

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (7): 1198.doi: 10.7503/cjcu20160960
• Organic Chemistry • Previous Articles Next Articles
YANG Jingjing, YU Yuewen, WANG Bingxiang*( ), JIANG Yuliang*(
), JIANG Yuliang*( )
)
Received:2016-12-29
Online:2017-07-10
Published:2017-04-11
Contact:
WANG Bingxiang,JIANG Yuliang
E-mail:wangbingxiang@njnu.edu.cn;07205@njnu.edu.cn
Supported by:CLC Number:
TrendMD:
YANG Jingjing, YU Yuewen, WANG Bingxiang, JIANG Yuliang. A Sensitive Fluorescent Probe for Cysteine Based on 1,8-Naphthalimide Derivative and Application in Bioimaging†[J]. Chem. J. Chinese Universities, 2017, 38(7): 1198.
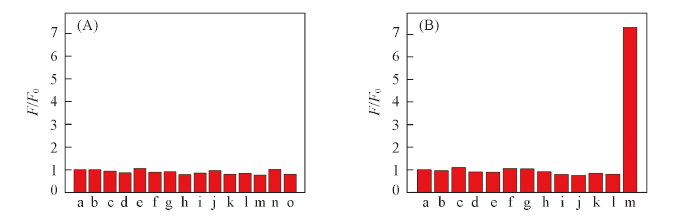
Fig.2 Various fluorescence intensity ratio(F/F0) of the probe NAD(50 μmol/L) in the absence and presence of various individual metal ions(A) and amino acids(B)F0 and F are the fluorescence intensity in the absence and presence of metal ions and amino acids, respectively. c(Metal ions)=c(amino acids)=10 μmol/L. (A) a. Blank; b. Co2+; c. Mn2+; d. Cr3+; e. Pb2+; f. Cu2+; g. Ni2+; h. Cd2+; i. Zn2+; j. Ca2+; k. Na+; l. Ag+; m. Fe3+; n. Mg2+; o. K+. (B) a. Blank; b. Met; c. His; d. Iso; e. Gly; f. Phe. g. Tyr; h. Val; i. Try; j. Leu; k. Ala; l. Thr; m. Cys.
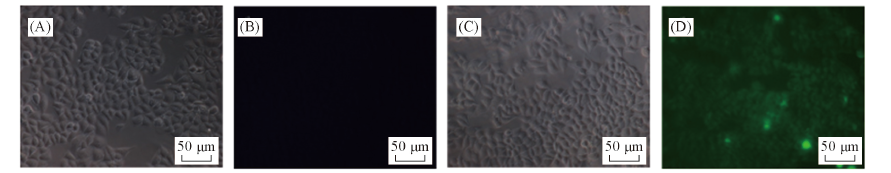
Fig.5 Fluorescence microscope images of HeLa cell with different treatmentBright-field image(A, C) and fluorescence mode(B, D) of HeLa cells treated with probe NAD(10 μmol/L)(A, B) and treated with probe NAD(10 μmol/L) and cysteine(10 μmol/L)(C, D). λex=488 nm.
