

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (6): 1185.doi: 10.7503/cjcu20170819
• Organic Chemistry • Previous Articles Next Articles
LIU Yubo, ZHANG Nana, CHEN Jinjiao, ZHU Tong, ZHANG Jianing, LI Wenli*( )
)
Received:2017-12-15
Online:2018-06-10
Published:2018-05-22
Contact:
LI Wenli
E-mail:biolwl@dlut.edu.cn
Supported by:CLC Number:
TrendMD:
LIU Yubo, ZHANG Nana, CHEN Jinjiao, ZHU Tong, ZHANG Jianing, LI Wenli. Discovery and Activity Verification of a O-GlcNAc Transferase Inhibitor by Structure-based Virtual Screening†[J]. Chem. J. Chinese Universities, 2018, 39(6): 1185.
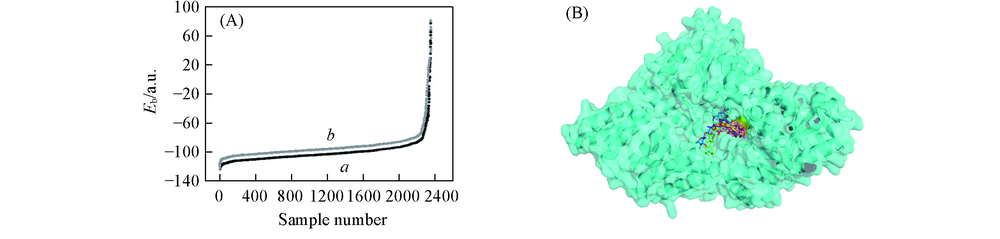
Fig.2 Identifcation of novel OGT inhibitors from a virtual screen(A) Eb distribution of the docking results about liginds, Lib-Dock(a) and C-Docker(b); (B) binding mode of ten hit compounds in human OGT by docking studies using Lib-Dock and C-Docker.
| No. | Compd. | Biological activity | Eb/(kJ·mol-1) | Hydrogen bond | |
|---|---|---|---|---|---|
| Lib-Dock | C-Docker | ||||
| 1 | Amentoflavone | Anti-inflammatory, anti-fungal | -146.7 | -137.5 | 7 |
| 2 | Robinin | Hydragogue | -130.5 | -124.3 | 3 |
| 3 | Griffipavixanthone | Cytotoxic | -127.5 | -115.6 | 3 |
| 4 | Dihydroxy-14-serraten-16-one | Cytotoxic | -126.3 | -116.1 | 2 |
| 5 | Daturametelin I | Anti-fungal, cytotoxic | -125.4 | -114.3 | 2 |
| 6 | Bisandrographolide A | Ion channelsagonist | -124.5 | -113.9 | 2 |
| 7 | Chloramultilide D | Anti-fungal, anti-inflammatory | -124.2 | -111.4 | 2 |
| 8 | Tetrahydroochnaflavone | Anti-fungal, anti-inflammatory | -123.1 | -110.9 | 2 |
| 9 | Pre-schisanartanin B | Cytotoxic | -122.9 | -111.2 | 1 |
| 10 | Isorhoifolin | Anti-fungal, anti-inflammatory, cytotoxic | -110.1 | -109.2 | 1 |
Table 1 Scoring results of virtual screening of candidate moleculars
| No. | Compd. | Biological activity | Eb/(kJ·mol-1) | Hydrogen bond | |
|---|---|---|---|---|---|
| Lib-Dock | C-Docker | ||||
| 1 | Amentoflavone | Anti-inflammatory, anti-fungal | -146.7 | -137.5 | 7 |
| 2 | Robinin | Hydragogue | -130.5 | -124.3 | 3 |
| 3 | Griffipavixanthone | Cytotoxic | -127.5 | -115.6 | 3 |
| 4 | Dihydroxy-14-serraten-16-one | Cytotoxic | -126.3 | -116.1 | 2 |
| 5 | Daturametelin I | Anti-fungal, cytotoxic | -125.4 | -114.3 | 2 |
| 6 | Bisandrographolide A | Ion channelsagonist | -124.5 | -113.9 | 2 |
| 7 | Chloramultilide D | Anti-fungal, anti-inflammatory | -124.2 | -111.4 | 2 |
| 8 | Tetrahydroochnaflavone | Anti-fungal, anti-inflammatory | -123.1 | -110.9 | 2 |
| 9 | Pre-schisanartanin B | Cytotoxic | -122.9 | -111.2 | 1 |
| 10 | Isorhoifolin | Anti-fungal, anti-inflammatory, cytotoxic | -110.1 | -109.2 | 1 |
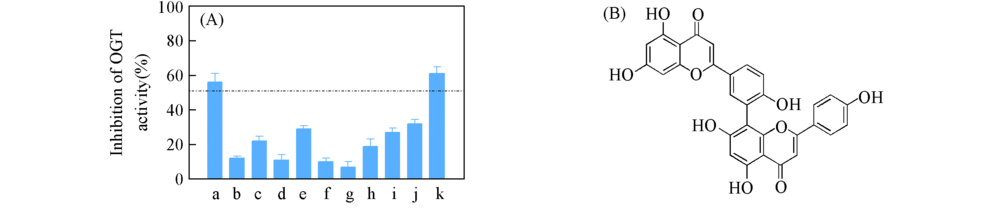
Fig.3 In vitro activity of candidate compounds(A) Effect of candidates on OGT activities in a cell free reaction system, all the compounds were used at 50 μmol/L. ST045849 was used as a positive control. a. Amentoflavone; b. robinin; c. griffipavixanthone; d. dihydroxy-14-serraten-16-one; e. daturametelin I; f. bisandrographolide A; g. chloramultilide D; h. tetrahydroochnaflavone; i. pre-schisanartanin B; j. isorhoifolin; k. ST045849. (B) the chemical structure of AF.
