

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (2): 366.doi: 10.7503/cjcu20180526
• Polymer Chemistry • Previous Articles Next Articles
ZHANG Xiaotao, WANG Yan’an, HUI Jia, SHI Yan*( ), FU Zhifeng, YANG Wantai
), FU Zhifeng, YANG Wantai
Received:2018-07-24
Online:2019-02-10
Published:2018-11-28
Contact:
SHI Yan
E-mail:Lshiyan@mail.buct.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Xiaotao,WANG Yan’an,HUI Jia,SHI Yan,FU Zhifeng,YANG Wantai. Reversible-deactivation Radical Solution Polymerization of Methyl Methacrylate Catalyzed by Tetrabutylammonium Iodide†[J]. Chem. J. Chinese Universities, 2019, 40(2): 366.
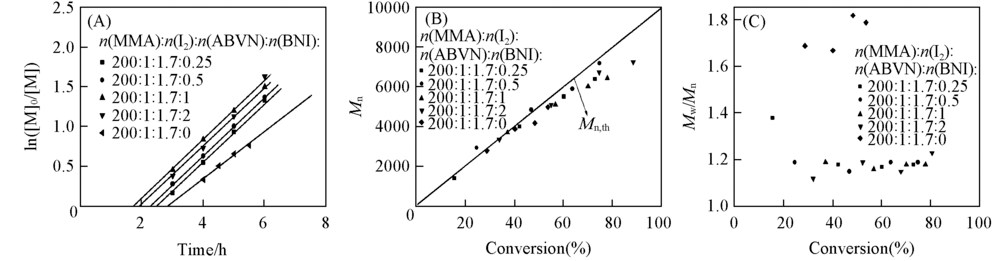
Fig.1 Plots of ln([M]0/[M]) vs. time for the MMA/I2/ABVN/BNI systems(in toluene) with different BNI concentrations(A), number-average molecular weight(B) and molecular weight distribution vs. conversion for RCMP with different BNI concentrations in toluene(C)Polymerization conditions: n(MMA):n(I2):n(ABVN)=200:1:1.7; temperature: 65 ℃; Mn,th=MA-I + 2[M]0/ [I2]0 × MMMA × conversion.
| n(MMA):n(I2):n(ABVN):n(BNI) | Time/h | Conversion(%) | Mn(Mn,th) | Mw/Mn |
|---|---|---|---|---|
| 200:1:1.7:0.25 | 3 | 15.4 | 1400(1800) | 1.38 |
| 4 | 42.2 | 4000(4500) | 1.18 | |
| 5 | 60.1 | 5500(6200) | 1.17 | |
| 6 | 69.2 | 6400(7000) | 1.18 | |
| 200:1:1.7:0.5 | 3 | 24.6 | 3000(2700) | 1.18 |
| 4 | 46.8 | 4900(4900) | 1.15 | |
| 5 | 63.6 | 5900(6500) | 1.19 | |
| 6 | 74.6 | 6700(7600) | 1.19 | |
| 200:1:1.7:1 | 3 | 37.1 | 3700(3900) | 1.19 |
| 4 | 53.9 | 5100(5600) | 1.14 | |
| 5 | 63.7 | 6000(6600) | 1.19 | |
| 6 | 70.8 | 6500(7300) | 1.21 | |
| 200:1:1.7:2 | 3 | 32.1 | 3400(3400) | 1.12 |
| 4 | 52.2 | 5100(5400) | 1.18 | |
| 5 | 67.8 | 6800(7000) | 1.23 | |
| 6 | 76.4 | 7300(7900) | 1.15 | |
| 200:1:1.7:0 | 3.5 | 28.7 | 2800(3100) | 1.60 |
| 4 | 40.1 | 3700(4200) | 1.67 | |
| 4.5 | 48.3 | 4200(5000) | 1.82 | |
| 5 | 53.6 | 5000(5600) | 1.79 | |
| 5.5 | 59.7 | 5400(6200) | 1.84 |
Table 1 Monomer conversion, Mn, Mn,th and Mw/Mn of PMMA with different BNI concentrations in toluene(at 65 ℃)
| n(MMA):n(I2):n(ABVN):n(BNI) | Time/h | Conversion(%) | Mn(Mn,th) | Mw/Mn |
|---|---|---|---|---|
| 200:1:1.7:0.25 | 3 | 15.4 | 1400(1800) | 1.38 |
| 4 | 42.2 | 4000(4500) | 1.18 | |
| 5 | 60.1 | 5500(6200) | 1.17 | |
| 6 | 69.2 | 6400(7000) | 1.18 | |
| 200:1:1.7:0.5 | 3 | 24.6 | 3000(2700) | 1.18 |
| 4 | 46.8 | 4900(4900) | 1.15 | |
| 5 | 63.6 | 5900(6500) | 1.19 | |
| 6 | 74.6 | 6700(7600) | 1.19 | |
| 200:1:1.7:1 | 3 | 37.1 | 3700(3900) | 1.19 |
