

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (2): 372.doi: 10.7503/cjcu20180500
• Polymer Chemistry • Previous Articles Next Articles
ZHANG Xinmu, CUI Xiangxu, YAOMA Kangyue, LI Tingting, ZHANG Zhiming*( )
)
Received:2018-07-14
Online:2019-02-10
Published:2018-11-16
Contact:
ZHANG Zhiming
E-mail:zhangzhiming1942@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Xinmu,CUI Xiangxu,YAOMA Kangyue,LI Tingting,ZHANG Zhiming. Electrospinning Preparation and Photocatalytic Activity of H4SiW12O40/Ethylene Vinyl Alcohol Copolymer Nanofibrous Membrane†[J]. Chem. J. Chinese Universities, 2019, 40(2): 372.
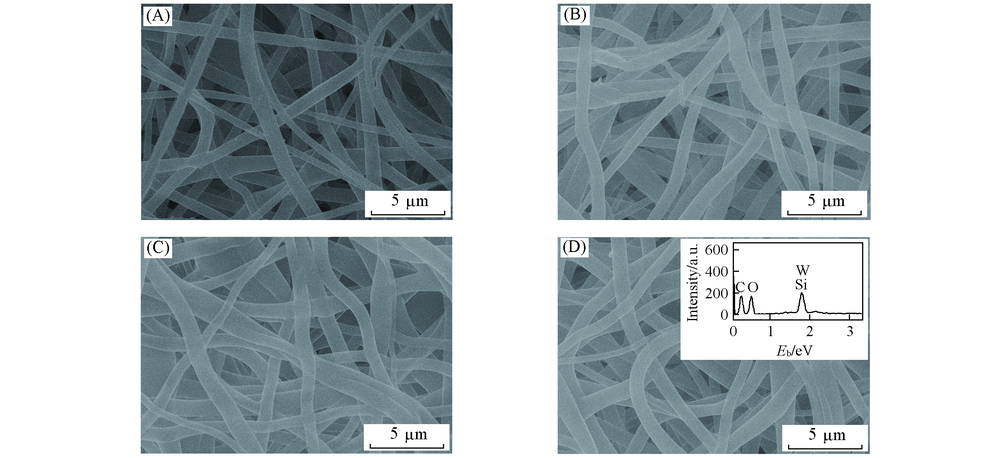
Fig.1 SEM images of SiW12/EVOH fibers with different mass ratios of EVOH to SiW12m(EVOH)/m(SiW12): (A) 1:0; (B) 4:1; (C) 3:1; (D) 2:1.Inset of (D) is corresponding EDX spectrum.
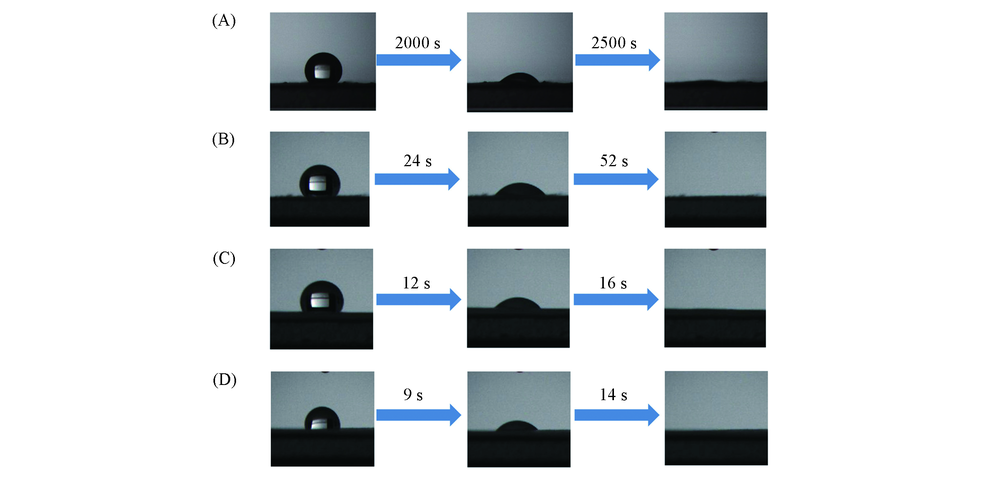
Fig.3 Dynamic measurements of water permeation on the surface of EVOH and SiW12/EVOH nanofibrous membranes with different mass ratios of EVOH to SiW12m(EVOH)/m(SiW12): (A) 1:0; (B) 4:1; (C) 3:1; (D) 2:1.
