

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (10): 1841.doi: 10.7503/cjcu20170065
• Physical Chemistry • Previous Articles Next Articles
TIAN Yi, LI Yuexiang*( ), PENG Shaoqin
), PENG Shaoqin
Received:2017-01-26
Online:2017-10-10
Published:2017-09-22
Contact:
LI Yuexiang
E-mail:liyx@ncu.edu.cn
Supported by:CLC Number:
TrendMD:
TIAN Yi, LI Yuexiang, PENG Shaoqin. Effect of Y2O3 Supporter on the Catalytic Hydrogen Production from an Aqueous Formaldehyde Solution Catalyzed by Metal Cu Loaded on Y2O3†[J]. Chem. J. Chinese Universities, 2017, 38(10): 1841.
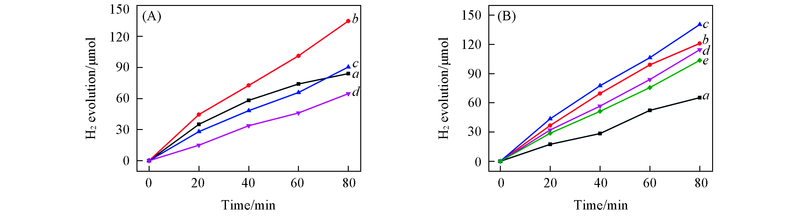
Fig.5 Effects of Cu loading of x-Cu/Y2O3(A) and formaldehyde concentration(B) on hydrogen generation under N2 atmosphere(A) x(%): a. 5; b. 10; c. 15; d. 20. Reaction conditions: 0.50 mol/L of NaOH; 0.60 mol/L of HCHO; a given amount of x-Cu/Y2O3 Cu containing 5 mg Cu. (B) cHCHO/(mol·L-1): a. 0.10; b. 0.30; c. 0.60; d. 0.90; e. 1.20. Reaction conditions: 0.50 mol/L of NaOH; 50 mg of 10-Cu/Y2O3.
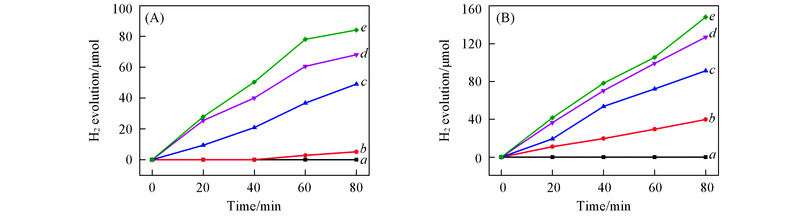
Fig.6 Effect of NaOH concentration on hydrogen generation over nano Cu(A) and 10-Cu/Y2O3(B) under N2 atmospherecNaOH/(mol·L-1): a. 0; b. 0.05; c. 0.10; d. 0.25; e. 0.50. Reaction conditions: 0.60 mol/L of HCHO;5 mg of nano Cu or 50 mg of 10-Cu/Y2O3.
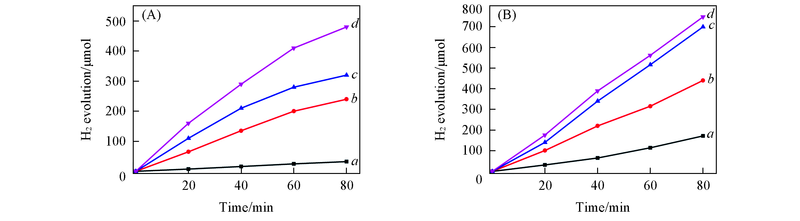
Fig.7 Effect of NaOH concentration on hydrogen generation over nano Cu(A) and 10-Cu/Y2O3(B) under air atmospherecNaOH/(mol·L-1): a. 0.05; b. 0.10; c. 0.25; d. 0.50. Reaction conditions are the same as those in Fig.6.