| 4 | 53.9 | 5100(5600) | 1.14 | |
| 5 | 63.7 | 6000(6600) | 1.19 | |
| 6 | 70.8 | 6500(7300) | 1.21 | |
| 200:1:1.7:2 | 3 | 32.1 | 3400(3400) | 1.12 |
| 4 | 52.2 | 5100(5400) | 1.18 | |
| 5 | 67.8 | 6800(7000) | 1.23 | |
| 6 | 76.4 | 7300(7900) | 1.15 | |
| 200:1:1.7:0 | 3.5 | 28.7 | 2800(3100) | 1.60 |
| 4 | 40.1 | 3700(4200) | 1.67 | |
| 4.5 | 48.3 | 4200(5000) | 1.82 | |
| 5 | 53.6 | 5000(5600) | 1.79 | |
| 5.5 | 59.7 | 5400(6200) | 1.84 |
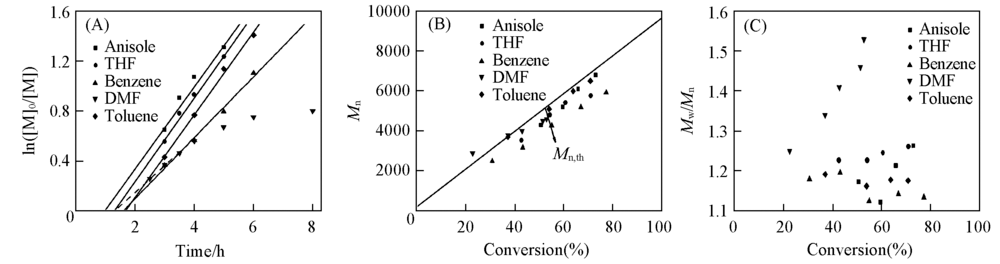
Fig.2 Plots of ln([M]0/[M]) vs. time for the RCMP of MMA in different solvents(A), Mn,GPC(B)and Mw/Mn vs. monomer conversion in various solvents(C)Polymerization conditions: n(MMA):n(I2):n(ABVN):n(BNI)=200:1:1.7:1; temperature: 65 ℃. Mn,th=MA-I + 2[M]0/ [I2]0 × MMMA × conversion.
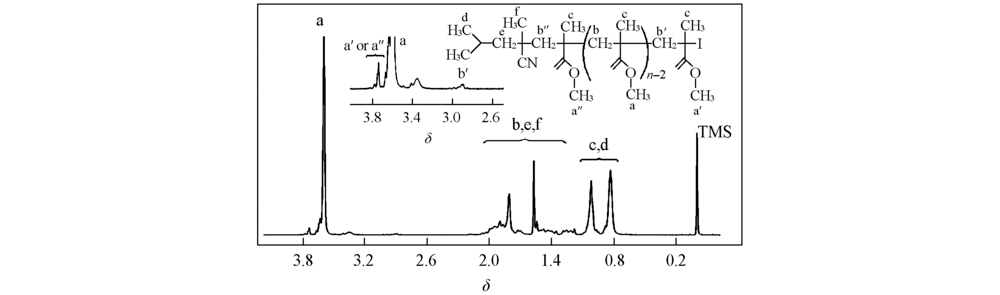
Fig.4 1H NMR spectrum of the PMMA sample(in CDCl3) synthesized by solution polymerization(50% toluene) with n(MMA):n(I2):n(ABVN):n(BNI)=200:1:1.7:1 at 65 ℃ for 3.0 h
| [1] | Otsu T., Yoshida M., Macromolecular Rapid Communications,1982, 26(3), 127-132 |
| [2] | Georges M. K., Veregin R. P. N., Kazmaier P. M., Hamer G. K., Macromolecules,1993, 26(11), 2987-2988 |
| [3] | Wang J. S., Matyjaszewski K., Journal of the American Chemical Society,1995, 117(20), 5614-5615 |
| [4] | Chiefari J., Chong Y. K., Ercole F., Krstina J., Jeffery J., Le T. P. T., Mayadunne R. T. A., Meijs G. F., Moad C. L., Moad G., Rizzardo E., Thang S. H., Macromolecules,1998, 31(16), 5559-5562 |
| [5] | Joseph R., Pallan P S., Sudalai A., Ravindranathan T., Tetrahedron Letters,1995, 36(4), 609-612 |
| [6] | Goto A., Fukuda T., Ohno K., Macromolecules,1998, 31(9), 2809-2814 |
| [7] | Clerc S., Tonnar J., Lacroix-Desmazes P., European Polymer Journal,2013, 49(3), 682-692 |
| [8] | Boyer C., Lacroix-Desmazes P., Jeanjacques R. A., Boutevin B., Macromolecules,2006, 39(12), 4044-4053 |
| [9] | Kim K., Na R. K., Rhee S. E., Lee B. H., Choe S., Polymer,2012, 53(19), 4054-4059 |
| [10] | Goto A., Zushi H., Hirai N., Kwak Y., Fukuda T., ACS Symposium Series,2006, 944(40), 595-603 |
| [11] | Goto A., Wakada T., Fukuda T., Tsujii Y., Macromolecular Chemistry & Physics,2010, 211(5), 594-600 |
| [12] | Goto A., Zushi H., Hirai N., Wakada T., Kwak Y., Fukuda T., Macromolecular Symposia,2007, 248(1), 126-131 |
| [13] | Goto A., Hirai N., Tsujii Y., Fukuda T., Macromolecular Symposia,2008, 261(1), 18-22 |
| [14] | Goto A., Hirai N., Tsujii Y., Fukuda T., Polymer,2008, 49(24), 5177-5185 |
