord. Chem. Rev., 2018,354, 28— 45
doi: 10.1016/j.ccr.2017.06.007 URL |
| [6] | Wu S., Min H., Shi W., Cheng P., Adv. Mater., 2019,e1805871 |
| [7] |
Mason J. A., Oktawiec J., Taylor M. K., Hudson M. R., Rodriguez J., Bachman J. E., Gonzalez M. I., Cervellino A., Guagliardi A., Brown C. M., Llewellyn P. L., Masciocchi N., Long J. R., Nature, 2015,527(7578), 357— 361
doi: 10.1038/nature15732 URL |
| [8] | Li B., Wen H. M., Yu Y., Cui Y., Zhou W., Chen B., Qian G., Mater. Today Nano, 2018,2, 21— 49 |
| [9] | Jiao L., Wang Y., Jiang H. L., Xu Q., Adv. Mater., 2018,30(37), e1703663 |
| [10] |
Dhakshinamoorthy A., Li Z. H., Garcia H., Chem. Soc. Rev., 2018,47(22), 8134— 8172
doi: 10.1039/C8CS00256H URL |
| [11] |
Zhang P. F., Yang G. P., Li G. P., Yang F., Liu W. N., Li J. Y., Wang Y. Y., Inorg. Chem., 2019,58(20), 13969— 13978
doi: 10.1021/acs.inorgchem.9b01954 URL |
| [12] |
Wang W., Gong N., Yin H., Zhang B., Guo P., Liu B., Wang Y. Y., Inorg. Chem., 2019,58(15), 10295— 10303
doi: 10.1021/acs.inorgchem.9b01465 URL |
| [13] |
Ebrahim F. M., Nguyen T. N., Shyshkanov S., Gladysiak A., Favre P., Zacharia A., Itskos G., Dyson P. J., Stylianou K. C., J. Am. Chem. Soc., 2019,141(7), 3052— 3058
doi: 10.1021/jacs.8b11907 URL |
| [14] |
Guo F., Inorg. Chem. Commun., 2019,102, 108— 112
doi: 10.1016/j.inoche.2019.02.026 URL |
| [15] | Li Y., Wei Z., Zhang Y., Guo Z., Chen D., Jia P., Chen P., Xing H ., ACS Sustainable Chemistry & Engineering, 2019,7(6), 6196— 6203 |
| [16] |
Tan L., Fan T., Xia T., Cui Y., Yang Y., Qian G ., J. Solid State Chem., 2019,272, 55— 61
doi: 10.1016/j.jssc.2019.01.027 URL |
| [17] |
Zhu X. D., Zhang K., Wang Y., Long W. W., Sa R. J., Liu T. F., Lu J., Inorg. Chem., 2018,57(3), 1060— 1065
doi: 10.1021/acs.inorgchem.7b02471 URL |
| [18] | Li Z., Li R., Li X., Chem. J. Chinese Universities, 2018,39(11), 2363— 2371 |
| ( 李铮, 李睿, 李夏 . 高等学校化学学报, 2018,39(11), 2363— 2371) | |
| [19] |
Xiao J., Liu J., Gao X., Ji G., Wang D., Liu Z ., Sens. Actuators B:Chem., 2018,269, 164— 172
doi: 10.1016/j.snb.2018.04.129 URL |
| [20] |
Yan X. L., Ma D. Y., Inorg. Chem. Commun., 2019,104, 31— 35
doi: 10.1016/j.inoche.2019.03.025 URL |
| [21] |
Sun Y. Q., Zhong J. C., Ding L., Chen Y. P., Dalton Trans., 2015,44(26), 11852— 11859
doi: 10.1039/C5DT01454A URL |
| [22] |
Zhang Y. Q., Blatov V. A., Lv X. X., Tang D. Y., Qian L. L., Li K., Li B. L., Acta Crystallogr. C: Struct. Chem., 2019,75(7), 960— 968
doi: 10.1107/S205322961900826X URL |
| [23] | Wang C., Liu X. M., Zhang M., Geng Y., Zhao L., Li Y. G., Su Z. M., ACS Sustainable Chemistry & Engineering, 2019,7(16), 14102— 14110 |
| [24] |
Munoz M., Garcia-Erce J. A., Remacha A. F., J. Clin. Pathol., 2011,64(4), 281— 286
doi: 10.1136/jcp.2010.079046 URL |
| [25] |
Zhang Q. Q., Ying G. G., Pan C. G., Liu Y. S., Zhao J. L., Environmental Science & Technology, 2015,49(11), 6772— 6782
doi: 10.1021/acs.est.5b00729 URL |
| [26] |
Liu W. N., Tong W. Q., Ma L. L., Wang Y., Wang J. M., Hou L., Wang Y. Y., Dalton Trans., 2019,48(22), 7786— 7793
doi: 10.1039/C9DT00933G URL |
| [27] |
Du Y., Yang H. Y., Shao C. Y., Liu J. W., Yan Y. T., Yu L. X., Zhu D. Q., Huang C. N., Yang L. R., J. Solid State Chem., 2019,277, 564— 574
doi: 10.1016/j.jssc.2019.07.012 URL |
| [28] |
Zhou Y., Yang Q., Zhang D., Gan N., Li Q., Cuan J ., Sens. Actuators B: Chem., 2018,262, 137— 143
doi: 10.1016/j.snb.2018.01.218 URL |
| [29] |
Wei F. H., Chen D., Liang Z., Zhao S. Q., Luo Y., RSC Adv., 2017,7(73), 46520— 46528
doi: 10.1039/C7RA09243A URL |
| [30] |
Zheng T. R., Qian L. L., Li M., Wang Z. X., Li K., Zhang Y. Q., Li B. L., Wu B., Dalton Trans., 2018,47(27), 9103— 9113
doi: 10.1039/C8DT01685B URL |
| [31] |
Xu Q. W., Wang Q. S., Li S. S., Li X., RSC Adv., 2019,9(29), 16305— 16312
doi: 10.1039/C9RA01496A URL |
| [32] | Sheldrick G. M., SHELXS-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, 1997 |
| [33] | Sheldrick G. M., SHELXL-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, 1997 |
| [34] | Hou B. W., Li K., Chinese J. Inorg. Chem., 2017,33(6), 1007— 1014 |
| ( 候不唯, 李恺. 无机化学学报, 2017,33(6), 1007— 1014) |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [3] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [4] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [5] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [6] | QIU Liqi, YAO Xiangyang, HE Liangnian. Visible-light-driven Selective Reduction of Carbon Dioxide Catalyzed by Earth-abundant Metalloporphyrin Complexes [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220064. |
| [7] | LIU Qingqing, WANG Pu, WANG Yongshuai, ZHAO Man, DONG Huanli. Synthesis and Topochemical Polymerization Study of Naphthalene/perylene Imides Substituted Diacetylene Derivatives [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220091. |
| [8] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| [9] | SHI Naike, ZHANG Ya, SANSON Andrea, WANG Lei, CHEN Jun. Uniaxial Negative Thermal Expansion and Mechanism in Zn(NCN) [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220124. |
| [10] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [11] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [12] | TAO Yu, OU Honghui, LEI Yongpeng, XIONG Yu. Research Progress of Single-atom Catalysts in Photocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220143. |
| [13] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [14] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [15] | MENG Xiangyu, ZHAN Qi, WU Yanan, MA Xiaoshuang, JIANG Jingyi, SUN Yueming, DAI Yunqian. Photothermal Enhanced Photocatalytic Hydrogenation Performance of Au/RGO/Na2Ti3O7 [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210655. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||