| Catalyst | Reaction condition | Minimum concentration/ (mol·L-1) | Optimal or used concentration/ (mol·L-1) | Optimal H2 evolution rate/ (mL·g-1·min-1) | Ref. |
|---|---|---|---|---|---|
| 10-Cu/Y2O3 | 0.60 mol/L HCHO; 50 mg catalyst | 0.050 | 0.25 | 39.8 | This work |
| Nano Cu | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 29.5 | [ |
| Nano Cu | 0.50 mol/L HCHO; 20 mg catalyst | 1.0 | 9.6 | [ | |
| Hollow Pd nanotube | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 168.0 | [ |
| Rh nanotube | 0.50 mol/L HCHO; 5 mg catalyst | 1.0 | 275.0 | [ | |
| Nano Au | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 29.5 | [ | |
| Au nanotube | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 154.0 | [ | |
| Nano Ag | 0.50 mol/L HCHO; 10 mg catalyst | 0.25 | 0.50 | 164.0 | [ |
| Ag/γ-Al2O3 | 0.87 mol/L HCHO; 50 mg catalyst | >0.10 | 2.0 | 415.0 | [ |
| AgPd4@C-72 | 0.26 mol/L HCHO; 15 mg catalyst | 0.50 | 1.0 | 237.0 | [ |
| Pd/TiO2 | 0.60 mol/L HCHO; 15 mg catalyst | 0.25 | 1.0 | 250.0 | [ |
Table 1 Comparison of minimum and optimal(used) NaOH concentrations and optimal H2 evolution rate under air atmosphere from formaldehyde solution over various catalysts at room temperature
| Catalyst | Reaction condition | Minimum concentration/ (mol·L-1) | Optimal or used concentration/ (mol·L-1) | Optimal H2 evolution rate/ (mL·g-1·min-1) | Ref. |
|---|---|---|---|---|---|
| 10-Cu/Y2O3 | 0.60 mol/L HCHO; 50 mg catalyst | 0.050 | 0.25 | 39.8 | This work |
| Nano Cu | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 29.5 | [ |
| Nano Cu | 0.50 mol/L HCHO; 20 mg catalyst | 1.0 | 9.6 | [ | |
| Hollow Pd nanotube | 0.48 mol/L HCHO; 10 mg catalyst | 0.25 | 1.0 | 168.0 | [ |
| Rh nanotube | 0.50 mol/L HCHO; 5 mg catalyst | 1.0 | 275.0 | [ | |
| Nano Au | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 29.5 | [ | |
| Au nanotube | 0.50 mol/L HCHO; 10 mg catalyst | 1.0 | 154.0 | [ | |
| Nano Ag | 0.50 mol/L HCHO; 10 mg catalyst | 0.25 | 0.50 | 164.0 | [ |
| Ag/γ-Al2O3 | 0.87 mol/L HCHO; 50 mg catalyst | >0.10 | 2.0 | 415.0 | [ |
| AgPd4@C-72 | 0.26 mol/L HCHO; 15 mg catalyst | 0.50 | 1.0 | 237.0 | [ |
| Pd/TiO2 | 0.60 mol/L HCHO; 15 mg catalyst | 0.25 | 1.0 | 250.0 | [ |
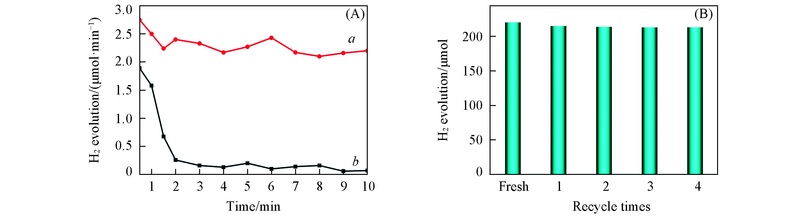
Fig.8 Time curves of 10-Cu/Y2O3(a) and nano Cu(b) for H2 production from formaldehyde solution(A) and reusability of 10-Cu/Y2O3 for H2 production from formaldehyde solutionReaction conditions are the same as those in Fig.5(A) except the reaction time. (B) Reaction conditions: 0.50 mol/L NaOH; 0.60 mol/L of HCHO; 100 mg 10-Cu/Y2O3, N2 atmosphere.