| [15] | Goto A., Hirai N., Wakada T., Nagasawa K., Tsujii Y., Fukuda T., Macromolecules,2008, 41(17), 6261-6264 |
| [16] | Kim J., Nomura A., Fukuda T., Goto A., Tsujii Y., Macromolecular Reaction Engineering,2010, 4(3/4), 272-277 |
| [17] | Wang C. G., Hanindita F., Goto A., ACS Macro Letters,2018, 7(2), 263-268 |
| [18] | Goto A., Sanada S., Lei L., Hori K., Macromolecules,2016, 49(7), 2511-2517 |
| [19] | Wang C. G., Goto A., Journal of the American Chemistry Society,2017, 139(30), 10551-10560 |
| [20] | Sarkar J., Xiao L., Goto A., Macromolecules,2016, 49(14), 5033-5042 |
| [21] | Lei L., Tanishima M., Goto A., Hironori K., Multidisciplinary Digital Publishing Institute, 2014, 6(3), 860-872 |
| [22] | Goto A., Ohtsuki A., Ohfuji H., Tanishima M., Hironori K., Journal of the American Chemical Society,2013, 135(30), 11131-11139 |
| [23] | Lacroix-Desmazes P., Severac R., Boutevin B., Macromolecules,2005, 38(38), 6299-6309 |
| [24] | Wang Y. A., Shi Y., Fu Z. F., Yang W. T., Polymer Chemistry,2017, 8(39), 6073-6085 |
| [25] | Chen K.L., Hui J., Shi Y., Yang W. T., Fu Z. F., Acta Polymerica Sinica, 2016, (1), 111-117 |
| (陈珂龙, 惠嘉, 石艳, 杨万泰, 付志峰. 高分子学报, 2016, (1), 111-117) | |
| [26] | Braunecker W. A., Itami Y., Matyjaszewski K., Macromolecules,2005, 38(23), 9402-9404 |
| [27] | Bai L., Wang W., Chen H., Wang M., Cheng Z., RSC Advances,2015, 5(44), 34769-34776 |
| [1] | LI Lun, ZHANG Jingyan, LUO Jing, LIU Ren, ZHU Yi. Synthesis and Properties of UV/Vis-LED Excitable Photoinitiators Based on Coumarin Pyridinium Salt [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220178. |
| [2] | SUN Guangdong, PAN Xiaopeng, ZHONG Yuhao, CHEN Enyu, HUANG Yi, SHAO Jianzhong. Photopolymerization Kinetics of Poly(Acrylate Acid) Hydrogels Induced by Blue Light † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2404. |
| [3] | ZHAO Ruiyang,YU Chunyan,HAN Jishu,FU Yunlei,LI Ming,HU Dehua,LIU Fusheng. Preparation of Photo-responsive Film by Electrochemical Deposition Method and the Application in Optical Information Storage† [J]. Chem. J. Chinese Universities, 2019, 40(2): 358. |
| [4] | YANG Jiarui, WANG Yifu, WANG Jilin, WANG Lulu, FENG Ruijiang. In situ Initiation, Polymerization and Construction of Cationic Active Sites of Gemini Molecules in Polysulfone Substrates† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1829. |
| [5] | LI Juan, DU Fanfan, FENG Rui, HU Qian, JIE Suyun, LI Bogeng. Synthesis of Cyclic and Linear Block Copolyesters via Ring-opening Copolymerization of ε-Caprolatone and L-Lactide Catalyzed by Zinc Complex† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1297. |