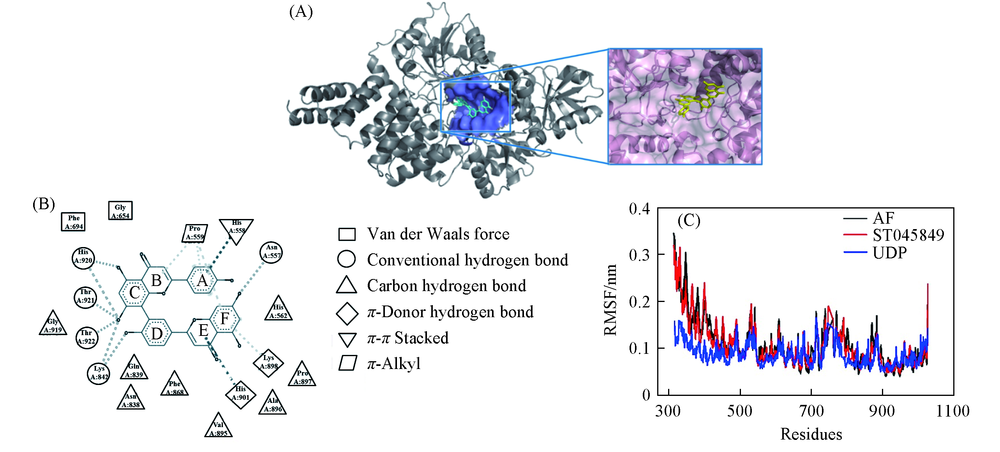
Fig.4 Binding mode analysis of AF and OGT by MD(A) Binding pose of AF with OGT after MD; the residues of binding pocket were shown by surface; AF interacts with residues in the UDP-binding pocket of OGT; (B) binding mode analysis of UDP-OGT; (C) the root mean square fluctuation(RMSF) of OGT over 20 ns MD simulation.
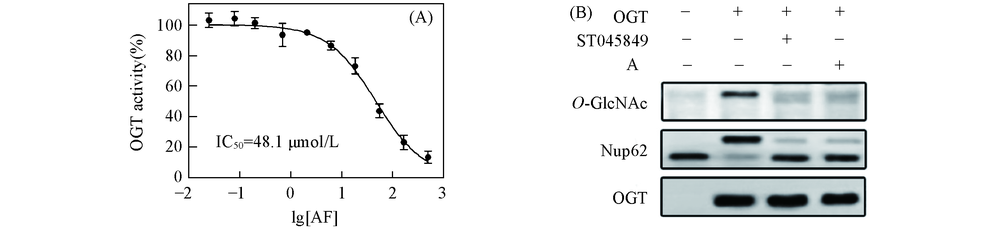
Fig.5 AF inhibits OGT in vitro(A) AF inhibited OGT activity in a dose-dependent manner; (B) AF(50 μmol/L) inhibited O-GlcNAc modification of recombinant Nup62 in a cell-free reaction system.