| [1] | Li Y. X., Hou Y. L., Fu Q. Y., Peng S. Q., Hu Y. H., Appl. Catal. B: Environ., 2017, 206, 726—733 |
| [2] | Li Y. X., Wang J. X., Peng S. Q., Lu G. X., Li S. B.,Int. J. Hydrogen Energy,2010, 35(13), 7116—7126 |
| [3] | Li Y. X., Wang H., Peng S. Q., J. Phys. Chem. C,2014, 118(34), 19842—19848 |
| [4] | Zhang W. Y., Li Y. X., Peng S. Q., ACS Appl. Mater. Interfaces,2016, 8(24), 15187—15195 |
| [5] | Zhang X., Zhong X., Yang Z., Song J., Lu H., Chem. Res. Chinese Universities,2016, 32(6), 1016—1018 |
| [6] | Ma Y., Li X. Z., Li Y. T., Liu D. M., Zhang Q. A., Si T. Z., Chem. J. Chinese Universities,2016, 37(10), 1776—1783 |
| (马勇, 李祥志, 李永涛, 柳东明, 张庆安, 斯庭智. 高等学校化学学报, 2016, 37(10), 1776—1783) | |
| [7] | Heim L. E., Schlörer N. E., Choi J. H., Prechtl M. H., Nat. Commun., 2014, 5, 4621 |
| [8] | Tsang M. H., Jwu T. C., Gene H. L., Yu W., J. Am. Chem. Soc., 1993, 115(3), 1170—1171 |
| [9] | Hu H. Y., Jiao Z. B., Ye J. H., Lu G. X., Bi Y. P., Nano Energy,2014, 8, 103—109 |
| [10] | Bi Y. P., Hu H., Li Q., Lu G. X., Int. J. Hydrogen Energy,2010, 35(13), 7177—7182 |
| [11] | Wang T., Liu L., Zhu Z., Papakonstantinou P., Hu J., Liu H., Li M., Energy Environ. Sci., 2013, 6, 625—633 |
| [12] | Li Y. W., Chen T., Wang T., Zhang Y. P., Lu G. X., Bi Y. P., Int. J. Hydrogen Energy,2014, 39, 9114—9120 |
| [13] | Pan X., Wang L., Ling F., Li Y., Han D., Pang Q., Jia L., Int. J. Hydrogen Energy,2015, 40(4), 1752—1759 |
| [14] | Bi Y. P., Lu G. X., Int. J. Hydrogen Energy,2008, 33(9), 2225—2232 |
| [15] | Preti D., Squarcialupi S., Fachinetti G. Aerobic., Angew. Chem., Int. Ed., 2009, 48(26), 4763—4766 |
| [16] | Patil N. S., Uphade B. S., McCulloh D. G., Bhargava S. K., Choudhary V. R., Catal. Commun., 2004, 5(11), 681—685 |
| [17] | Biesinger M. C., Lau L. W. M., Gerson A. R., Appl. Surf. Sci., 2010, 257(3), 887—898 |
| [18] | Nakamura T., Tomizuka H., Takahashi M., J. Surf. Sci. Soc. Jpn., 1995, 16(8), 515—520 |
| [19] | Craciun V., Howard J., Lambers E. S., Singh R. K., Craciun D., Perriere J., Appl. Phys. A,1999, 69(1), 535—538 |
| [20] | Nefedov V. I., Firsov M. N., Shaplygin I. S., J. Electron. Spectrosc. Relat. Phenom., 1982, 26(1), 65—78 |
| [21] | Barreca D., Battiston G. A., Berto D., Gerbasi R., Tondello E., Surf. Sci. Spectra,2001, 8(3), 234—239 |
| [22] | Kuroda Y., Hamano H., Mori T., Yoshikawa Y., Nagao M., Langmuir,2000, 16(17), 6937—6947 |
| [23] | Li Y. X., Guo M. M., Peng S. Q., Lu G. X., Li S. B., Int. J. Hydrogen Energy,2009, 34(14), 5629—5636 |
| [24] | Işik M., Colloids Surf. B, 2008, 62(1), 97—104 |
| [25] | Bi Y. P., Lu G. X., Chem. Commun., 2008, 47, 6402—6404 |
| [26] | Bi Y. P., Lu G. X., Mater. Lett., 2008, 62(17), 2696—2699 |
| [27] | Bi Y. P., Lu G. X., Nanotechnology,2008, 19(27), 275306 |
| [28] | Gao S., Feng T., Wu Q., Feng C., Shang N., Wang C., RSC Adv., 2016, 6(107), 105638—105643 |
| [29] | Li S., Hu H., Bi Y. P., J. Mater. Chem. A,2016, 4(3), 796—800 |
| [30] | Walker J.F., Formaldehyde, 3th. Ed., Reinhold Publishing Corporation, London, 1964, 59—73 |
| [31] | Li Y. X., Lü G. X., Li S. B., Yu F., J. Mol. Catal.(China), 2002, 16(4), 241—246 |
| (李越湘, 吕功煊, 李树本, 俞飞. 分子催化, 2002, 16(4), 241—246) | |
| [32] | Azizi S. N., Ghasemi S., Amiripour F., Sens. Actuators B,2016, 227, 1—10 |
| [33] | Zhao C., Li M., Jiao K., J. Anal. Chem., 2006, 61(12), 1204—1208 |
| [34] | Starodubov S. S., Nechaev I. V., Vvedenskii A. V., Russ. J. Phys. Chem. A,2016, 90(1), 122—129 |
