

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (2): 351.doi: 10.7503/cjcu20170255
• Physical Chemistry • Previous Articles Next Articles
DAI Hongyan1,2, YANG Huimin1, LIU Xian3, JIAN Xuan1, GUO Minmin1, CAO Lele1, LIANG Zhenhai1,*( )
)
Received:2017-04-21
Online:2018-02-10
Published:2017-12-23
Contact:
LIANG Zhenhai
E-mail:liangzhenh@sina.com
Supported by:CLC Number:
TrendMD:
DAI Hongyan, YANG Huimin, LIU Xian, JIAN Xuan, GUO Minmin, CAO Lele, LIANG Zhenhai. Preparation and Electrochemical Evaluation of MoS2/graphene as a Catalyst for Hydrogen Evolution in Microbial Electrolysis Cell†[J]. Chem. J. Chinese Universities, 2018, 39(2): 351.
| Serial number | m(GO)/mg | m[(NH4)2MoS4]/mg | V(HHA)/mL | V(H2O)/mL | m(MoS2)/m(Gr) |
|---|---|---|---|---|---|
| 1# | 30 | 120 | 2 | 30 | 2.46∶1 |
| 2# | 40 | 80 | 2 | 40 | 1.23∶1 |
| 3# | 40 | 40 | 2 | 40 | 0.62∶1 |
| 4# | 80 | 40 | 2 | 80 | 0.31∶1 |
| 5# | 80 | 20 | 2 | 80 | 0.15∶1 |
Table 1 Material ratio for MoS2/Gr composites
| Serial number | m(GO)/mg | m[(NH4)2MoS4]/mg | V(HHA)/mL | V(H2O)/mL | m(MoS2)/m(Gr) |
|---|---|---|---|---|---|
| 1# | 30 | 120 | 2 | 30 | 2.46∶1 |
| 2# | 40 | 80 | 2 | 40 | 1.23∶1 |
| 3# | 40 | 40 | 2 | 40 | 0.62∶1 |
| 4# | 80 | 40 | 2 | 80 | 0.31∶1 |
| 5# | 80 | 20 | 2 | 80 | 0.15∶1 |
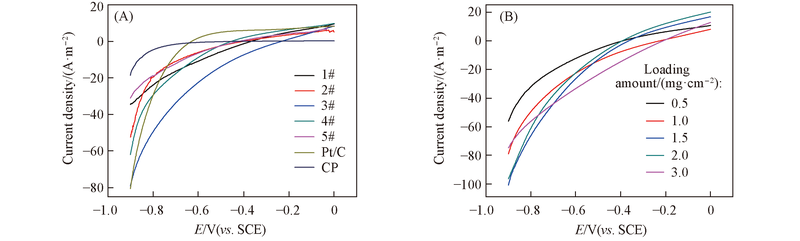
Fig.3 LSV curves of the electrodes loaded with 1 mg/cm2 composites(1#—5#), Pt/C and CP cathode(A) and the electrodes with different loading amounts of 3# composite(B)
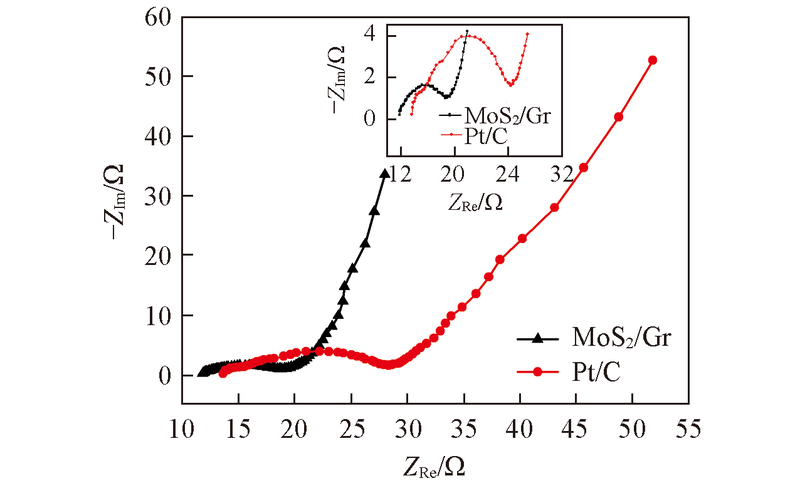
Fig.5 Nyquist plots of 3#MoS2/Gr(1.5 mg/cm2) and Pt/CEIS tests were conducted under the condition of open circuit voltage with a potential amplitude of 10 mV over a frequency range of 100 kHz—10 mHz. Inset: Medium-high frequency part.