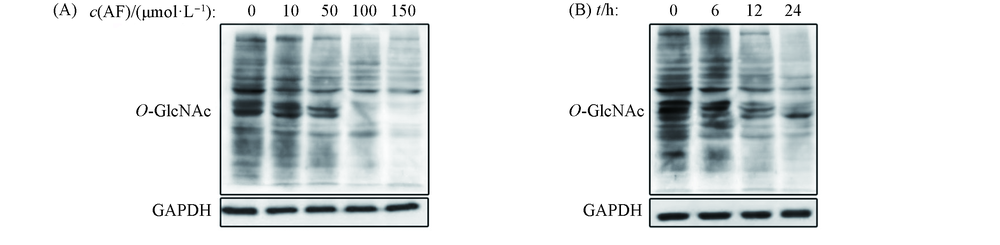
Fig.6 AF inhibites OGT in cells(A) Western blots of Cos7 cell lysates after AF treatment at different doses(0—150 μmol/L) for 24 h; (B) western blots of Cos7 cell lysates after 50 μmol/L AF treatment for 6—24 h.
| [1] | Moremen K. W., Tiemeyer M., Nairn A. V., Nature Reviews Molecular Cell Biology, 2012, 13(7), 448—462 |
| [2] | Wells L., Vosseller K., Hart G.W., Science, 2001, 291(5512), 2376—2378 |
| [3] | Torres C. R., Hart G. W., J. Biol. Chem., 1984, 259(5), 3308—3317 |
| [4] | Levine Z. G., Walker S., Ann. Rev. Biochem., 2016, 85, 631—657 |
| [5] | Zheng J. P., Liu H. T., Du Y. G., Acta Microbiologica Sinica, 2017, 57(8), 1141—1151 |
| (郑军平, 刘洪涛, 杜昱光. 微生物学报, 2017, 57(8), 1141—1151) | |
| [6] | Caldwell S. A., Jackson S. R., Shahriari K. S., Lynch T. P., Sethi G., Walker S., Vosseller K., Reginato M. J., Oncogene, 2010, 29(19), 2831—2842 |
| [7] | Brownlee M., Nature, 2001, 414(6865), 813—820 |
| [8] | Bond M. R., Hanover J. A., Ann. Rev. Nutrition, 2013, 33, 205—229 |
| [9] | Trapannone R., Rafie K., van Aalten D. M., Biochemical Society Transactions, 2016, 44(1), 88—93 |
| [10] | Wang Y., Zheng Q. C., Zhang J. L., Xie M., Zhan J. Y., Zhang H. X., Chem. Res. Chinese Universities, 2015, 31(6), 1029—1038 |
| [11] | Cheng S. Q., Liang G. D., Jiang X. F., Wang C., Liu K. L., Chem. J. Chinese Universities, 2016, 37(7), 1287—1292 |
| (程思绮, 梁国栋, 王潮, 刘克良. 高等学校化学学报, 2016, 37(7), 1287—1292) | |
| [12] | Ortiz R. F., Jiang J., Lazarus M. B., Orman M., Janetzko J., Fan C., Duveau D. Y., Tan Z. W., Thomas C. J., Walker S., ACS Chemical Biology, 2015, 10(6), 1392—1397 |
| [13] | Lazarus M. B., Nam Y., Jiang J., Sliz P., Walker S., Nature, 2011, 469(7331), 564—567 |
| [14] | Duan Y. B., Yin Y., Meng F. L., Zhao L. H., Liu Y. K., Yuan Z., Feng Y. B., Chem. J. Chinese Universities, 2017, 38(9), 1568—1577 |
| (段永斌, 殷燕, 孟凡丽, 赵连花, 刘玉坤, 袁哲, 冯阳波. 高等学校化学学报, 2017, 38(9), 1568—1577) | |
| [15] | Wang Q., He J., Wu D., Wang J., Yan J., Li H., Journal of Luminescence, 2015, 164, 81—85 |
| [16] | Totrov M., Abagyan R., Current Opinion in Structural Biology, 2008, 18(2), 178—184 |
| [17] | Xiong Z. J., Du P., Li B., Xu L. L., Zhen X. C., Fu W., Chem. Res. Chinese Universities, 2011, 27(4), 655—660 |
| [18] | Meraj K., Mahto M. K., Christina N. B., Desai N., Shahbazi S., Bhaskar M., Bioinformation, 2012, 8(23), 1139—1146 |
| [19] | Han Y., Zhu L. L., Zhang Y. Y.,Chem. Res. Chinese Universities, 2016, 32(4), 641—646 |
| [20] | Lee E., Shin S., Kim J. K., Woo E. R., Kim Y., Bulletin of the Korean Chemical Society, 2012, 33(9), 2878—2882 |
| [21] | Gloster T. M., Zandberg W. F., Heinonen J. E., Shen D. L., Deng L., Vocadlo D. J., Nature Chemical Biology, 2011, 7(3), 174—181 |
| [1] | HUAN Xinyu, LAI Ganqiang, HUANG Yue, YANG Caiguang. Research Progress on Chemical Intervention of N6-methyladenosine Modification [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220340. |
| [2] | YE Chenghao, LIANG Heng, LI Enmin, XU Liyan, LI Peng, CHEN Guanghui. High-throughput Virtual Screening of CDK2/Cyclin A2 Target Inhibitors [J]. Chem. J. Chinese Universities, 2021, 42(10): 3135. |