| [1] | Reddie K. G., Carroll K. S., Curr. Opin. Chem. Biol., 2008, 12, 746—754 |
| [2] | Kim K. B., Kim H., Song E. J., Kim S., Noh I., Kim C., Dalton Trans., 2013, 42, 16569—16577 |
| [3] | Jo H. Y., Park G. J., Na Y. J., Choi Y. W., You G. R., Kim C., Dyes Pigments,2014, 109, 127—134 |
| [4] | Wu Y. R., Wang W. Q., Messing J., BMC Plant Biol., 2012, 12, 1—9 |
| [5] | Dai J., Ren W. L., Wang H. N., Shi Y., Org. Biomol. Chem., 2015, 13, 8429—8432 |
| [6] | Kawasaki T., Takamatsu N., Aiba S., Tokuna Y., J. Chem. Commun., 2015, 51, 14377—14380 |
| [7] | Rodríguez-Sanz A. A., Cabaleiro-Lago E. M., Rodríguez-Otero J., Org. Biomol. Chem., 2015, 13, 7961—7972 |
| [8] | Kirchhecker S., Tröger-Müller S., Bake S., Antonietti M., Taubert A., Esposito D., Green Chem., 2015, 17, 4151—4156 |
| [9] | Ozeryanskii V. A., Gorbacheva A. Y., Pozharskii A. F., Vlasenko M. P., Tereznikov A. Y., Org. Biomol. Chem., 2015, 13, 8524—8532 |
| [10] | Jia H. L., Ren K., Wang X., Li L. M., Sensor. Actuat.B,2016, 228, 308—316 |
| [11] | Yan L. Q., Kong Z. N., Shen W., Du W. Q., Zhou Y., Qi Z. J., Anal. Biochem., 2016, 500, 1—5 |
| [12] | Wald D. S., Law M., Morris J. K., BMJ-Br. Med. J., 2002, 325, 1202—1206 |
| [13] | Levine J., Timinsky I., Vishne T., Dwolatzky T., Roitman S., Kaplan Z., Kotler M., Sela B. A., Spivak B., Depress.>Anxiety,2008, 25, 154—157 |
| [14] | Li Q., Guo Y., Yue Y., Shao S., Sensor. Actuat.B,2012, 173, 797—801 |
| [15] | Yang Y., Soyoung S., Jinsung T., Chem. Commun., 2010, 46, 7766—7768 |
| [16] | Bobrowski A., Nowak K., Zarebski J., Anal. Bioanal. Chem., 2005, 382, 1691—1697 |
| [17] | Ohashi A., Ito H., Kanai C., Imura H., Ohashi K., Talanta,2005, 65, 525—530 |
| [18] | Lunvongsa S., Oshima M., Motomizu S., Talanta,2006, 68, 969—973 |
| [19] | Tesfaldet Z. O., Van Staden J. F., Stefan R. I., Talanta,2004, 64, 1189—1195 |
| [20] | Ma Q. J., Li H. P., Yang F., Zhang J., Wu X. F., Bai Y., Li X. F., Sensor. Actuat.B,2012, 166, 68—74 |
| [21] | Yang L., Yang W., Xu D. M., Zhang Z. Y., Liu A. F., Dyes Pigments,2013, 97, 168—174 |
| [22] | Kim H., Lee S., Lee J., Tae J., Org. Lett., 2010, 12, 5343—5345 |
| [23] | Silva A. P. D., Gunaratne H. Q. N., Gunnlaugsson T., Huxley A. J. M., McCoy C. P., Rademacher J. T., Chem. Rev., 1997, 97, 1515—1566 |
| [24] | Quang D. T., Kim J. S., Chem. Rev., 2007, 107, 3780—3799 |
| [25] | Yue Y., Guo Y., Xu J. A., Shao S. J., New J. Chem., 2011, 35, 61—64 |
| [26] | Li H. L., Fan J. L., Liu X. J., Sun S. G., Peng X. J., Chem. J. Chinese Universities,2010, 31(9), 1725—1728 |
| (李宏林, 樊江莉, 刘晓键, 孙世国, 彭孝军.高等学校化学学报, 2010, 31(9), 1725—1728) | |
| [27] | McGehee M. D., Heeger A. J., Adv. Mater., 2000, 12, 1655—1668 |
| [28] | Gao Y.Q., Marous R. A., J. Phys. Chem. A, 2002, 106, 1956—1960 |
| [29] | Kolosov D., Adamovich V., Djurovich P., Thompson M. E., Adachi C., J. Am. Chem. Soc., 2002, 124, 9945—9954 |
| [30] | Zhang Z. Y., Lu S. Z., Sha C. M., Xu D. M., Sensor. Actuat.B,2015, 208, 258—266 |
| [31] | Du W. W., Xu J., Li H. X., Feng C. C., Yu M. M., Li Z. X., Wei L. H., RSC Adv., 2015, 5, 15077—15083 |
| [32] | NiuC. G., Guan A. L., Zeng G. M., Liu Y. G., Huang G. H., Gao P. F., Gui X. Q., Anal. Chim.Acta,2005, 547, 221—228 |
| [33] | Liu C. X., Xu J., Yang F., Zhou W., Li Z. X., Wei L. H., Yu M. M., Sensor. Actuat.B,2015, 212, 364—370 |
| [34] | Zhou Y. M., Zhou H., Ma T. S., Zhang J. L., Niu J. Y., Spectrochimica Acta Part A,2012, 88, 56—59 |