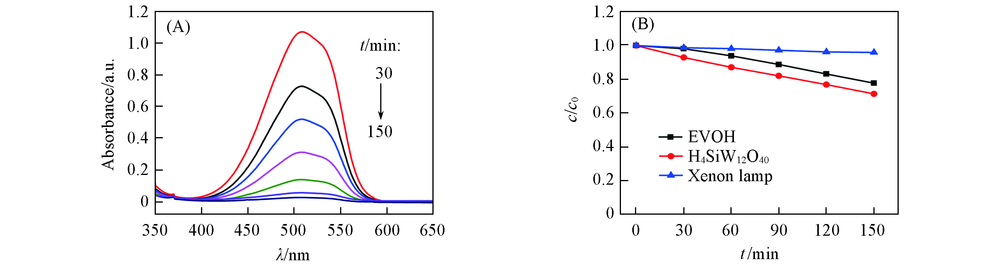
Fig.5 UV-Vis spectra of MO vs. photoreaction time catalyzed by SiW12/EVOH nanofibrous membrane under xenon lamp irradiation(A) and the blank controlled experiments(B)
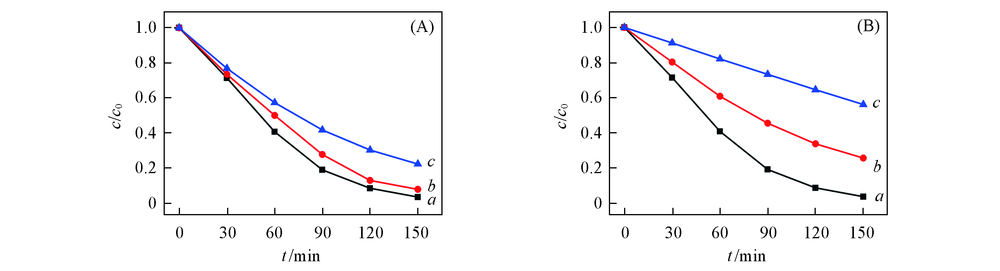
Fig.6 Photodegradation of MO over SiW12/EVOH nanofibrous membranes with different mass ratio of EVOH to SiW12(A) and effect of pH on photocatalytic activity of SiW12/EVOH nanofibrous membrane(B)(A) m(EVOH)/m(SiW12): a. 2:1; b. 3:1; c. 4:1. (B) pH: a. 1; b. 3; c. 5.
| Catalyst | Catalytic condition | MO degradation(%) | Ref. |
|---|---|---|---|
| Ag/TiO2 film | [TiO2]=120 mg/L, [AgNO3]=10-3 mol/L, initial | 90 | [ |
| pH=9.2, after 1 h illumination | |||
| Sulfate-modified titania(S | [Catalyst]=1.0 g/L, [S | 61 | [ |
| PSt-grafted ZnO nanoparticles | [Catalyst]=1.5 g/L, pH=7, 30 ℃, 5 h | 83 | [ |
| ZnFe2O4/TiO2 photocatalysts | [TiO2]=5 g/L, [ZnFe2O4]=1.5%, 4 h | 84 | [ |
| Natural rutile sample containing substituting | [Rutile]=1 g/L, [H2O2]=3.8 mmol/L, [V2O5]= | 61 | [ |
| metal ions as V5+ and Fe3+ | 12.2 mg/mL, [FeO]=3.9 mg/mL, pH=3, 1 h | ||
| SiW12/EVOH composite nanofibrous membrane | [H4SiW12O40]=0.5 g/L, pH=1, 2.5 h | 96.3 | This work |
Table 1 Comparison of photocatalytic efficiency of different catalysts for the degradation of MO
| Catalyst | Catalytic condition | MO degradation(%) | Ref. |
|---|---|---|---|
| Ag/TiO2 film | [TiO2]=120 mg/L, [AgNO3]=10-3 mol/L, initial | 90 | [ |
| pH=9.2, after 1 h illumination | |||
| Sulfate-modified titania(S | [Catalyst]=1.0 g/L, [S | 61 | [ |
| PSt-grafted ZnO nanoparticles | [Catalyst]=1.5 g/L, pH=7, 30 ℃, 5 h | 83 | [ |
| ZnFe2O4/TiO2 photocatalysts | [TiO2]=5 g/L, [ZnFe2O4]=1.5%, 4 h | 84 | [ |
| Natural rutile sample containing substituting | [Rutile]=1 g/L, [H2O2]=3.8 mmol/L, [V2O5]= | 61 | [ |
| metal ions as V5+ and Fe3+ | 12.2 mg/mL, [FeO]=3.9 mg/mL, pH=3, 1 h | ||
| SiW12/EVOH composite nanofibrous membrane | [H4SiW12O40]=0.5 g/L, pH=1, 2.5 h | 96.3 | This work |
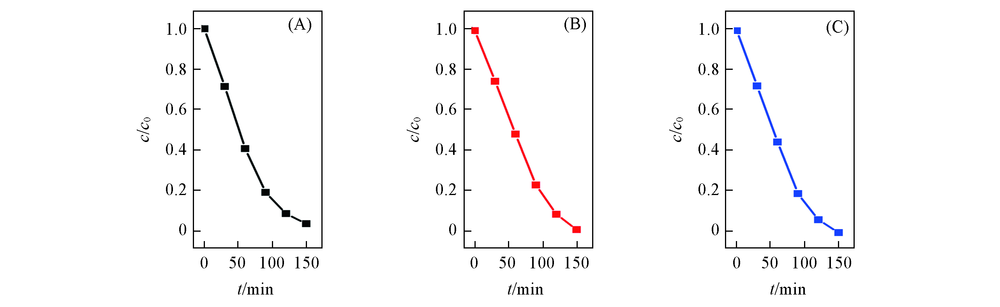
Fig.7 Photocatalytic activity of SiW12/EVOH nanofibrous membrane for MO degradation with three times of cycling useTimes of cycling use: (A) 1; (B) 2; (C) 3.