| [1] | CHEN Wangsong, LUO Lan, LIU Yuguang, ZHOU Hua, KONG Xianggui, LI Zhenhua, DUAN Haohong. Recent Progress in Photoelectrochemical H2 Production Coupled with Biomass-derived Alcohol/aldehyde Oxidation [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210683. |
| [2] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [3] | LI Shurong, WANG Lin, CHEN Yuzhen, JIANG Hailong. Research Progress of Metal⁃organic Frameworks on Liquid Phase Catalytic Chemical Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210575. |
| [4] | XUE Jinbo, GAO Guoxiang, SHEN Qianqian, LIU Tianwu, LIU Xuguang, JIA Husheng. Construction of a Novel S-scheme CdS-BiVO4 Heterojunction Photoelectrodes and Research on Hydrogen Production [J]. Chem. J. Chinese Universities, 2021, 42(8): 2493. |
| [5] | WU Qiliang, MEI Jinghao, LI Zheng, FAN Haidong, ZHANG Yanwei. Photo-thermal Coupling Water Splitting over Fe-doped TiO2 with Various Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(6): 1837. |
| [6] | XU Anqi, LI Bin, DU Fanglin. Synthesis of Ordered Mesoporous TiO2 and Their Application for Hydrogen Production from Photocatalytic Water-splitting [J]. Chem. J. Chinese Universities, 2021, 42(4): 978. |
| [7] | WANG Yishu, LI Xue, YAN Li, XU Hongyun, ZHU Yuxin, SONG Yanhua, CUI Yanjuan. Photocatalytic Reduction Performance of Z-scheme Two-dimensional BCN/Sn3O4 Composite Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3722. |
| [8] | NING Qiuyang, FENG Wei, WU Guoguang. Preparation of InN-In2O3 Nanocomposite with Bottle-shaped Structure and Its Enhanced Formaldehyde Gas Sensitivity [J]. Chem. J. Chinese Universities, 2020, 41(12): 2804. |
| [9] | DUAN Yajun,CHENG Yanyan,SUI Guanghui,ZHU Yanchao,WANG Xiaofeng,GUO Yupeng,WANG Zichen. Lignin Impacts on the Lignin-urea-formaldehyde Copolymer Resin and the Reaction Mechanism† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1058. |
| [10] | WANG Zhipeng,NIU Zhuzhu,BAN Lijun,HAO Quanai,ZHANG Hongxi,LI Haitao,ZHAO Yongxiang. Formaldehyde Ethynylation Reaction over Cu2O Supported on TiO2 with Different Phases† [J]. Chem. J. Chinese Universities, 2019, 40(2): 334. |
| [11] | SUN Dawei,LI Yuejun,CAO Tieping,ZHAO Yanhui,YANG Diankai. Preparation of Dy 3+-doped YVO4/TiO2 Composite Nanofibers with Three-dimensional Net-like Structure and Enhanced Photocatalytic Activity for Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2348. |
| [12] | DAI Hongyan, YANG Huimin, LIU Xian, JIAN Xuan, GUO Minmin, CAO Lele, LIANG Zhenhai. Preparation and Electrochemical Evaluation of MoS2/graphene as a Catalyst for Hydrogen Evolution in Microbial Electrolysis Cell† [J]. Chem. J. Chinese Universities, 2018, 39(2): 351. |
| [13] | WANG Xuan, JIN Tao, WANG Haowei, LIAO Shengzhi, YANG Huaiyu. Preparation and Characterization of Polysulfide Sealant Microcapsules Based on in situ Polymerization of Urea and Formaldehyde† [J]. Chem. J. Chinese Universities, 2018, 39(2): 397. |
| [14] | MENG Qingnan, WANG Kai, TANG Yufei, ZHAO Kang. Facile Synthesis of Hollow Silica Particles and Fine Tuning Their Structures† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2550. |
| [15] | CHAO Wei, YANG Xiaomin, ZHOU Yu, ZHU Yanchao, WANG Zichen. Lignin Impacts on the Preparation of Phenolic-resin/Lignin Block Copolymer Resin and the Reaction Mechanism† [J]. Chem. J. Chinese Universities, 2017, 38(2): 312. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||