| Cathode | RCE(%) | Rcat(%) | ηW(%) | ηW+S(%) | ||
|---|---|---|---|---|---|---|
| CP | 19.18±2.97 | 3.48±0.61 | 17.41±2.26 | 0.021±0.004 | 45.60±4.90 | 3.97±0.55 |
| Pt/C | 83.19±11.77 | 62.75±8.67 | 71.40±9.03 | 0.377±0.052 | 228.39±18.91 | 83.46±10.16 |
| MoS2/Gr | 89.11±5.87 | 70.47±6.78 | 78.86±2.49 | 0.424±0.041 | 227.59±15.55 | 81.92±5.86 |
Table 2 Energy efficiencies and hydrogen production in the MEC with different cathodes
| Cathode | RCE(%) | Rcat(%) | ηW(%) | ηW+S(%) | ||
|---|---|---|---|---|---|---|
| CP | 19.18±2.97 | 3.48±0.61 | 17.41±2.26 | 0.021±0.004 | 45.60±4.90 | 3.97±0.55 |
| Pt/C | 83.19±11.77 | 62.75±8.67 | 71.40±9.03 | 0.377±0.052 | 228.39±18.91 | 83.46±10.16 |
| MoS2/Gr | 89.11±5.87 | 70.47±6.78 | 78.86±2.49 | 0.424±0.041 | 227.59±15.55 | 81.92±5.86 |
| [1] | Zhang J. J., Li L., Hao Y. T., Sun L. L., Zhang X. Y., Chem. J. Chinese Universities, 2017, 38(2), 238—245 |
| (张晶晶, 李莉, 郝玉婷, 孙雷蕾, 张鑫悦. 高等学校化学学报, 2017,38(2), 238—245) | |
| [2] | Liu H., Grot S., Logan B. E., Environ. Sci. Technol., 2005, 39(11), 4317—4320 |
| [3] | Lupi C.,Dell'Era A., Pasquali M., Int. J. Hydrogen Energy, 2014, 39,1932—1940 |
| [4] | Kundu A., Sahu J. N., Redzwan G., Hashim M. A., Int. J. Hydrogen Energy, 2013, 38, 1745—1757 |
| [5] | Xiang Z. C., Zhang Z., Xu X. J., Zhang Q., Yuan C. W., Carbon,2016, 98, 84—89 |
| [6] | Deng J., Yuan W. T., Ren P. J., Wang Y., Deng D. H., Zhan Z., Bao X. H., RSC Adv., 2014, 4, 34733—34738 |
| [7] | Tokash J. C., Logan B. E., Int. J. Hydrogen Energy, 2011, 36, 9439—9445 |
| [8] | Li G. Q., Zhang D., Qiao Q., Yu Y. F., Peterson D., Zafar A., Kumar R., Curtarolo S., Hunte F., Shannon S., Zhu Y. M., Yang W. T., Cao L. Y., J. Am. Chem. Soc., 2016, 138(51), 16632—16638 |
| [9] | Laursen A. B., Kegnaes S., Dahl S., Chorkendorff I., Energy & Environmental Science, 2012, 5, 5577—5591 |
| [10] | Kong D. S., Wang H. T., Cha J. J., Pasta M., Koski K. J., Yao J., Cui Y., Nano Lett., 2013, 13, 1341—1347 |
| [11] | Huang X., Zeng Z. Y., Fan Z. X., Liu J. Q., Zhang H., Adv. Mater., 2012, 24(45), 5979—6004 |
| [12] | Ding M. J., Huang W., Yang P., Chem. J. Chinese Universities, 2015, 36(5), 238—245 |
| (丁敏娟, 黄徽, 杨平. 高等学校化学学报, 2015,36(5), 238—245) | |
| [13] | Liao L., Zhu J., Bian X. J., Zhu L. N., Scanlon M., Girault H. H., Liu B. H., Adv. Funct. Mater., 2013, 23(42), 5326—5333 |
| [14] | Logan B. E., Call D., Cheng S., Hamelers H. V. M., Sleutels T. H. J. A., Jeremiasse A. W., Rozendal R. A., Environ. Sci. Technol., 2008, 42, 8630—8640 |
| [15] | Xia X. H., Zheng Z. X., Zhang Y., Zhao X. J., Wang C. H., Int. J. Hydrogen Energy, 2014, 39, 9638—9650 |