| [3] | YOU Yipeng, NIE Lin, LIU Jinbiao, FENG Yahui, LU Gui. Design, Synthesis and Anti-influenza Activities of Novel Neuraminidase Inhibitors† [J]. Chem. J. Chinese Universities, 2020, 41(10): 2279. |
| [4] | GAO Yang, LI Daixi, LIU Baolin, GUO Baisong, WEI Dongqing. Inhibitory Mechanism of Glycerol on the Growth of Ice Crystals by Molecular Dynamics† [J]. Chem. J. Chinese Universities, 2019, 40(4): 763. |
| [5] | ZHANG Juanrong,YOU Huimei,JING Yuxing,ZHAO Jiaowen,WANG Wei,LIU Wenxing,ZHOU Min,JIANG Zhiyong. Three New Phenolic Compounds from Salacia cochinchinensis Lour and Their α-Glucosidase Inhibitory Activities† [J]. Chem. J. Chinese Universities, 2019, 40(3): 456. |
| [6] | Qiuchen DONG,Guanghua ZHANG,Wanbin ZHANG,Xue ZHANG,Jing LIU. Corrosion Inhibition of Q235 Steel by Ionic Liquid Based on the 2-(Dimethylamino)ethyl Methacrylate † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2556. |
| [7] | Yanjie LI,Ensi WANG,Xiaowei SHAO,Xingmin ZHANG,Shengxiu NIU,Lijuan YANG,Yi WU. Synthesis and Biological Activity in vitro of Imidazo[4,5-c]quinoline Derivatives † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2502. |
| [8] | DONG Qiuchen,ZHANG Guanghua,ZHANG Wanbin,LIU Jing. Experimental and Theoretical Analysis of Quinoline Diquaternary Ammonium Salt as Corrosion Inhibitor † [J]. Chem. J. Chinese Universities, 2019, 40(10): 2195. |
| [9] | ZHANG Guanghua,DONG Qiuchen,ZHANG Wanbin,WANG Shuang. Corrosion Inhibition of Q235 Steel by Octyl Dimethyl Benzyl Quaternary Ammonium Salt Ionic Liquid† [J]. Chem. J. Chinese Universities, 2019, 40(1): 130. |
| [10] | LIU Haichun, LU Shuai, ZHANG Yanmin, ZHOU Weineng, YIN Lingfeng, ZHU Lu, ZHAO Junnan, LU Tao, CHEN Yadong. Molecular Dynamics Simulation of the Selectivity of Fedratinib Complex with JAK2/JAK3† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1540. |
| [11] | DONG Shigang, GAO Yingbo, GUAN Zichao, WANG Haipeng, WANG Xia, DU Ronggui, SONG Guangling. Inhibition Effect of Polyvinylpyrrolidone on Corrosion of Reinforcing Steel in Simulated Concrete Pore Solutions† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1260. |
| [12] | LU Tong, WANG Chunyang, ZHU Zhihui, JIANG Wei, HUO Mingnan, LI Fei. Polyethyleneimine Functionalized Graphene Oxide Against hIAPP Amyloid Aggregation† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1274. |
| [13] | SHI Yanli, LIU Yubo, WU Sijin, LIU Yajun, ZHANG Jianing, LI Wenli. Virtual Screening and Activity Verification of Novel Inhibitors of ApIV 3C Protease† [J]. Chem. J. Chinese Universities, 2018, 39(4): 701. |
| [14] | WANG Lei, ZHENG Guojun, JI Qi, CHEN Bo, GONG Longlong, GAO Congmin, DU Zhenjian, ZHANG Xingmin. Synthesis and Biological Activity of Novel PI3K/mTOR Inhibitors† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1590. |
| [15] | DUAN Yongbin, YIN Yan, MENG Fanli, ZHAO Lianhua, LIU Yukun, YUAN Zhe, FENG Yangbo. Design, Synthesis and Biological Evaluation of Benzothiazoles as Highly Potent ROCK Inhibitors Through Molecular Docking and Free Energy Calculations† [J]. Chem. J. Chinese Universities, 2017, 38(9): 1568. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||