| [1] | ZHAO Yongmei, MU Yeshu, HONG Chen, LUO Wen, TIAN Zhiyong. Bis-naphthalimide Derivatives for Picronitric Acid Detection in Aqueous Solution [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210765. |
| [2] | TANG Qian, DAN Feijun, GUO Tao, LAN Haichuang. Synthesis and Application of Quinolinone-coumarin-based Colorimetric Fluorescent Probe for Recognition of Hg2+ [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210660. |
| [3] | WANG Di, ZHONG Keli, TANG Lijun, HOU Shuhua, LYU Chunxin. Synthesis of Schiff-based Covalent Organic Framework and Its Recognition of I ‒ [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220115. |
| [4] | LI Anran, ZHAO Bing, KAN Wei, SONG Tianshu, KONG Xiangdong, BU Fanqiang, SUN Li, YIN Guangming, WANG Liyan. ON-OFF-ON Double Colorimetric and Fluorescent Probes Based on Phenanthro[9,10-d]imidazole Derivatives and Their Living Cells Imaging [J]. Chem. J. Chinese Universities, 2021, 42(8): 2403. |
| [5] | HUANG Shan, YAO Jiandong, NING Gan, XIAO Qi, LIU Yi. Efficient Determination of Alkaline Phosphatase Activity Based on Graphene Quantum Dots Fluorescent Probes [J]. Chem. J. Chinese Universities, 2021, 42(8): 2412. |
| [6] | YANG Xinjie, LAI Yanqiong, LI Qiuyang, ZHANG Yanli, WANG Hongbin, PANG Pengfei, YANG Wenrong. An Enzyme-free and Label-free Fluorescent Probe for Detection of Microcystin-LR Based on Circular DNA-Silver Nanoclusters [J]. Chem. J. Chinese Universities, 2021, 42(12): 3600. |
| [7] | CHEN Weiju, CHEN Shiya, XUE Caoye, LIU Bo, ZHENG Jing. Fluorescent Probe for Hypoxia-triggered Imaging and Cancer Therapy [J]. Chem. J. Chinese Universities, 2021, 42(11): 3433. |
| [8] | WANG Bodong, PAN Meichen, ZHUO Ying. Construction of Electrochemiluminescence Sensing Interface Based on Silver Nanoclusters-Silica Nanoparticles and Biomolecular Recognition [J]. Chem. J. Chinese Universities, 2021, 42(11): 3519. |
| [9] | HUANG Jialing,LIU Fengjiao,WANG Tingting,LIU Cuie,ZHENG Fengying,WANG Zhenhong,LI Shunxing. Nitrogen and Sulfur co-Doped Carbon Quantum Dots for Accurate Detection of pH in Gastric Juice† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1513. |
| [10] | FU Kefei, LIAN Huiting, WEI Xiaofeng, SUN Xiangying, LIU Bin. Construction of Cyclodextrin-based Impedance Sensor for Recognition of L-Cysteine † [J]. Chem. J. Chinese Universities, 2020, 41(4): 706. |
| [11] | PENG Yuyu,WANG Yu,YU Xinyao,ZENG Julan,XIAO Zhongliang,CAO Zhong. Rapid and Sensitive Detection of L-Cysteine Based on Mono(6-mercapto-6-deoxy)-β-cyclodextrin Modified Gold Electrode † [J]. Chem. J. Chinese Universities, 2020, 41(2): 268. |
| [12] | WU Qian, CHENG Dan, LÜ Yun, YUAN Lin, ZHANG Xiaobing. Monitoring of Peroxynitrite Variation During Liver Injury Adopting a Far Red to Near-infrared Fluorescent Probe with Large Stokes Shift [J]. Chem. J. Chinese Universities, 2020, 41(11): 2426. |
| [13] |
WANG Jinjin,QI Shaolong,DU Jianshi,YANG Qingbiao,SONG Yan,LI Yaoxian.
Synthesis of Benzothiazole Fluorescent Probe for Detection of N2H4·H2O and HS |
| [14] | Yong ZHANG,Cheng SHEN,Zhirong XING,Guiqi CHEN,Zi LU,Zhibing HOU,Xuemei CHEN. Benzimidazole-Derived Fluorescence Enhancement Probe for Visual Detection of HClO † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2480. |
| [15] | XIA Yuting, JIANG Bo, WU Qing, HU Qinghong, YUAN Zeli. Synthesis and Application of Colorimetric Probe for the Simultaneous Detection of Sulphion and Homocysteine/Cysteine in the Serum Samples† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1647. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||