| [16] | Yan Y., Xia B., Ge X., Liu Z., Wang J. Y., Wang X., Acs Appl. Mater. Inter., 2013, 5(24), 12794—12798 |
| [17] | Zhao S. Y., Li C. X., Wang L. P., Liu N. Y., Qiao S., Liu B. B., Huang H., Liu Y., Kang Z. H., Carbon,2016, 99, 599—606 |
| [18] | Lu Y. Z., Jiang Y. Y., Wei W. T., Wu H. B., Liu M. M., Niu L., Chen W., J. Mater. Chem., 2012, 22, 2929—2934 |
| [19] | Liu M. M., Chen W., Nanoscale,2013, 5, 12558—12564 |
| [20] | Hu W. H., Shang X., Han G. Q., Dong B., Liu Y. R., Li X., Chai Y. M., Liu Y. Q., Liu C. G., Carbon,2016, 100, 236—242 |
| [21] | Yan Y., Ge X. M., Liu Z. L., Wang J. Y., Lee J. M., Wang X., Nanoscale,2013, 5(17), 7768—7771 |
| [22] | Vrubel H., Merki D., Hu X. L., Energy & Environmental Science, 2012, 5(3), 6136—6144 |
| [23] | Zheng X. L., Xu J. B., Yan K. Y., Wang H., Wang Z. L., Yang S. H., Chem. Mater., 2014, 26, 2344—2353 |
| [24] | Merki D., Hu X. L., Energy & Environmental Science, 2011, 4(10), 3878—3888 |
| [25] | Gao M. R., Liang J. X., Zheng Y. R., Nat. Commun., 2015, 6, 5982 |
| [26] | Lu L., Hou D. X., Fang Y. F., Huang Y. P., Ren Z. Y. J., Electrochim. Acta, 2016, 206, 381—387 |
| [27] | Li Y. G., Wang H. L., Xie L. M., Liang Y. Y., Hong G. S., Dai H. J., J. Am. Chem. Soc., 2011, 133, 7296—7299 |
| [28] | Kong D. S., Wang H. T., Lu Z. Y., Cui Y., J. Am. Chem. Soc., 2014, 136, 4897—4900 |
| [29] | Wen Z. H., Ci S. Q., Mao S., Cui S. M., Lu G. H., Yu K. H., Luo S. L., He Z., Chen J. H., J. Power Sources, 2013, 234, 100—106 |
| [30] | De S., Muňoz L., Bergel A., Féron D., Basséguy R., Int. J. Hydrogen Energy, 2010, 35, 8561—8568 |
| [31] | Wang A. J., Liu W. Z., Cheng S. A., Xing D. F., Zhou J. Z., Logan B. E., Int. J. Hydrogen Energy, 2009, 34, 3653—3658 |
| [1] | CHEN Wangsong, LUO Lan, LIU Yuguang, ZHOU Hua, KONG Xianggui, LI Zhenhua, DUAN Haohong. Recent Progress in Photoelectrochemical H2 Production Coupled with Biomass-derived Alcohol/aldehyde Oxidation [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210683. |
| [2] | JIANG Shan, SHEN Qianqian, LI Qi, JIA Husheng, XUE Jinbo. Pd-loaded Defective TiO2 Nanotube Arrays for Enhanced Photocatalytic Hydrogen Production Performance [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220206. |
| [3] | LI Shurong, WANG Lin, CHEN Yuzhen, JIANG Hailong. Research Progress of Metal⁃organic Frameworks on Liquid Phase Catalytic Chemical Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210575. |
| [4] | XUE Jinbo, GAO Guoxiang, SHEN Qianqian, LIU Tianwu, LIU Xuguang, JIA Husheng. Construction of a Novel S-scheme CdS-BiVO4 Heterojunction Photoelectrodes and Research on Hydrogen Production [J]. Chem. J. Chinese Universities, 2021, 42(8): 2493. |