| [1] | Petrie B., Barden R., Kasprzyk-Hordern B., Water Res.,2015, 72, 3-27 |
| [2] | Zhu Z., Lu Z., Wang D., Tang X., Yan Y., Shi W., Wang Y., Gao N., Yao X., Dong H., Appl. Catal. B: Environ.,2016, 182, 115-122 |
| [3] | Upadhyay R. K., Soin N., Roy S. S., RSC Adv.,2014, 4, 3823-3851 |
| [4] | Wang Y., Lu K., Feng C., J. Rare Earth,2013, 31(4), 360-365 |
| [5] | Bafana A., Devi S. S., Chakrabarti T., Environ. Rev.,2011, 19(NA), 350-371 |
| [6] | Li W., Li T., Ma X., Li Y., An L., Zhang Z., RSC Adv.,2016, 6(15), 12491-12496 |
| [7] | Habiba U., Islam M. S., Siddique T. A., Afifi A. M., Ang B. C., Carbohyd. Polym.,2016, 149, 317-331 |
| [8] | Wang H., Yuan X., Wu Y., Huang H., Peng X., Zeng G., Zhong H., Liang J., Ren M., Adv. Colloid Interfac.,2013, 195/196, 19-40 |
| [9] | Wang H., Yuan X., Wu Y., Zeng G., Dong H., Chen X., Leng L., Wu Z., Peng L., Appl. Catal. B: Environ.,2016, 186, 19-29 |
| [10] | Yao T., Guo S., Zeng C., Wang C., Zhang L., J. Hazard. Mater.,2015, 292, 90-97 |
| [11] | Yue L., Wang S., Shan G., Wu W., Qiang L., Zhu L., Appl. Catal. B: Environ.,2015, 176/177, 11-19 |
| [12] | Zhao G., Wu X., Tan X., Wang X., Open Colloid Sci. J.,2010, 4(1), 19-31 |
| [13] | Zhang N., Liu S., Xu Y. J., Nanoscale,2012, 4(7), 2227-2238 |
| [14] | Leal Marchena C., Lerici L., Renzini S., Pierella L., Pizzio L., Appl. Catal. B: Environ.,2016, 188, 23-30 |
| [15] | Hong B., Liu L., Wang S. M., Han Z. B., J. Clust. Sci.,2016, 27(2), 563-571 |
| [16] | Pruethiarenun K., Isobe T., Matsushita S., Nakajima A., Appl. Catal. A: Gen.,2012, 445/446(2), 274-279 |
| [17] | Corma A., García H., Llabrési Xamena F. X., Chem. Rev.,2010, 110(8), 4606-4655 |
| [18] | Abdal-hay A., Mousa H. M., Khan A., Vanegas P., Lim J. H., Colloid. Surfaces A,2014, 457, 275-281 |
| [19] | Ner Y., Asemota C., Olson J. R., Sotzing G. A., ACS Appl. Mater. Inter.,2009, 1(10), 2093-2097 |
| [20] | Sui C., Li C., Guo X., Cheng T., Gao Y., Zhou G., Gong J., Du J., Appl. Surf. Sci.,2012, 258, 7105-7111 |
| [21] | Li T., Zhang Z., Li W., Liu C., Zhou H., An L., J. Appl. Polym. Sci.,2016, 133(11), 43193 |
| [22] | Fu Q., Wang X., Si Y., Liu L., Yu J., Ding B., ACS Appl. Mater. Inter.,2016, 8(18), 11819-11829 |
| [23] | Hori H., Takano Y., Koike K., Takeuchi K., Einaga H., Environ. Sci. Technol.,2003, 37(2), 418-422 |
| [24] | Troupis A., Triantis T. M., Gkika E., Hiskia A., Papaconstantinou E., Appl. Catal. B: Environ.,2009, 86(1/2), 98-107 |
| [25] | Arabatzis I., Stergiopoulos T., Bernard M., Labou D., Neophytides S., Falaras P., Appl. Catal. B: Environ.,2003, 42, 187-201 |
| [26] | Parida K., Sahu N., Biswal N., Naik B., Pradhan A., J. Colloid Interf. Sci.,2008, 318, 231-237 |
| [27] | Hong R., Li J., Chen L., Liu D., Li H., Zheng Y., Ding J., Powder Technol.,2009, 189, 426-432 |
| [28] | Cheng P., Deng C., Gu M., Shangguan W., J. Mater. Sci.,2007, 42, 9239-9244 |
| [29] | Lu A., Li Y., Lv M., Wang C., Yang L., Liu J., Wang Y., Wong K., Wong P., Sol. Energ. Mat. Sol. C,2007, 91, 1849-1855 |