| [6] | GUAN Mingming,CHEN Jia,TANG Shaokun. Synthesis and Performance of Novel Bis-functional Cross-linked Anion Exchange Membranes† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2054. |
| [7] | YANG Qinghua, WANG Longgang, LIU Jie, LU Yong, CHEN Tianyun. Preparation and Characterization of Star-shaped β-Cyclodextrin Based Polymer† [J]. Chem. J. Chinese Universities, 2018, 39(4): 793. |
| [8] | ZHANG Dingjun, BAI Xue, CHEN Yuxian, WU Yanfei, LI Xianwen, CHEN Zhenbin, MA Yingxia. Preparation and Performance of Magnetic Polymer-composite Microspheres Profile Control Agent by Multilayer Assembly† [J]. Chem. J. Chinese Universities, 2018, 39(3): 583. |
| [9] | WANG Yifu, DONG Jinxin, WANG Jilin, WANG Lulu, FENG Ruijiang. Ordered Self-assembly of Gemini Molecules in Mesoporous Silica Channels Constructing Normal Micelles to Assist the Migration of OH-† [J]. Chem. J. Chinese Universities, 2017, 38(1): 141. |
| [10] | JIANG Hongbo, GONG Chengbin, WANG Qiang, TANG Qian, MA Xuebing. Synthesis and Characterization of Novel Photo- and pH-Responsive Poly[8-1] [J]. Chem. J. Chinese Universities, 2014, 35(9): 2043. |
| [11] | GAO Xu, LU Ping, MA Yuguang. Ultrasound-assisted Synthesis and Characterization of Silicon Centered Porous Aromatic Frameworks† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1795. |
| [12] | HAN Ke-Fei, YANG Qi-Tao, YU Shu-Ping, YU Jing-Hua, ZHU Hong, WANG Zhong-Ming. Preparation and Characterization of Novel Poly(2, 6-dimethyl-1, 4-phenylene oxide) Anion-exchange Membranes for Alkaline Fuel Cells [J]. Chem. J. Chinese Universities, 2013, 34(10): 2437. |
| [13] | XU Yan-Yan, YU Shu-Ping, HAN Ke-Fei, YU Jing-Hua, ZHU Hong, WANG Zhong-Ming. Microwave-Assisted Synthesis of Novel Polybenzimidazole Resin and Performance of Its Proton Exchange Membranes [J]. Chem. J. Chinese Universities, 2013, 34(6): 1547. |
| [14] | HUANG Yao, ZHU Zhao-Jin, XU Jing-Kun, LU Bao-Yang, YUE Rui-Rui. Novel Copolymers Synthesis by Second Polymerization of Acrylate Acid Grafted 1,2-Dihydroxylbenzene Derivatives [J]. Chem. J. Chinese Universities, 2012, 33(03): 608. |
| [15] | XIE Mei-Ran*, MA Zhuo, HAN Hui-Jing, SHI Jia-Xin, WANG Wei-Zhen, LI Jin-Xin, ZHANG Yi-Qun. Synthesis of Pyridyl-based Ionic Liguid Supported Ruthenium Complex and Kinetics of Ring-opening Metathesis Polymerization in Ionic Liguid [J]. Chem. J. Chinese Universities, 2009, 30(2): 396. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||