| [5] | WU Qiliang, MEI Jinghao, LI Zheng, FAN Haidong, ZHANG Yanwei. Photo-thermal Coupling Water Splitting over Fe-doped TiO2 with Various Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(6): 1837. |
| [6] | XU Anqi, LI Bin, DU Fanglin. Synthesis of Ordered Mesoporous TiO2 and Their Application for Hydrogen Production from Photocatalytic Water-splitting [J]. Chem. J. Chinese Universities, 2021, 42(4): 978. |
| [7] | WANG Yishu, LI Xue, YAN Li, XU Hongyun, ZHU Yuxin, SONG Yanhua, CUI Yanjuan. Photocatalytic Reduction Performance of Z-scheme Two-dimensional BCN/Sn3O4 Composite Materials [J]. Chem. J. Chinese Universities, 2021, 42(12): 3722. |
| [8] | SUN Dawei,LI Yuejun,CAO Tieping,ZHAO Yanhui,YANG Diankai. Preparation of Dy 3+-doped YVO4/TiO2 Composite Nanofibers with Three-dimensional Net-like Structure and Enhanced Photocatalytic Activity for Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2348. |
| [9] | TIAN Yi, LI Yuexiang, PENG Shaoqin. Effect of Y2O3 Supporter on the Catalytic Hydrogen Production from an Aqueous Formaldehyde Solution Catalyzed by Metal Cu Loaded on Y2O3† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1841. |
| [10] | LU Yonghong, WU Pingxiao, HUANG Junyi, TRAN Lytuong, ZHU Nengwu, DANG Zhi. Alkaline-assisted Hydrothermal Fabrication of CdZnS with Enhanced Visible-light Photocatalytic Performance† [J]. Chem. J. Chinese Universities, 2015, 36(8): 1563. |
| [11] | QU Yang, ZHOU Wei, REN Zhiyu, PAN Kai, JIANG Le, FU Honggang. Controllable Preparation of CdTiO3 Nanorods and Their Photocatalytic Properties for Hydrogen Production† [J]. Chem. J. Chinese Universities, 2014, 35(5): 995. |
| [12] | ZHAO Weiliang, LI Cong, HAN Xu, LI Tingting, ZHANG Guiju, GAN Xin, LI Fumin, FU Wenfu. Syntheses, Properties and Photocatalytic Hydrogen Evolution Efficiency of Cyclometalated Pt(Ⅱ) Complexes Bearing S-methylphenyl Group† [J]. Chem. J. Chinese Universities, 2014, 35(10): 2214. |
| [13] | HAO Wei-Chang*, ZHAI Ting-Ting, WANG Xu, WANG Tian-Min. Fabrication and Catalytic Activity of Nickel Core-shell Structure [J]. Chem. J. Chinese Universities, 2010, 31(6): 1213. |
| [14] | ZHANG Xin-Rong, WANG Lu-Cun, YAO Cheng-Zhang, CAO Yong, DAI Wei-Lin, FAN Kang-Nian, WU Dong, SUN Yu-Han . Highly Effective Hydrogen Production from Steam Reforming of CH3OH over Cu/ZnO/Al2O3 Catalysts Promoted by Nanostructured Carbon Materials [J]. Chem. J. Chinese Universities, 2004, 25(11): 2125. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||