| [30] | Zhang P., Shao C., Li X., Zhang M., Zhang X., Sun Y., Liu Y., J. Hazard. Mater.,2012, 237, 331-338 |
| [31] | Troupis A., Gkika E., Triantis T., Hiskia A., Papaconstantinou E., J. Photoch. Photobio. A,2007, 188(2/3), 272-278 |
| [1] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [2] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [3] | WU Yu, LI Xuan, YANG Hengpan, HE Chuanxin. Construction of Cobalt Single Atoms via Double-confinement Strategy for High-performance Electrocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220343. |
| [4] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [5] | ZHAO Yingzhe, ZHANG Jianling. Applications of Metal-organic Framework-based Material in Carbon Dioxide Photocatalytic Conversion [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220223. |
| [6] | QIU Liqi, YAO Xiangyang, HE Liangnian. Visible-light-driven Selective Reduction of Carbon Dioxide Catalyzed by Earth-abundant Metalloporphyrin Complexes [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220064. |
| [7] | XIA Wu, REN Yingyi, LIU Jing, WANG Feng. Chitosan Encapsulated CdSe QDs Assemblies for Visible Light-induced CO2 Reduction in an Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220192. |
| [8] | WANG Guangqi, BI Yiyang, WANG Jiabo, SHI Hongfei, LIU Qun, ZHANG Yu. Heterostructure Construction of Noble-metal-free Ternary Composite Ni(PO3)2-Ni2P/CdS NPs and Its Visible Light Efficient Catalytic Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220050. |
| [9] | TAO Yu, OU Honghui, LEI Yongpeng, XIONG Yu. Research Progress of Single-atom Catalysts in Photocatalytic Reduction of Carbon Dioxide [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220143. |
| [10] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [11] | MENG Xiangyu, ZHAN Qi, WU Yanan, MA Xiaoshuang, JIANG Jingyi, SUN Yueming, DAI Yunqian. Photothermal Enhanced Photocatalytic Hydrogenation Performance of Au/RGO/Na2Ti3O7 [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210655. |
| [12] | GUO Biao, ZHAO Chencan, LIU Xinxin, YU Zhou, ZHOU Lijing, YUAN Hongming, ZHAO Zhen. Effects of Surface Hydrothermal Carbon Layer on the Photocatalytic Activity of Magnetic NiFe2O4 Octahedron [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220472. |
| [13] | LI Chenchen, NA Yong. g-C3N4/CdS/Ni Composite as a Bifunctional Photocatalyst for H2 Generation and 5-Hydroxymethylfurfural Oxidation [J]. Chem. J. Chinese Universities, 2021, 42(9): 2896. |
| [14] | WANG Peng, YANG Min, TANG Sengpei, CHEN Feitai, LI Youji. Preparation of Cellular C3N4/CoSe2/GA Composite Photocatalyst and Its CO2 Reduction Activity [J]. Chem. J. Chinese Universities, 2021, 42(6): 1924. |
| [15] | YANG Sixian, ZHONG Wenyu, LI Chaoxian, SU Qiuyao, XU Bingjia, HE Guping, SUN Fengqiang. Photochemical Fabrication and Performance of Polyaniline Nanowire/SnO2 Composite Photocatalyst [J]. Chem. J. Chinese Universities, 2021, 42(6): 1942. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||